Supplemental Digital Content is available in the text.
Abstract
Objective:
The increased incidence of multidrug-resistant organisms is associated with increased morbidity, mortality, hospital length of stay, and cost. Estimates show that up to 50% of antimicrobial use is inappropriate. This initiative focuses on inappropriate use of antibiotics in respiratory syncytial virus (RSV) infections. This virus is the most common cause of bronchiolitis during childhood.
Methods:
Baseline data from the 2011–2012 RSV season showed that 56.2% of our RSV-positive patients received antibiotics. To decrease inappropriate antibiotic use in RSV infections, we established an antimicrobial stewardship program (ASP). This process improvement initiative aimed to decrease exposure to antibiotics and days of antibiotic therapy per 1,000 patient days (DOT/1000PD) in hospitalized RSV-positive patients by 25%. Key drivers included building health-care knowledge, proactive interventions using prospective audit and feedback, emergency department engagement, and performance dashboards.
Results:
We included a total of 290 children in the final analysis. After full implementation of the ASP, there was a significant reduction of antibiotic exposure from 56.2% to 30.9% (P < 0.001), an absolute reduction of 25% and a relative reduction of 45%. There was also a significant decrease in DOT/1000PD from 432.7 to 268.1 days (P = 0.017). This change represents a reduction of 164.6 DOT/1000PD from baseline after full ASP implementation.
Conclusion:
Despite the lack of a unified hospitalist group in our institution, we were successful in reducing inappropriate antibiotic use by focusing on standardizing care among different private pediatricians in the community. A multifaceted strategy and well-designed quality improvement methodology led to a sustained reduction in antibiotic use.
What’s known on this subject: 50% of antimicrobial utilization is inappropriate, which includes misuse in viral infections. RSV is the most common cause of pediatric bronchiolitis. Per AAP guidelines, antibiotics should only be used if there are specific indications of coexisting bacterial infection in RSV.
What this study adds: Utilization of antibiotics in RSV is common, despite published guidelines. Implementation of ASP through ramping PDSA cycles led to a safe reduction in antibiotic utilization in RSV. This article demonstrates that by narrowing the scope of the project to RSV initially, foundation for a successful and sustained formal ASP is possible.
INTRODUCTION
The increased incidence of multidrug-resistant organisms (MDRO) over the last decade is a global medical crisis. Infections associated with MDROs increase morbidity, mortality, length of stay (LOS), and health-care costs.1 Evidence shows an association between the emergence of resistance pattern and the misuse/overuse of antibiotics.2 Estimates show that up to 50% of antimicrobial use is inappropriate.3 Antimicrobial stewardship programs (ASPs) are designed to improve appropriate antimicrobial use, lower costs, prevent the development of antimicrobial resistance, and improve therapeutic outcomes.1,4 Before this project, there was no formal ASP at our institution; we initiated this project as a first step toward the establishment of an organization-wide ASP. We targeted the inappropriate use of antibiotics in pediatric patients admitted with viral bronchiolitis due to respiratory syncytial virus (RSV). RSV is the most common cause of lower respiratory tract infections in infants and young children, accounting for more than 126,000 US hospitalizations annually.5 RSV is seasonal in the Northeast, beginning in late fall and continuing through early spring. Serious bacterial infections complicating RSV are rare where the rate of subsequent bacterial infection of children hospitalized with RSV bronchiolitis is 0.6–1.2%.6,7 Existing evidence-based guidelines from the American Academy of Pediatrics (AAP) discourage routine use of antibiotics in children admitted with bronchiolitis unless there is a specific documentation of coexistent bacterial infection.8,9 Despite these guidelines, antibiotics continue to be prescribed in 45–60% of children with viral bronchiolitis.8
We observed that antibiotic use in RSV bronchiolitis was frequent in our institution. Baseline data from our 2011–2012 RSV season showed that 56.2% of our RSV patients received antibiotics. The primary aim of this initiative was to reduce the percentage of patients with RSV bronchiolitis exposed to antibiotics in the subsequent RSV season by 25%. Exposure to antibiotics includes the receipt of antibiotic in the emergency department (ED) and the inpatient unit. The secondary aim was to reduce the number of days of antibiotic therapy per thousand RSV patient days (DOT/1000PD).
METHODS
This quality improvement project was conducted at the Children’s Medical Center at Winthrop University Hospital, a suburban, tertiary-care academic hospital. There are 95 pediatric inpatient beds and 19,000 pediatric ED visits per year and 48 pediatric medical residents. As a residency training program, teaching physicians are assigned to conduct daily multidisciplinary rounds on all patients. Many admissions on the pediatric inpatient unit are admitted under the private pediatricians from our local community as our institution does not have a full-time hospitalist model.
Our institution admits an average of 200 patients with bronchiolitis per year, of which 70% are confirmed to have RSV by testing. In our hospital, viral testing is routine during peak respiratory seasons. This practice facilitates patient cohorting and enhances infection prevention practices. We focused our initial interventions during November to February, when 72% of confirmed RSV-positive patients are admitted.
We assembled a multidisciplinary team, which included physicians, nurses, clinical pharmacists, representatives from microbiology, information technology, the pediatric ED, and quality improvement department. This quality improvement initiative was reviewed by the institutional review board (IRB) and determined “not subject to IRB human research protocol review.”
Our project included all pediatric patients admitted with viral bronchiolitis or viral pneumonia confirmed to be RSV-positive by RSV rapid testing or the respiratory multiplex Polymerase Chain Reaction BioFire Diagnostics Film Array (Biofire Diagnostics, LLC, Salt Lake City, Utah), used at our institution since May 2013.
We targeted patients with confirmed RSV infections by viral testing. We excluded high-risk RSV-positive patients with underlying comorbid conditions such as extreme prematurity (gestational age <28 wk), postnatal age <28 days of age, immunodeficiencies, underlying cardiac or chronic pulmonary disease, neuromuscular diseases, patients admitted to the pediatric intensive care unit, and patients with documented evidence of otitis media or urinary tract infections.
Our primary outcome measures included the percentage of patient infected with RSV exposed to antibiotics and DOT/1000PD in RSV-positive hospitalized patients. To calculate the percentage of patients exposed to antibiotics, we divided the number of patients receiving antibiotics by the total number of all RSV-infected patients per observation period. The secondary outcome measure is the number of days of antibiotic therapy per 1,000 RSV patient days (DOT/1000PD). This measure is the aggregate sum of administration days for each antibiotic agent divided by total RSV patient days and multiplied by 1,000. We performed our measurements from November to February of each year. Each observation period consisted of 20 consecutively admitted patients.
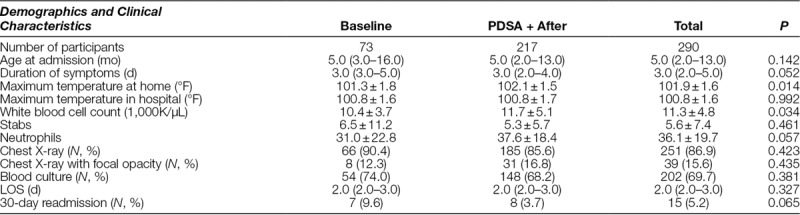
Data on age, chest radiograph, blood culture results, LOS, and readmissions were collected and compared between RSV seasons (Table 1).
Table 1.
Demographics and Clinical Characteristics
Establishment of Baseline Data
Retrospective chart reviews were performed to establish baseline utilization rates of antibiotics. We reviewed hospital discharge data using International Classification of Diseases (ICD-9) codes for RSV pneumonia/bronchiolitis (ICD 466.11 and 480.1) from the previous RSV season, November 2011 through February 2012.
Intervention
The interdisciplinary team identified a set of key drivers and a series of interventions to decrease antibiotic use (Fig. 1). We reviewed the literature on published guidelines to develop strategies in establishing our ASP.1
Fig. 1.
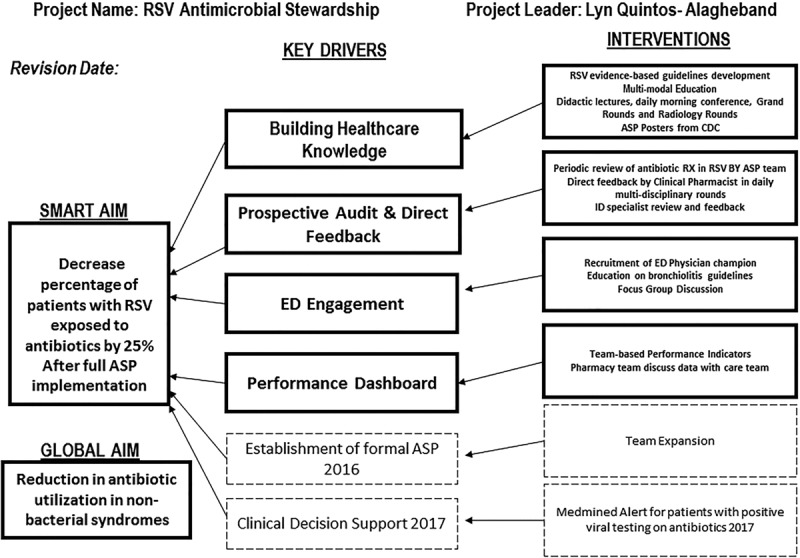
Key driver diagram used to achieve the desired goal.
Because education is essential for ASP acceptance, our initial key drivers were building health-care knowledge and prospective audit and feedback, with the goal to influence prescribing behaviors. Prospective audit with feedback to the provider was chosen as our proactive approach. This method is one of the core evidence-based strategies for promoting ASP identified by the Infectious Diseases Society of America (IDSA) and the Society for Healthcare Epidemiology of America (SHEA).1,10,11 Our third key driver focused on engaging the ED, where the majority of antibiotics are initiated.
To communicate performance standards and engage the frontline, we posted dashboards reflecting data on antibiotic prescribing in visible unit-based locations. We chose these cost-effective initial interventions as a necessary foundation for a formal ASP. We implemented our ASP interventions using a series of rapid Plan-Do-Study-Act (PDSA) cycles.
Intervention I (November 2012): Development of Guidelines and Educational Interventions
We distributed guidelines for management of RSV consistent with evidence-based literature from the AAP guidelines of 20068 (revised consistent with AAP 2014 guidelines)9 to attending physicians and house staff before the start of RSV seasons (see Appendix, Supplemental Digital Content 1, http://links.lww.com/PQ9/A18). We provided just in time monthly education sessions during house staff rotations. We focused our intervention on RSV-positive patients with a low-risk clinical profile for serious bacterial infections. We excluded patients with comorbid conditions outlined in our exclusion criteria, identified through review of medical records. Education was provided with lectures, morning case conferences, and pediatric grand rounds. Because our institution does not have a hospitalist-based system, it was critical to include the community pediatricians in our education campaign. We used our monthly quality improvement reports during general faculty grand rounds as a forum to educate primary care providers on RSV guidelines and the ASP program.
The pediatric radiologists were instrumental in the education process. During daily interdisciplinary radiology teaching rounds, emphasis was placed on focal findings consistent with viral processes. These findings were correlated with clinical summaries to allow decisions for early discontinuation of antibiotic therapy.
Intervention II (December 2012): Prospective Audit and Direct Feedback
The ASP team conducted periodic review of antibiotic prescribing in RSV-positive patients. A pediatric pharmacist participating in daily multidisciplinary rounds provided direct feedback to the team regarding discontinuation of antimicrobial therapy in RSV-positive patients. Various members of the ASP team, especially the pediatric infectious disease attendings, gave direct feedback regarding antibiotic use during clinical consultations and educational encounters.
Intervention III (January 2013)
Interim data review showed that the ED initiated the majority of antibiotics. Therefore, the focus of our third intervention was frontline engagement in the ED. We recruited a pediatric ED physician champion to the team. We disseminated bronchiolitis guidelines via group discussions and electronic mail. Education was also given to emphasize the importance of an ASP, and feedback on antibiotic usage in the ED for RSV cases was provided during focus group discussions.
Intervention IV (February 2013)
We displayed performance dashboards showing daily antibiotic use in RSV-positive patients in the pediatric resident conference room. Poster boards on antimicrobial stewardship from the Center for Disease Control were displayed in the pediatric inpatient unit and ED.
These 4 key drivers were the foundation for our ASP and were carried out over the course of 1 continuous RSV season (November 2012 to February 2013). Performance measures were continuously monitored during the same period of initial ASP implementation to assess the impact of each intervention. After initial implementation, all interventions were sustained and rolled into the development of a formal ASP. To measure sustainability, we monitored performance during peak RSV seasons (November 2013 to February 2014; November 2014 to February 2015 RSV season). In 2014, our hospital transitioned from paper to electronic medical record (EMR); however, we continued to manually review patient records for antibiotic use in the electronic health record.
Analyses
Control charts were used to measure the impact of the interventions on the percentage of antibiotic exposure and DOT/1000PD. Control charts were generated using Minitab software to track performance and analyze process shifts. Data before and after ASP implementation were compared. The Fisher’s exact test was used to analyze the percentage of patients exposed to antibiotics, and the 2 sample t test was used for DOT/1000PD data. A P value of <0.05 was considered to be statistically significant.
RESULTS
A total of 386 children were admitted to the Winthrop University Hospital pediatric unit with confirmed RSV infection from November 2011 through February 2015, and 290 children met criteria for our ASP intervention. There were 73 patients identified during the first year, which formed the baseline group, and 217 during the ASP period. The majority 93% (269 of 290) of the patients received a diagnosis of RSV bronchiolitis (ICD9 466.11). The percentage of patients identified with a diagnosis of RSV Pneumonia (ICD9 480.1) was 4% in the baseline group (3 of 73) and 8% during the intervention period (18 of 217).
The median age of the population was 5 months (interquartile range: 2–13 mo). Seventy percent of patients had blood cultures drawn. There was no significant change in the percentage of patients who received a blood culture from baseline. Two patients in the preintervention group had positive blood cultures: 1 patient with coagulase-negative Staphylococcus and 1 with Corynebacterium species. Both were determined to be contaminants by clinicians managing the patients. There were no positive blood cultures during the ASP period. The proportion of chest x-rays (CXRs) performed at baseline compared with post-ASP implementation was not significantly different. Overall, 15.6% of CXRs performed revealed focal opacities. There was no difference in mean LOS between baseline and post-ASP implementation (3 d). There was a decrease in 30-day readmissions in the ASP group to 3.7% from a baseline of 9.6%, which was not statistically significant (P = 0.065) (Table 1).
After the establishment of the ASP, there was a significant reduction in antibiotic exposure in RSV-positive patients, from 56.2% (N = 41/73) to 30.9% (N = 46/149), P < 0.001. This change represents a 25% absolute reduction or a 45% relative reduction. In our cohort, third-generation cephalosporins, N = 118 (82%), were the most commonly prescribed antibiotics for patients with possible pneumonia or sepsis. Other antibiotics were azithromycin, N = 5 (3%), amoxicillin, N = 7 (5%), ampicillin, N = 12 (9%), and clindamycin, N = 1 (1%). There were 432.7 (+477.8 SD) DOT/1000PD in the baseline group, which decreased to 268.1 (+476.5 SD) DOT/1000PD in the ASP group, P =0.017, reflecting a reduction from baseline of 164.6 DOT/1000PD after full ASP implementation.
The P-chart for the percentage of patients who received antibiotics (Fig. 2) shows that 7 of 8 data points were below the mean after full implementation of the ASP. The control chart for DOT/1000PD (Fig. 3) demonstrates 8 consecutive points below the mean reflecting a favorable center line shift after full ASP implementation (Shewhart Rules for Control Chart #2; Institute for Healthcare Improvement).12
Fig. 2.
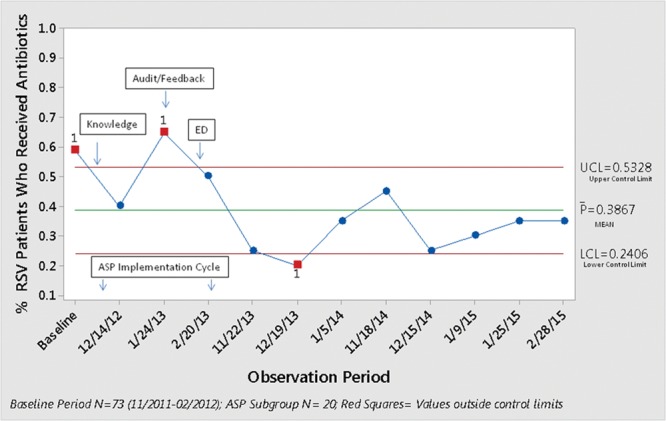
A control chart showing a reduction of patients exposed to antibiotics. The bar represents the average number of patients exposed to antibiotics during each season.
Fig. 3.
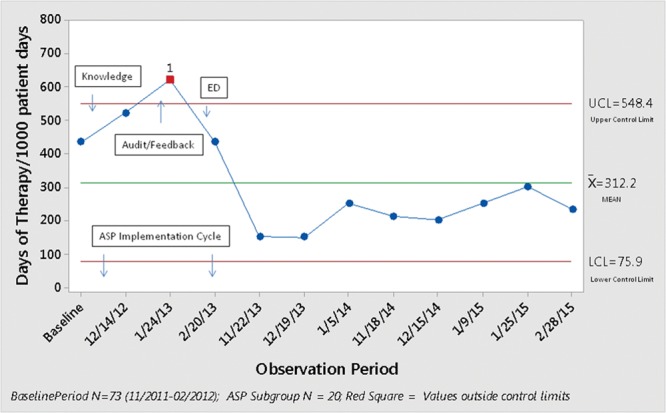
A control chart showing the reduction in days of antibiotic therapy/1,000 patient days.
DISCUSSION
Despite published guidelines, use of antibiotics in viral bronchiolitis is common.8,9 This article demonstrates that antibiotic use in RSV infections can be safely and effectively reduced. Through an ASP program, we were able to achieve a reduction in antibiotic exposure and DOT/1000PD.
Appropriate reduction in antibiotic use is possible with a comprehensive quality improvement program using a well-designed ramping of PDSA cycles. We started our first intervention with guidelines implementation and education before the anticipated start of peak RSV season. There was a reduction in antibiotic prescribing after the intervention; however, this was not sustained. Dellitt et al. described that although education is important to raise awareness and build knowledge foundation, it is not sufficient to change prescribing behaviors.1
We then implemented active interventions in the form of prospective audits and direct feedback to providers during interdisciplinary rounds by our pediatric clinical pharmacists. The clinical pharmacists’ input often led to successful discontinuation of antibiotics. We did not see a decrease in antibiotics use immediately after this intervention. In-depth analyses of the data showed that the majority of our patients received their first dose of antibiotics in the ED and 60% of patients continued to receive subsequent doses during the admission. This required active intervention from the ASP team to discontinue further doses. To ensure success, we needed to improve engagement with the ED staff. A crucial lesson learned is the need to incorporate ED personnel full force early on in the process and recruit a local champion. Serving as the gateway to the majority of pediatric admissions, the ED generates a first-dose antibiotic effect. Education of the ED providers was critical and also addressing their concerns on careful monitoring and subsequent follow-up of pending test results.
Together, these three initial interventions affected a downward trend in both percentages of antibiotic exposure and days of antibiotic therapy. This trend signals positive effects on prescribing behavior and earlier discontinuation of therapy.
We started performance dashboards and poster board displays on antimicrobial stewardship as visual reminders to engage the frontline staff. Continuously engaging the frontline through data discussions and weekly 10-minute huddles was a key to ensure success and sustainability.
One of the barriers we anticipated is a perceived threat to clinical decision autonomy from attending physicians. When implementing an ASP, it is important to identify those who are most likely to resist the ASP, understand and address these concerns, and highlight the benefit of proposed change.13 We set out early to keep the pediatricians informed through a series of grand round presentations on our initiative and engaged them on multidisciplinary rounds.
The use of routine viral testing in our institution gave us objective data when recommending discontinuation of antibiotics with providers. Doan et al.14, in their Cochrane review, concluded that the use of viral testing in the ED might reduce inappropriate antibiotic usage. A similar retrospective study in US children’s hospitals found an association with RSV testing and decreased use of antibiotics.15
Continuous quality improvement and data monitoring after a carefully planned implementation of the ASP led to a significant decrease in the use of inappropriate antibiotics for RSV bronchiolitis. The use of PDSA cycles, a well-designed QI tool, provided us the ability to study the effect of each intervention. Our control charts reflect improvement in the percentage of antibiotic prescribing and days of antibiotic therapy after these 4 key driver interventions. As in any process improvement, changing behavior takes time and requires continuous engagement. With our initial success, we continued all our interventions and rolled this foundation toward the development of a formal ASP at our institution. Our goal is to reduce further antibiotic prescribing in bronchiolitis according to the recent benchmark from the literature based on the Pediatric Health Information System which quotes 19% of children receiving antibiotics for bronchiolitis as the optimal target.16 With the recent implementation of MedMined (Bectin, Dickinson, and Co., Franklin Lakes, N.J.) infection surveillance software, we established alerts and clinical decision support for all patients with positive viral studies on antibiotics, as outlined in our key driver diagram. This will help us build the necessary infrastructure to accelerate our process improvement goals.
One limitation of our study is that it was performed at a single institution, and findings may not be readily applied to other settings. Another limitation includes the transition from paper to EMR at our institution during the project. The hybrid medical record during this transition necessitated brief retraining of team members on data abstraction. Both manual and electronic reviews of antibiotic prescribing were done to ensure data accuracy. The absence of routine confirmatory testing for RSV during off-respiratory seasons at our institution made it a challenge to identify our target patient population during those time periods. This led to a gap in data tracking during off-seasons, which may have impacted our results. Expanding the scope of this project to include all patients with clinical diagnosis of viral bronchiolitis in the future will bridge this gap. We cannot exclude the effects of other ongoing quality initiatives in our hospital that aim at decreasing unnecessary antibiotics. For example, the clinical effectiveness program for asthma management was a quality initiative started at our hospital in 2015. One of the components of the guidelines addresses decreasing inappropriate use of antibiotics in patients with asthma.
In conclusion, we have demonstrated a significant reduction in inappropriate use of antibiotics in hospitalized pediatric patients with RSV infection through our ongoing ASP. One of the biggest challenges in a setting with no full-time hospitalists is the need to standardize care among an extensive network of private pediatricians. We were able to overcome this barrier by engaging them in multiple settings throughout this ASP improvement project. Engaging the ED early on was also crucial as unnecessary antibiotics are often initiated there; hospitals with a similar structure could benefit by implementing these strategies.
With the recent updates on bronchiolitis guidelines, areas for future quality improvement initiatives should focus on decreasing unnecessary treatments such as albuterol and unnecessary testing such as chest radiographs and blood cultures. Future areas of efforts should also target inappropriate use of antibiotics in pediatric patients with other nonbacterial syndromes such as non-RSV bronchiolitis, viral syndromes, and acute viral gastrointestinal disorders.
DISCLOSURE
The authors have no financial interest to declare in relation to the content of this article.
Supplementary Material
Footnotes
Published online December 1, 2017
Supplemental digital content is available for this article. Clickable URL citations appear in the text.
REFERENCES
- 1.Dellit T, Owens R, McGowan J, et al. Diseases Society of America and Society for Healthcare Epidemiology of America Guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44:159–177.. [DOI] [PubMed] [Google Scholar]
- 2.Metjian TA, Prasad PA, Kogon A, et al. Evaluation of an antimicrobial stewardship program at a pediatric teaching hospital. Pediatr Infect Dis J. 2008;27:106–111.. [DOI] [PubMed] [Google Scholar]
- 3.Fishman N.Antimicrobial Stewardship. Am J Med. 2006;119(6 Suppl 1:S53–S61.. [DOI] [PubMed] [Google Scholar]
- 4.Hersh AL, Beekmann SE, Polgreen PM, et al. Antimicrobial stewardship programs in pediatrics. Infect Control Hosp Epidemiol. 2009;30:1211–1217.. [DOI] [PubMed] [Google Scholar]
- 5.Simões EA.RSV disease in the pediatric population: epidemiology, seasonal variability, and long-term outcomes. Manag Care 2008;17(11 Suppl 12):3–6, discussion 18.. [PubMed] [Google Scholar]
- 6.Hall CB, Powell KR, Schnabel KC, et al. Risk of secondary bacterial infection in infants hospitalized with respiratory syncytial viral infection. J Pediatr. 1988;113:266–271.. [PubMed] [Google Scholar]
- 7.Ralston S, Hill V, Waters A.Occult serious bacterial infection in infants younger than 60 to 90 days with bronchiolitis: a systematic review. Arch Pediatr Adolesc Med. 2011;165:951–956.. [DOI] [PubMed] [Google Scholar]
- 8.American Academy of Pediatrics Subcommittee on Diagnosis and Management of Bronchiolitis. Diagnosis and management of bronchiolitis. Pediatrics 2006;118:1774–1793.. [DOI] [PubMed] [Google Scholar]
- 9.Ralston SL, Lieberthal AS, Meissner HC, et al. American Academy of Pediatrics. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics 2014;134: e1474–e1502.. [DOI] [PubMed] [Google Scholar]
- 10.Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antibiotic stewardship program: Guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016;62:e51–e77.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drew RH.Antimicrobial stewardship programs: how to start and steer a successful program. J Manag Care Pharm. 2009;15(2 Suppl):S18–S23.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.http://www.ihi.org/education/WebTraining/OnDemand/Run_ControlCharts.
- 13.Goff DA.Antimicrobial stewardship: bridging the gap between quality care and cost. Curr Opin Infect Dis. 2011;24(Suppl 1):S11–S20.. [DOI] [PubMed] [Google Scholar]
- 14.Doan Q, Enarson P, Kissoon N, et al. Rapid viral diagnosis for acute febrile illness in children in the emergency department. Cochrane Database Syst Rev. 2012;5:CD006452. [DOI] [PubMed] [Google Scholar]
- 15.Christakis DA, Cowan CA, Garrison MM, et al. Variation in inpatient diagnostic testing and management of bronchiolitis. Pediatrics 2005;115:878–884.. [DOI] [PubMed] [Google Scholar]
- 16.Ralston S, Parikh K, Goodman D.Benchmarking overuse of medical interventions for bronchiolitis. JAMA Pediatr. 2015;169:805–806.. [DOI] [PubMed] [Google Scholar]


