Abstract
Background:
Postoperative hyperglycemia related to stress has been shown to be an independent risk factor for periprosthetic joint infection. In a non-intensive care, general-surgery setting, a standardized postoperative insulin protocol has been shown to decrease the rate of wound infections. We hypothesized that the use of a similar protocol is both safe and effective for controlling hyperglycemia in patients who have undergone total joint replacement.
Methods:
We performed a retrospective cohort study of 489 consecutive patients who underwent primary or revision total hip or knee arthroplasty between January 2008 and April 2013. All patients were tested with point-of-care (finger-stick) glucose determinations postoperatively and were started on a subcutaneous insulin protocol if they had postoperative stress hyperglycemia of >140 mg/dL when fasting or >180 mg/dL after meals. Insulin was discontinued when blood glucose decreased to <100 mg/dL.
Results:
Of the 489 patients, 301 (62%) qualified for the insulin protocol. Thirty-seven (17%) of the 220 patients for whom the hemoglobin A1c level was available were diabetic, and 21 (11%) of the 187 patients for whom body mass index data were available were morbidly obese (body mass index, ≥40 kg/m2). Diabetes (p < 0.001), revision surgery (p < 0.001), male sex (p = 0.0110), and obesity (including morbid obesity) (p = 0.0051) were independent factors resulting in significant glycemic elevation. A trend toward hyperglycemia occurred in younger patients but did not reach significance (p = 0.063). The glucose levels of patients in all of these groups responded well to insulin. None of the patients who were managed with the insulin experienced a periprosthetic joint infection. There were no injuries related to hypoglycemia.
Conclusions:
The findings of the present study suggest that hyperglycemia is a common link between seemingly disparate factors related to the increased prevalence of periprosthetic joint infection. The standardized subcutaneous insulin protocol was both safe and effective for the treatment of hyperglycemia for nondiabetic as well as diabetic patients. Patients who have undergone total joint replacement, especially those with revision procedures, male sex, morbid obesity, and diabetes, should be evaluated for hyperglycemia starting in the post-anesthesia care unit and should be managed with the insulin protocol when that risk is identified.
Level of Evidence:
Therapeutic Level IV. See Instructions for Authors for a complete description of levels of evidence.
The detrimental effect of elevated glucose levels on the immune system has long been recognized1,2, and postoperative elevation of glucose increases the risk of periprosthetic joint infections among patients who have undergone total knee or total hip replacement3-5. In an effort to preoperatively identify patients at high risk for periprosthetic joint infection, hemoglobin A1c (HbA1c) has been used. However, recent studies have suggested that this measure does not have good predictive validity for periprosthetic joint infection6-8. Recent research has suggested that postoperative hyperglycemia is an independent risk factor for postoperative infection among orthopaedic patients5,9. Furthermore, nondiabetic patients may be at a higher risk for stress-related periprosthetic joint infection than diabetic patients at a given level of hyperglycemia3. Previous studies also have suggested that male sex10-13, younger age14,15, obesity4,11,15-17, and revision procedures7,18 are related to higher rates of periprosthetic joint infection. The present study suggests that hyperglycemia represents a common link between these factors.
Two strategies are commonly used to prevent operative wound infections. The first is to decrease bacterial load at the time of surgery through time-honored strategies such as skin decolonization, sterile technique, wound irrigation, and decreasing operative time. The second is to optimize the host response to that bacterial load. The latter strategy can be implemented preoperatively, through the selection of healthy patients, or postoperatively, through the routine use of perioperative antibiotics and, more recently, the use of insulin for the postoperative treatment of stress-induced hyperglycemia to improve white blood-cell phagocytic function19.
The use of intravenous (IV) insulin to decrease wound infection was pioneered by cardiovascular surgeons managing patients in intensive care units (ICUs)20-22. The Rabbit-2 Trial (randomized study of basal-bolus insulin therapy in the inpatient management of patients with type-2 diabetes) involved a strategy involving the use of basal-bolus subcutaneous insulin to reduce wound infections among patients undergoing non-ICU general surgery procedures23; this strategy is applicable to patients undergoing total joint replacements.
The Mercy Health System introduced a Subcutaneous Basal-Bolus Insulin Protocol for use across all of its hospitals in 2007. In light of literature demonstrating increased risk of infection related to stress-induced hyperglycemia in nondiabetic as well as diabetic patients24, this protocol was initiated in 2008 to reduce the incidence of periprosthetic joint infection. The protocol was adapted for postoperative use by adding start and stop parameters (triggers) to ensure that insulin was initiated in a timely manner and was discontinued when appropriate to avoid hypoglycemia. In order to assess the effectiveness of this protocol, after obtaining approval by the Jewish Hospital-Mercy Health institutional review board, we conducted a retrospective cohort study of 301 patients who had undergone total joint replacement and were managed with insulin according to this protocol.
We hypothesized that the standardized insulin protocol would reduce hyperglycemia without resultant injury related to hypoglycemia caused by insulin.
Materials and Methods
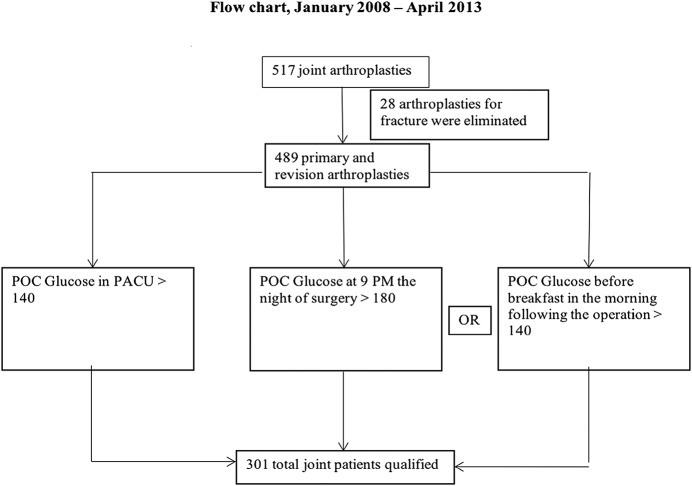
The present report describes a retrospective single-surgeon cohort study that was performed at a single institution. A review of the hospital database revealed that the senior author (J.M.G.) performed 517 total hip and knee arthroplasties between January 2008 and April 2013. All arthroplasties that were performed for the treatment of fracture, including periprosthetic fracture, were excluded, leaving 489 primary and revision total hip and knee replacements available for the study (Fig. 1). The patients were routinely given normal saline solution (without dextrose) intravenously during the operation and in the recovery room (post-anesthesia care unit [PACU]). Steroids were not routinely utilized for surgery unless the patient was steroid-dependent preoperatively.
Fig. 1.
Flow chart illustrating the selection of patients for the study. POC = point of care.
Three trigger points for starting the insulin were included in standard order sets, including a fasting glucose in the PACU, a random glucose test done at 9 p.m. after the operation, and a second fasting glucose test done prior to breakfast on the morning after the operation. Glucose determinations were made at the point of care with use of finger-sticks and measured with Accu-Chek instruments, which were rigorously maintained by the Mercy Central Laboratory and calibrated daily. Any patient who had glucose values of >140 mg/dL on fasting or >180 mg/dL on testing after meals was started on insulin as per American Association of Clinical Endocrinologists/American Diabetes Association (AACE/ADA) Consensus Guidelines25. Glucose determinations were continued 4 times a day—at the 3 standard meals and at bedtime—once the insulin was initiated. When any glucose value fell to <100 mg/dL, the use of basal and prandial insulin was discontinued. Point-of-care glucose determinations were continued until discharge. Patients who were known to have diabetes were managed with resumption of their preadmission diabetic medications, including insulin if appropriate.
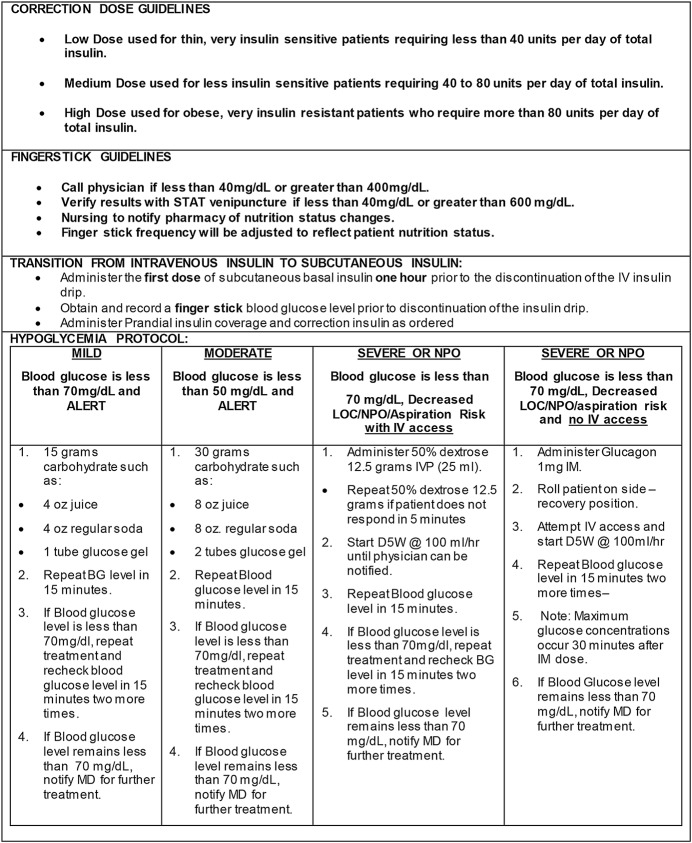
According to these criteria, 301 patients (62%) were managed with the insulin protocol (Figs. 1, and 2-A, 2-B). The protocol had 3 components. A basal dose of long-acting insulin (Lantus [insulin glargine]) was given in the evening according to the following formula: dose in units = 0.25 units/kg × body weight in kg as calculated by pharmacy. A prandial dose of short-acting insulin (Humalog [lispro] or aspart) was given just prior to or at the time of meals as long as the patient was eating at least 50% of his or her food. This dose was calculated as 0.25 units/kg× body weight in kg, divided in 3 doses (for 3 standard meals). Finally, a correction dose of lispro was added to the prandial dose if the blood sugar was >140, with half a dose given at bedtime if glucose was >200 mg/dL. The protocol included detailed nursing instructions for the treatment of any hypoglycemia that occurred. All oral diabetic medications were stopped during the patient’s time on the insulin protocol to prevent hypoglycemia.
Figs. 2-A and 2-B: The Mercy Health System insulin protocol.
Fig. 2-A.
Fig. 2-B.
The inclusion of patients was discontinued once the decision was made to initiate the study. A nurse performed a chart review for any complications or readmissions. Demographic and glycemic laboratory data were determined through a review of electronic medical records. Analysis was performed with use of the SAS statistical software package (JMP version 12.1, 2015).
Results
Demographic Data
Of the 301 patients, 183 were women. The mean age (and standard deviation) was 69.1 ± 10.7 years, and the mean length of stay in the orthopaedic unit was 2.9 days. The procedures included 187 primary total knee replacements (62%), 83 primary total hip replacements (28%), 19 revision total hip replacements (6%), and 12 revision total knee replacements (4%). Of the 187 patients for whom body mass index (BMI) data were available, 21 (11%) were morbidly obese (BMI, ≥40 kg/m2), 86 (46%) were obese (BMI, ≥30 to 40 kg/m2), 48 (26%) were overweight (BMI, ≥25 to 29.9 kg/m2), and 32 (17%) were a healthy weight (BMI, 18.5 to 24.9 kg/m2).
On the average, age at the time of the operation was linearly related to weight, with morbidly obese patients having surgery at 62.2 years; obese patients, at 67.6 years; overweight patients, at 70.8 years; and healthy-weight patients, at 75.4 years. The great majority of patients (246 patients; 82%) who triggered the protocol did so in the PACU.
Glycemic Data
For the purposes of the present study, diabetic status was based only on the perioperative HbA1c data obtained by Mercy Central Laboratory within 14 days before the operation or during the hospitalization. This definition of diabetes was a simplification as the HbA1c level can decrease below 6.5% in patients with well-controlled diabetes. Of the 220 patients for whom the HbA1c level was available, 37 (17%) were diabetic (HbA1c, ≥6.5%), 105 (48%) were prediabetic (HbA1c, ≥5.8 to 6.4%), and 78 (35%) were normal from a diabetes standpoint (HbA1c <5.8%). The preoperative glucose determinations were available for all 301 patients and were obtained by means of venipuncture as part of chemistry laboratory studies within 14 days before admission.
For the purpose of analysis, the point-of-care glucose determinations (4,139 in all) were converted to time-weighted mean daily glucose levels (hereafter referred to as glucose levels) for the 301 patients and were used to examine the postoperative glucose differences according to sex, type of procedure (primary or revision), BMI category, diabetic status, and age at the time of admission. Statistical methods used in this analysis included linear regression, nonparametric Wilcoxon rank-sum tests (because of the presence of outlying glucose levels and the non-normality of data within comparison groups), and t tests when normality of data within the comparison groups was observed.
When the glucose levels were compared according to BMI category, significantly higher glucose levels were observed on the day of surgery when morbidly obese patients (median, 163.76; interquartile range [IQR], 53.76; n = 21) were compared with healthy patients (median, 138.29; IQR, 31.32; n = 32) (p = 0.0051, Wilcoxon test) (Fig. 3). When the 37 patients with known diabetes were excluded, significantly higher glucose levels were again observed when overweight patients (median, 148.72; IQR, 29.12; n = 42) were compared with healthy patients (median, 135.14; IQR, 24.45; n = 28), when obese patients (median, 150.26; IQR, 33.03; n = 77) were compared with healthy patients, and when morbidly obese patients (median, 156.80; IQR, 37.28; n = 17) were compared with healthy patients (p = 0.0333, 0.0303, and 0.003, respectively). When preoperative glucose levels were compared with the levels on the day of surgery, morbidly obese patients also showed a significantly greater elevation (median, 55.75; IQR, 40.26; n = 20) than healthy patients (median, 34.5; IQR, 42.28; n = 25) (p = 0.0144).
Fig. 3.
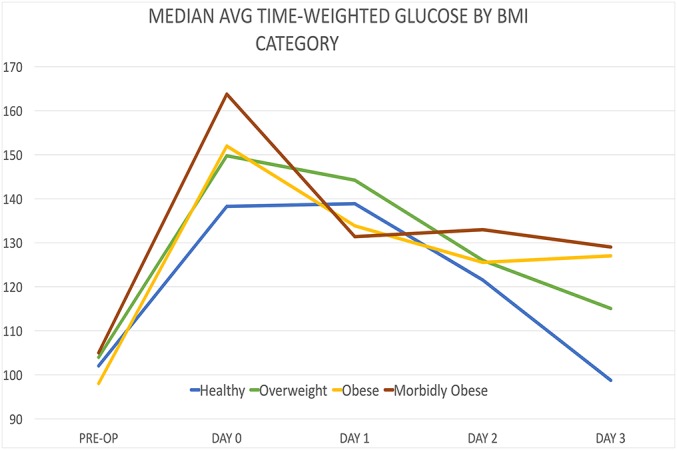
Line graph illustrating glucose by BMI category. (Note: for Figures 2 through 5, PRE-OP = within 14 days before surgery, DAY 0 = day of the operation, DAY 1 = first day after the operation, DAY 2 = second day after the operation, DAY 3 = third day after the operation.)
In the present study, male patients were significantly younger and had a significantly higher average daily glucose level compared with female patients (Fig. 4). Although the elevation in glucose levels on the day of surgery did not reach clinical significance, when the values were evaluated across the period of hospitalization, male patients (median, 133.97; IQR, 29.31; n = 118) had a significant persistent elevation of glucose in comparison with female patients (median, 127.4; IQR, 2.17; n = 183) (p = 0.0110).
Fig. 4.
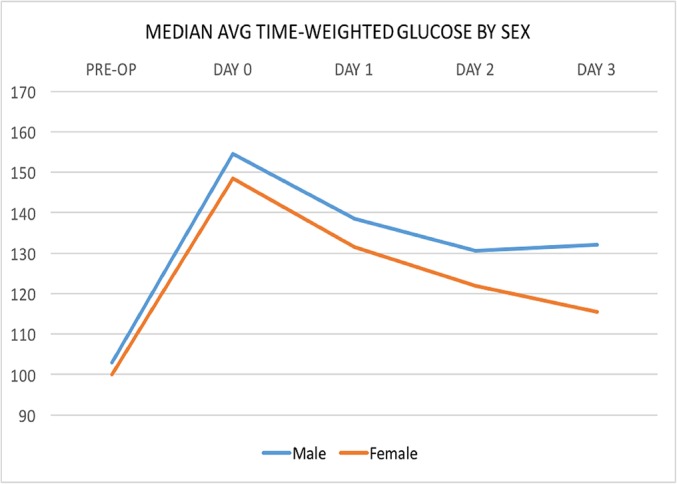
Line graph illustrating glucose by sex.
With respect to the type of surgery, revision procedures generally were associated with a greater risk of infection than primary procedures (Fig. 5). On the day of surgery, patients who had revision procedures (median, 166.89; IQR, 42.17; n = 31) had significantly higher glucose levels than those who underwent primary surgery (median, 148.57; IQR, 31.20; n = 270) (p < 0.001).
Fig. 5.
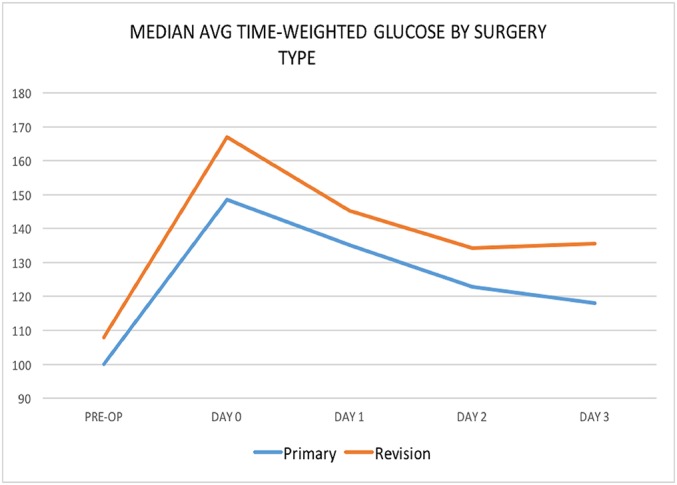
Line graph illustrating glucose by surgery type.
When diabetic patients were compared with normal and prediabetic patients, the natural log of the glucose levels on the day of surgery was analyzed because of the large variation of glucose values in the diabetic group (Fig. 6). These groups were compared with use of t tests, which indicated that diabetic patients had significantly higher log mean glucose levels (mean and standard deviation, 5.257 ± 0.237; n = 37) when compared with normal patients (4.967 ± 0.137; n = 78) and prediabetic patients (4.985 ± 0.178; n = 105) (p < 0.001 for both comparisons). These results are not surprising; however, when the change in glucose from preoperatively to the day of surgery was evaluated with use of the t test, there were no significant differences between diabetic patients (49.96 ± 51.37; n = 34) and normal patients (49.48 ± 25.03; n = 67) or between prediabetic patients (43.88 ± 26.21; n = 100) and normal patients (p > 0.31 for both comparisons).
Fig. 6.
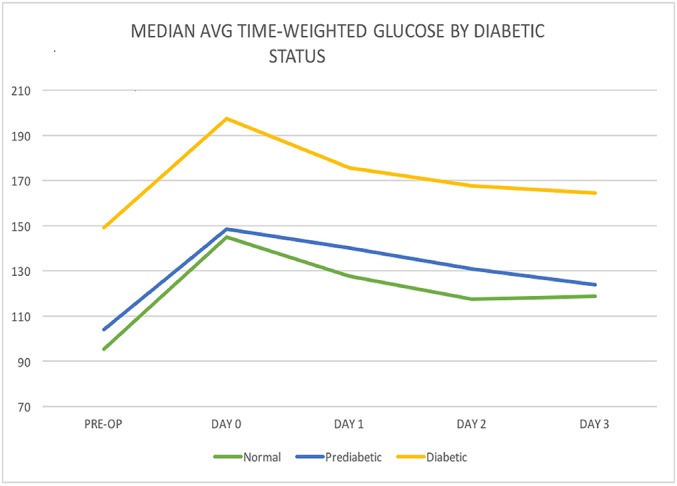
Line graph illustrating glucose by diabetic status.
Finally, the relationship between age and glucose level on the day of surgery was examined with use of linear regression. Glucose levels were transformed with use of the natural log to satisfy the assumptions of this model, and younger age was almost significant (p = 0.063). On the basis of the results of this model, for every year increase in age, the natural log of the glucose level decreased by 0.00198. Therefore, there was a trend for younger patients to have higher glucose levels after surgery, but this finding did not reach significance.
Complications
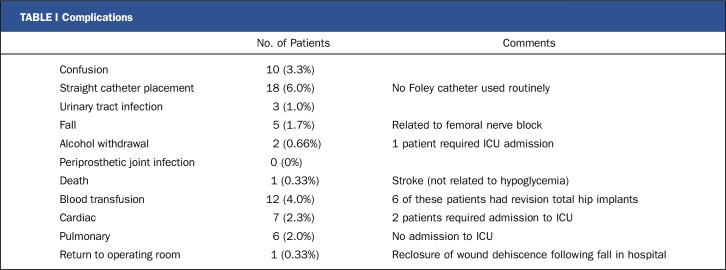
There were no periprosthetic joint infections in this study population. Twenty-five patients (8.3%) had a single episode of hypoglycemia with a glucose level of <70 mg/dL, including 4 patients (1.3%) with a glucose level of <50 mg/dL. This rate of hypoglycemia compares favorably with that in the Rabbit-2 study, in which hypoglycemia occurred in 21% of patients23. This improvement is attributed to the stop-insulin trigger that was added to the protocol. Female patients tend to become hypoglycemic more often than male patients. There were no injuries related to these hypoglycemic episodes. Complications included 5 falls, all of which were related to use of femoral nerve blocks. One of the 5 patients who fell while in the hospital was returned to the operating room for irrigation and reclosure of a traumatic wound dehiscence.
One patient in the treatment group died as the result of a cerebrovascular accident while in the hospital. She did not have a blood glucose level of <70 mg/dL, so the death was not related to any hypoglycemic event (Table I).
TABLE I.
Complications
Discussion
The present study demonstrates that a standardized protocol involving the use of subcutaneous insulin for the treatment of postoperative stress-induced hyperglycemia was both safe to use on an orthopaedic ward and effective for the correction of hyperglycemia in both diabetic and nondiabetic patients. The components of the protocol, including standard order sets for the use of basal-bolus subcutaneous insulin and point-of-care glucose monitors, are commonly available in most hospitals.
The barriers to the use of the insulin protocol have been a reluctance to use insulin from a safety standpoint (that is, a fear of the effects of hypoglycemia) and the lack of realization, until recently, that nondiabetic patients also are at risk for hyperglycemia-related periprosthetic joint infection3. The findings of the present study showed that hyperglycemia in nondiabetic patients responded to treatment with insulin in a predictable manner and that the stress-induced phenomenon generally subsided spontaneously within 48 hours, allowing for the discontinuation of insulin before the time of discharge. Although discontinuation of the insulin protocol at a threshold of 100 mg/dL is supported in the literature26, the stop trigger has now been changed to 110 mg/dL on the basis of the experience in the present study. This change, along with strict hypoglycemia guidelines, has led to increased safety. Diabetic patients respond more erratically (i.e., they have increased glycemic variability) and tend to have a slower decrease in hyperglycemia, occasionally requiring a longer time on the protocol. For a patient with a history of particularly poor glycemic control, a preoperative consultation with an endocrinologist would be in order.
The reason for the increased risk of infection in nondiabetic as compared with diabetic patients for a given level of hyperglycemia is not well understood; however, it appears to be related to an adjustment by the immune system of diabetic patients to chronic glycemic swings. The immune system of nondiabetic patients, on the other hand, appears to shut down in response to the unaccustomed hyperglycemia.
The primary limitation of the present study is that it was a retrospective cohort study involving a relatively small patient population. The absence of periprosthetic joint infection during this study is suggestive of efficacy against infection (with a national average incidence of approximately 0.5% to 1.5% for patients undergoing primary total joint replacement and at least double that for those undergoing revision total joint replacement7,18); however, because of the lack of a control group, the efficacy cannot be considered to have been proven.
The first strength of the present study is that it was based on the largest and most complete collection of postoperative glycemic data on patients with total hip and total knee replacements that we are aware of in the literature, with >4,000 point-of-care glucose levels for the 301 patients. Statistical analysis revealed correspondence of these data with known risk factors for infection already in the literature, including male sex10-13, morbid obesity 4,11,15-17, revision surgery7,8, and diabetes3-5. A trend toward hyperglycemia was noted in younger patients, which also been cited as a risk factor14,15. It is important to understand that the data do not represent the natural history of the stress-related glycemic response beyond the day of surgery but instead show the response to treatment, with all treatment groups responding in a salutary manner to the use of insulin. To understand the natural history of the glycemic stress response in patients undergoing total joint replacement, it is necessary to evaluate the work of Maeda et al.27, who closely collected glycemic data but did not manage their patients with insulin. In their study of 236 patients who had undergone total joint replacement, Maeda et al. demonstrated a persistence of hyperglycemia through the first 2 days postoperatively, with peak glucose levels occurring in the afternoon of the second day. All 3 of the infections in that study occurred in patients each of whom had an average glucose level of >200 mg/dL, regardless of their diabetic status.
The second strength of the present study is its unique approach to the problem of periprosthetic joint infection caused by hyperglycemia. Despite the widespread use of insulin protocols in other surgical disciplines such as open-heart surgery, general surgery, and colorectal surgery, there has been no apparent previous standardized use, especially for nondiabetic patients, in total joint surgery. While most authors in the field of orthopaedic surgery have focused on the preoperative evaluation of diabetes and hyperglycemia, the protocol described in the present study takes direct aim at the source of the risk: postoperative stress-induced hyperglycemia.
The use of this protocol requires cooperation on the part of the pharmacy, laboratory, hospitalists, and especially nursing staff for its safe and effective execution. The protocol can be safely managed on the orthopaedic ward without the need for monitoring in the ICU. Patients should be prepared for the possibility that if postoperative hyperglycemia is identified, a 2-day stay in the acute-care setting may be necessary.
The successful use of a standardized postoperative insulin protocol in the present study should encourage orthopaedists to join surgeons in other disciplines, not only those involved in total joint surgery but also those involved in other areas such as spine surgery and major trauma surgery, in treating the risks of postoperative hyperglycemia. A larger randomized, controlled study (similar to the Rabbit-2 study in general surgery) to test the efficacy against periprosthetic joint infection is warranted.
Disclosure of Potential Conflicts of Interest
Footnotes
Investigation performed at the Department of Orthopaedic Surgery, Mercy Health System, Cincinnati, Ohio
Disclosure: Funding through Mercy Health Administration. No external funding was received. The Disclosure of Potential Conflicts of Interest forms are provided with the online version of the article (http://links.lww.com/JBJSOA/A4).
References
- 1.Bagdade JD, Root RK, Bulger RJ. Impaired leukocyte function in patients with poorly controlled diabetes. Diabetes. 1974. January;23(1):9-15. [DOI] [PubMed] [Google Scholar]
- 2.Marhoffer W, Stein M, Maeser E, Federlin K. Impairment of polymorphonuclear leukocyte function and metabolic control of diabetes. Diabetes Care. 1992. February;15(2):256-60. [DOI] [PubMed] [Google Scholar]
- 3.Mraovic B, Suh D, Jacovides C, Parvizi J. Perioperative hyperglycemia and postoperative infection after lower limb arthroplasty. J Diabetes Sci Technol. 2011. March 01;5(2):412-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jämsen E, Nevalainen P, Eskelinen A, Huotari K, Kalliovalkama J, Moilanen T. Obesity, diabetes, and preoperative hyperglycemia as predictors of periprosthetic joint infection: a single-center analysis of 7181 primary hip and knee replacements for osteoarthritis. J Bone Joint Surg Am. 2012. July 18;94(14):e101-9. [DOI] [PubMed] [Google Scholar]
- 5.Stryker LS, Abdel MP, Morrey ME, Morrow MM, Kor DJ, Morrey BF. Elevated postoperative blood glucose and preoperative hemoglobin A1C are associated with increased wound complications following total joint arthroplasty. J Bone Joint Surg Am. 2013. May 01;95(9):808-14: S1-2. [DOI] [PubMed] [Google Scholar]
- 6.Iorio R, Williams KM, Marcantonio AJ, Specht LM, Tilzey JF, Healy WL. Diabetes mellitus, hemoglobin A1C, and the incidence of total joint arthroplasty infection. J Arthroplasty. 2012. May;27(5):726-9.e1. Epub 2011 Nov 4. [DOI] [PubMed] [Google Scholar]
- 7.Maradit Kremers H, Lewallen LW, Mabry TM, Berry DJ, Berbari EF, Osmon DR. Diabetes mellitus, hyperglycemia, hemoglobin A1C and the risk of prosthetic joint infections in total hip and knee arthroplasty. J Arthroplasty. 2015. March;30(3):439-43. Epub 2014 Oct 15. [DOI] [PubMed] [Google Scholar]
- 8.Chrastil J, Anderson MB, Stevens V, Anand R, Peters CL, Pelt CE. Is hemoglobin A1c or perioperative hyperglycemia predictive of periprosthetic joint infection or death following primary total joint arthroplasty? J Arthroplasty. 2015. July;30(7):1197-202. Epub 2015 Jan 31. [DOI] [PubMed] [Google Scholar]
- 9.Richards JE, Kauffmann RM, Zuckerman SL, Obremskey WT, May AK. Relationship of hyperglycemia and surgical-site infection in orthopaedic surgery. J Bone Joint Surg Am. 2012. July 03;94(13):1181-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh JA, Kwoh CK, Richardson D, Chen W, Ibrahim SA. Sex and surgical outcomes and mortality after primary total knee arthroplasty: a risk-adjusted analysis. Arthritis Care Res (Hoboken). 2013. July;65(7):1095-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bozic KJ, Ward DT, Lau EC, Chan V, Wetters NG, Naziri Q, Odum S, Fehring TK, Mont MA, Gioe TJ, Della Valle CJ. Risk factors for periprosthetic joint infection following primary total hip arthroplasty: a case control study. J Arthroplasty. 2014. January;29(1):154-6. Epub 2013 May 20. [DOI] [PubMed] [Google Scholar]
- 12.Poultsides LA, Ma Y, Della Valle AG, Chiu YL, Sculco TP, Memtsoudis SG. In-hospital surgical site infections after primary hip and knee arthroplasty—incidence and risk factors. J Arthroplasty. 2013. March;28(3):385-9. Epub 2012 Nov 8. [DOI] [PubMed] [Google Scholar]
- 13.Kurtz SM, Ong KL, Lau E, Bozic KJ, Berry D, Parvizi J. Prosthetic joint infection risk after TKA in the Medicare population. Clin Orthop Relat Res. 2010. January;468(1):52-6. Epub 2009 Aug 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meehan JP, Danielsen B, Kim SH, Jamali AA, White RH. Younger age is associated with a higher risk of early periprosthetic joint infection and aseptic mechanical failure after total knee arthroplasty. J Bone Joint Surg Am. 2014. April 02;96(7):529-35. [DOI] [PubMed] [Google Scholar]
- 15.Malinzak RA, Ritter MA, Berend ME, Meding JB, Olberding EM, Davis KE. Morbidly obese, diabetic, younger, and unilateral joint arthroplasty patients have elevated total joint arthroplasty infection rates. J Arthroplasty. 2009. September;24(6)(Suppl): 84-8. Epub 2009 Jul 15. [DOI] [PubMed] [Google Scholar]
- 16.Pulido L, Ghanem E, Joshi A, Purtill JJ, Parvizi J. Periprosthetic joint infection: the incidence, timing, and predisposing factors. Clin Orthop Relat Res. 2008. July;466(7):1710-5. Epub 2008 Apr 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wagner ER, Kamath AF, Fruth KM, Harmsen WS, Berry DJ. Effect of body mass index on complications and reoperations after total hip arthroplasty. J Bone Joint Surg Am. 2016. February 03;98(3):169-79. [DOI] [PubMed] [Google Scholar]
- 18.Bohl DD, Samuel AM, Basques BA, Della Valle CJ, Levine BR, Grauer JN. How much do adverse event rates differ between primary and revision total joint arthroplasty? J Arthroplasty. 2016. March;31(3):596-602. Epub 2015 Sep 28. [DOI] [PubMed] [Google Scholar]
- 19.Ata A, Lee J, Bestle SL, Desemone J, Stain SC. Postoperative hyperglycemia and surgical site infection in general surgery patients. Arch Surg. 2010. September;145(9):858-64. [DOI] [PubMed] [Google Scholar]
- 20.Furnary AP, Zerr KJ, Grunkemeier GL, Starr A. Continuous intravenous insulin infusion reduces the incidence of deep sternal wound infection in diabetic patients after cardiac surgical procedures. Ann Thorac Surg. 1999. February;67(2):352-60; discussion 360-2. [DOI] [PubMed] [Google Scholar]
- 21.Zerr KJ, Furnary AP, Grunkemeier GL, Bookin S, Kanhere V, Starr A. Glucose control lowers the risk of wound infection in diabetics after open heart operations. Ann Thorac Surg. 1997. February;63(2):356-61. [DOI] [PubMed] [Google Scholar]
- 22.Doenst T, Wijeysundera D, Karkouti K, Zechner C, Maganti M, Rao V, Borger MA. Hyperglycemia during cardiopulmonary bypass is an independent risk factor for mortality in patients undergoing cardiac surgery. J Thorac Cardiovasc Surg. 2005. October;130(4):1144. [DOI] [PubMed] [Google Scholar]
- 23.Umpierrez GE, Smiley D, Jacobs S, Peng L, Temponi A, Mulligan P, Umpierrez D, Newton C, Olson D, Rizzo M. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes undergoing general surgery (RABBIT 2 surgery). Diabetes Care. 2011. February;34(2):256-61. Epub 2011 Jan 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab. 2002. March;87(3):978-82. [DOI] [PubMed] [Google Scholar]
- 25.Umpierrez GE, Hellman R, Korytkowski MT, Kosiborod M, Maynard GA, Montori VM, Seley JJ, Van den Berghe G; Endocrine Society. Management of hyperglycemia in hospitalized patients in non-critical care setting: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012. January;97(1):16-38. [DOI] [PubMed] [Google Scholar]
- 26.Flory JH, Aleman JO, Furst J, Seley JJ. Basal insulin use in the non-critical care setting: is fasting hypoglycemia inevitable or preventable? J Diabetes Sci Technol. 2014. March;8(2):427-8. Epub 2014 Jan 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maeda Y, Nakamura N, Hawawaju M. Perioperative hyperglycemia and post operative infection after total knee and hip arthroplasty. Presented as a poster exhibit at the American Academy of Orthopaedic Surgery Annual Meeting; 2015. March 24-27; Las Vegas, Nevada. Poster no. P058. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.