Abstract
Background:
Propionibacterium species are commonly cultured from specimens harvested at the time of revision shoulder arthroplasty. These bacteria reside in normal sebaceous glands, out of reach of surgical skin preparation. The arthroplasty incision transects these structures, which allows Propionibacterium to inoculate the wound and to potentially lead to the formation of a biofilm on the inserted implant. To help identify patients who are at increased risk for wound inoculation, we investigated whether preoperative cultures of the specimens from the unprepared skin surface were predictive of the results of intraoperative cultures of dermal wound-edge specimens obtained immediately after incision of the surgically prepared skin.
Methods:
Sixty-six patients (mean age, 66.1 ± 9.4 years [range, 37 to 82 years]; 73% male) undergoing primary shoulder arthroplasty had preoperative cultures of the unprepared skin surface and intraoperative cultures of the freshly incised dermis using special culture swabs. For the first 50 patients, a control swab was opened to air during the same time that the dermal specimen was obtained. The results for female and male patients were characterized as the Specimen Propionibacterium Value (SpPV). We then determined the degree to which the results of cultures of the skin surface specimens were predictive of the results of culture of the dermal specimens.
Results:
The skin-surface SpPV was ≥1 in 3 (17%) of the 18 female patients and 34 (71%) of the 48 male patients (p < 0.001). The dermal SpPV was ≥1 in 0 (0%) of the 18 female patients and 19 (40%) of the 48 male patients (p < 0.001). None of the control samples had an SpPV of ≥1. The predictive characteristics of a skin-surface SpPV of ≥1 for a dermal SpPV of ≥1 were as follows: sensitivity, 1.00 (95% confidence interval [CI], 0.82 to 1.00); specificity, 0.62 (95% CI, 0.46 to 0.75); positive predictive value, 0.51 (95% CI, 0.34 to 0.68); and negative predictive value, 1.00 (95% CI, 0.88 to 1.00).
Conclusions:
A preoperative culture of the unprepared skin surface can help to predict whether the freshly incised dermal edge is likely to be positive for Propionibacterium. This test may help to identify patients who may merit more aggressive topical and systemic antibiotic prophylaxis.
Clinical Relevance:
This study shows that surgeons have the opportunity to use preoperative skin cultures to determine the likelihood that the shoulder arthroplasty wound will be culture-positive for Propionibacterium.
Shoulder arthroplasty failure is often associated with cultures of deep specimens, harvested at the time of revision surgery, that are positive for common commensal skin organisms such as Propionibacterium and coagulase-negative Staphylococcus1-3. While we are aware that the term Cutibacterium has recently been introduced to refer to these bacteria4, we retain the term Propionibacterium because of its familiarity to orthopaedic surgeons. These bacteria inhabit the dermal sebaceous glands under the skin surface, particularly in males5-7. Standard means of prophylaxis, including skin-surface preparation and systemic antibiotics, are unable to eliminate Propionibacterium from these dermal organs8-11. As a result, the surgical incision for shoulder arthroplasty is likely to transect these bacteria-containing structures, inoculating the wound with Propionibacterium. Once in the wound, Propionibacterium species have the potential to form a biofilm on the surface of the arthroplasty implants that may contribute to failure of the arthroplasty months or years after the joint replacement12.
Up to the present time, there has been no means to predict the risk of wound inoculation with Propionibacterium. If such a means were available, it could be useful for determining which patients merit extra efforts toward perioperative prophylaxis and longitudinal surveillance for the delayed presentation of Propionibacterium-related arthroplasty failure.
The purpose of the present study was to determine whether the results of preoperative cultures of specimens obtained from the unprepared skin at the site of the planned incision are predictive of the results of intraoperative cultures of freshly incised dermal wound-edge specimens obtained at the time of primary shoulder arthroplasty.
Materials and Methods
This study was approved by the University’s institutional review board (#50408). Between October 2016 and March 2017, 68 patients between the ages of 18 and 90 years undergoing primary shoulder arthroplasty without a history of shoulder surgery and without antibiotic use within the prior 3 weeks were approached for participation in this study. Two patients were excluded because they had received antibiotics within 3 weeks of the procedure. The remaining 66 consenting patients included 48 men (73%) and 18 women (27%) with an average age (and standard deviation) of 66.1 ± 9.4 years (range, 37 to 82 years).
On the day of surgery, a standardized swab specimen (Eswab #480C; Copan Diagnostics) was obtained from the unprepared skin at the site of the planned incision. Four passes were taken over a 2-cm area, with the swab being rotated 90° for each pass. After the administration of intravenous antibiotic prophylaxis (2 g of ceftriaxone and 1 g of vancomycin) and standard skin preparation with use of a 70% alcoholic chlorhexidine solution, an incision was made over the deltopectoral interval. Immediately after the incision, 2 culture swabs were opened. A control swab was held open to the air by a surgical assistant for the same length of time that the surgeon passed the other swab along the freshly exposed edge of the dermal incision. To reduce cost, control swabs were obtained only for the first 50 patients. The 2 swabs were capped simultaneously and were sent to the microbiology laboratory. All 3 specimens were processed by the laboratory in a Class-2 laminar flow biological safety cabinet within 1 hour after surgery. Specimens were inoculated onto the following microbiological media: blood agar (trypticase soy agar with 5% sheep blood), chocolate agar, Brucella agar (with blood, hemin, and vitamin K), and brain-heart infusion broth. All media except for the Brucella agar were incubated at 37°C with 5% CO2 for 21 days. The Brucella agar plates were incubated anaerobically at 37°C for 21 days. Plates were sealed in a manner that allowed sterile aeration without desiccation. Media were visually examined daily for growth but were only opened if growth was noted. Rather than categorizing the results as “positive” or “negative,” culture results were recorded in a semiquantitative manner and were categorized in terms of the 21-day specimen Propionibacterium value (SpPV), with 0 representing no growth; 0.1 representing 1 colony; 0.2 representing growth in broth only; and 1, 2, 3, and 4 representing 1+, 2+, 3+, and 4+ growth, respectively, as described previously6,13. Representative culture result reports are shown for the skin surface (Fig. 1-A), dermal (Fig. 1-B), and control samples (Fig. 1-C). The culture results for coagulase-negative Staphylococcus were also recorded in terms of specimen coagulase-negative Staphylococcus values (SpCV).
Figs. 1-A, 1-B, and 1-C Results of the 21-day cultures of specimens from a 42-year-old man who presented for a shoulder arthroplasty.
Fig. 1-A.
Results of the culture of a specimen from the unprepared skin surface. Combining the culture positivity for the 2 different species of Propionibacterium yielded an SpPV of 2. The SpCV value is 1. The Corynebacterium result was not considered in our analysis. Note that, despite the presence of these bacteria, the Gram smear was negative. C and S w/Gram, Ortho = culture and sensitivity with Gram stain as per orthopaedic protocol.
Fig. 1-B.
Results of the culture of a specimen from the freshly incised dermis. The SpPV was 3, and the SpCV was 0.
Fig. 1-C.
Results of the culture of the control swab. The SpPV was 0, and the SpCV was 0.
Descriptive statistics for the female and male patients were documented and were compared with use of the 2-sample t test, Wilcoxon rank-sum test, or Fisher exact test, as appropriate. Sensitivity, specificity, positive predictive value, and negative predictive value were calculated to determine the extent to which a dermal wound SpPV of ≥1 was predicted by an unprepared skin surface SpPV of ≥1. A receiver operating characteristic (ROC) curve was determined to illustrate the extent to which a freshly incised dermal wound SpPV of ≥1 was predicted by the skin-surface SpPV. Multivariate logistic regression fitted with use of the Firth penalized likelihood method14 assessed the collective effects of age, sex, and skin-surface culture on the likelihood of a dermal wound SpPV of ≥1. Statistical analysis was performed with R (version 3.4.0; R Foundation for Statistical Computing).
Results
Propionibacteria were often recovered from cultures of specimens from the unprepared skin surface and the fresh dermal wound (Table I). The culture results for women were significantly different from those for men. The skin surface SpPV was ≥1 in 3 (17%) of the 18 female patients, compared with 34 (71%) of the 48 male patients (p < 0.001). The dermal SpPV was ≥1 in 0 (0%) of the 18 female patients, compared with 19 (40%) of the 48 male patients (p < 0.001). While 2 of the control samples had SpPVs of >0, none of the control samples had an SpPV of ≥1. The predictive characteristics of a skin surface SpPV of ≥1 for a dermal SpPV of ≥1 were as follows: sensitivity, 1.00 (95% confidence interval [CI], 0.82 to 1.00); specificity, 0.62 (95% CI, 0.46 to 0.75); positive predictive value, 0.51 (95% CI, 0.34 to 0.68); and negative predictive value, 1.00 (95% CI, 0.88 to 1.00). An ROC curve showed good discriminative statistics for both an SpPV of ≥1 and an SpPV of ≥2 (Fig. 2). After controlling for the result of the skin surface culture SpPV, a multivariate model using logistic regression with use of the Firth penalized likelihood method14 showed that younger patients and male patients tended to have a greater risk of a dermal culture SpPV of ≥1, although this trend was not significant.
Fig. 2.
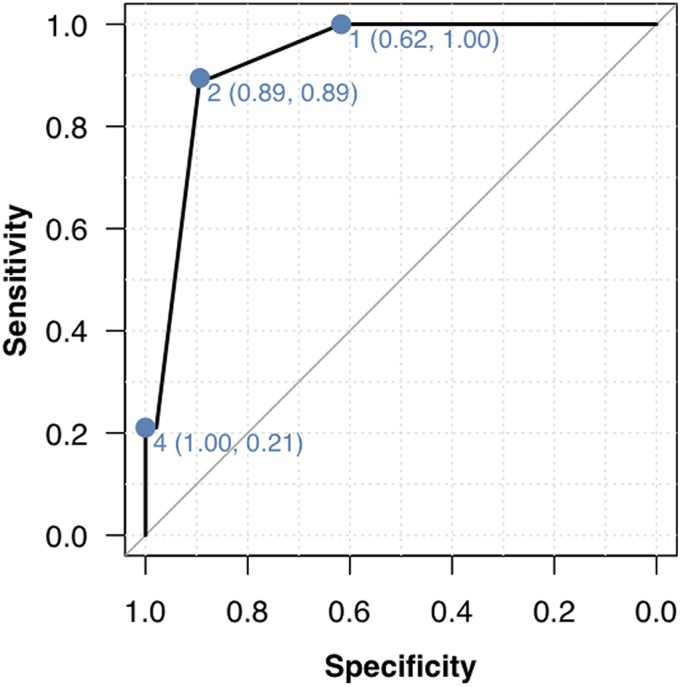
ROC curve for predicting a fresh dermal-wound SpPV of ≥1 from the result of the culture of a specimen from the unprepared skin surface. An ROC curve is a graphical plot that illustrates the performance of a binary classifier system (in this case, whether the dermal wound SpPV is ≥1) as the discrimination threshold (in this case, the skin-surface SpPV) is varied. In this plot, the values next to each dot are the skin-surface SpPV, with the specificity and sensitivity shown in parentheses. Thus, for a threshold of skin-surface SpPV of 4, the specificity for a dermal wound SpPV of ≥1 is 1 and the sensitivity is 0.21. The area under the curve (0.93) is high (a perfect predictor would have an area under the curve of 1).
TABLE I.
Characteristics of Patients Undergoing Primary Shoulder Arthroplasty
| Female (N = 18) | Male (N = 48) | P Value† | |
| Age* (yr) | 67.8 ± 9.3 | 65.5 ± 9.5 | 0.384 |
| Unprepared skin surface | |||
| SpPV* | 0.19 ± 0.39 | 1.38 ± 1.22 | <0.001 |
| SpPV >0 (no. of patients) | 7 (39%) | 36 (75%) | 0.009 |
| SpPV ≥1 (no. of patients) | 3 (17%) | 34 (71%) | <0.001 |
| SpCV* | 0.23 ± 0.50 | 0.57 ± 0.92 | 0.309 |
| SpCV >0 (no. of patients) | 12 (67%) | 31 (65%) | >0.99 |
| SpCV ≥1 (no. of patients) | 2 (11%) | 14 (29%) | 0.198 |
| Freshly incised dermis | |||
| SpPV* | 0.00 ± 0.00 | 0.78 ± 1.15 | <0.001 |
| SpPV >0 (no. of patients) | 0 (0%) | 22 (46%) | <0.001 |
| SpPV ≥1 (no. of patients) | 0 (0%) | 19 (40%) | <0.001 |
| SpCV* | 0.00 ± 0.00 | 0.06 ± 0.32 | 0.290 |
| SpCV >0 (no. of patients) | 0 (0%) | 3 (6%) | 0.556 |
| SpCV ≥1 (no. of patients) | 0 (0%) | 2 (4%) | >0.99 |
The values are given as the mean and the standard deviation.
2-sample t test for age, Wilcoxon rank-sum test for ordinal culture variables, and Fisher exact test for percentages.
The culture results for coagulase-negative Staphylococcus were distinct from those for Propionibacterium. While preoperative cultures of specimens from the unprepared skin surface for coagulase-negative Staphylococcus often had a positive SpCV, cultures of specimens from the freshly incised dermal wound edge usually did not (Table I). For cultures of the unprepared skin surface, the percentage with positive SpCVs were similar in male (65%) and female (67%) patients. With the small number of cultures of specimens from the freshly incised dermal wound edge that had positive SpCVs, the differences between male and female patients were inconclusive.
Discussion
We investigated the relationship between preoperative cultures of specimens from the unprepared skin surface and intraoperative cultures of specimens from the freshly incised dermis in patients undergoing primary shoulder arthroplasty. In this series of patients, a preoperative SpPV of ≥1 for the unprepared skin had fair positive predictive values and excellent negative predictive values for an SpPV of ≥1 for the freshly incised dermal wound. Surgeons may wish to use the results of preoperative cultures of specimens from the unprepared skin surface when considering additional forms of prophylaxis against Propionibacterium, such as preoperative topical benzoyl peroxide and clindamycin15, in-wound topical antibiotics, and prolonged postoperative antibiotics. These culture results also may suggest the need for special longitudinal clinical observation of patients identified as being at a higher risk for wound inoculation.
Both Propionibacterium and coagulase-negative Staphylococcus are common commensals on the skin surface, but Propionibacterium—by virtue of its anaerobic nature and its ability to live on fatty acids in the sebum secreted by sebaceous glands—is better able to live in the dermis below the skin surface. As a result, solutions for surgical skin preparation can reduce the presence of both Propionibacterium and coagulase-negative Staphylococcus on the skin surface but are less effective against Propionibacterium in the dermis8,10,16.
There is concern for specimen contamination when cultures for Propionibacterium are held for extended periods. Mook et al.17 reported a 13.0% culture-positive rate when using a sterile sponge as a control, whereas Sabetta et al.18 reported a 4% culture-positive rate when using a cotton swab exposed to air as a control. Horneff et al.19 reported a 3.2% positive-culture rate for tissue samples from the sites of primary shoulder arthroscopies. Each of those studies categorized the culture result as “positive” or “negative.” Our study suggests that the application of the threshold SpPV may help to interpret the clinical importance of the control-culture result. We found that the control swabs exposed to the air while the dermal cultures were obtained were positive for a threshold SpPV of ≥1 at a rate of 0%.
Our results need to be considered in light of certain limitations. First, the culturing and reporting protocols of our microbiology laboratory may be different from those of other medical centers; such differences potentially could limit the generalizability of our approach, which involves a 21-day observation of specimens cultured on 3 media, with the results reported as the SpPV. Second, it is possible that preoperative showers taken by the patients with use of antibacterial soaps may have lowered the rate of positive preoperative cultures of specimens from the unprepared skin surface. Third, it is possible that the pre-incision administration of prophylactic intravenous antibiotics may have reduced the rate of positivity of the dermal wound cultures. Fourth, while positive cultures from control specimens (2 of 50 with an SpPV of >0) potentially may confound the interpretation of culture results, none of the 50 control samples had an SpPV of ≥1; this outcome gives us greater confidence in the importance of skin-surface or dermal cultures when using this SpPV threshold. Fifth, the relationship between skin-surface and dermal cultures for coagulase-negative Staphylococcus and the relationship between cultures positive for Propionibacterium and for coagulase-negative Staphylococcus remain unclear and deserve further study. Sixth, the clinical utility of the present study lies in the opportunity to obtain predictive cultures of specimens from the unprepared skin surface in the surgeon’s office 3 weeks prior to surgery, with ample time for the culture results to be finalized for Propionibacterium. While one might assume that the results of cultures of specimens from the unprepared skin surface obtained 3 weeks prior to surgery would be the same as the results of similar cultures of specimens obtained immediately before surgery, we did not test this assumption in our study.
The present study needs to be put in the perspective of the rapidly rising number of shoulder arthroplasties being performed each year20 and the high percentage of surgical revisions for failed arthroplasties that have positive deep cultures1. The risk of positive wound cultures is reported to be increased for male patients, younger patients, and those who have had prior surgery17. Our findings suggest that additional information on the risk of wound inoculation can be obtained by culturing the unprepared skin surface prior to surgery. This information could assist the surgeon when discussing surgical risks with the patient and when considering the extent of anti-Propionibacterium prophylaxis.
In conclusion, the present study showed that the results of preoperative cultures of specimens from the unprepared skin surface may be helpful for anticipating the risk of positive intraoperative dermal wound-edge cultures that may, in turn, have a bearing on the risk of prosthetic bacterial colonization. This test may help to identify patients who may or may not merit more aggressive topical and systemic antibiotic prophylaxis.
Disclosure of Potential Conflicts of Interest
Footnotes
Investigation performed at the Department of Orthopaedics and Sports Medicine, University of Washington, Seattle, Washington
Disclosure: There was no external source of funding for this study. On the Disclosure of Potential Conflicts of Interest forms, which are provided with the online version of the article, one or more of the authors checked “yes” to indicate that the author had a relevant financial relationship in the biomedical arena outside the submitted work (http://links.lww.com/JBJSOA/A30).
References
- 1.Pottinger P, Butler-Wu S, Neradilek MB, Merritt A, Bertelsen A, Jette JL, Warme WJ, Matsen FA., 3rd Prognostic factors for bacterial cultures positive for Propionibacterium acnes and other organisms in a large series of revision shoulder arthroplasties performed for stiffness, pain, or loosening. J Bone Joint Surg Am. 2012. November 21;94(22):2075-83. [DOI] [PubMed] [Google Scholar]
- 2.Hsu JE, Gorbaty JD, Whitney IJ, Matsen FA., 3rd Single-stage revision is effective for failed shoulder arthroplasty with positive cultures for Propionibacterium. J Bone Joint Surg Am. 2016. December 21;98(24):2047-51. [DOI] [PubMed] [Google Scholar]
- 3.Hsu JE, Bumgarner RE, Matsen FA., 3rd Propionibacterium in shoulder arthroplasty: what we think we know today. J Bone Joint Surg Am. 2016. April 06;98(7):597-606. [DOI] [PubMed] [Google Scholar]
- 4.Scholz CF, Kilian M. The natural history of cutaneous Propionibacteria, and reclassification of selected species within the genus Propionibacterium to the proposed novel genera Acidipropionibacterium gen. nov., Cutibacterium gen. nov. and Pseudopropionibacterium gen. nov. Int J Syst Evol Microbiol. 2016. November;66(11):4422-32. Epub 2016 Aug 2. [DOI] [PubMed] [Google Scholar]
- 5.Falconer TM, Baba M, Kruse LM, Dorrestijn O, Donaldson MJ, Smith MM, Figtree MC, Hudson BJ, Cass B, Young AA. Contamination of the surgical field with Propionibacterium acnes in primary shoulder arthroplasty. J Bone Joint Surg Am. 2016. October 19;98(20):1722-8. [DOI] [PubMed] [Google Scholar]
- 6.Matsen FA, 3rd, Butler-Wu S, Carofino BC, Jette JL, Bertelsen A, Bumgarner R. Origin of Propionibacterium in surgical wounds and evidence-based approach for culturing Propionibacterium from surgical sites. J Bone Joint Surg Am. 2013. December 04;95(23):e1811-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hsu JE, Somerson JS, Vo KV, Matsen FA., 3rd What is a “periprosthetic shoulder infection”? A systematic review of two decades of publications. Int Orthop. 2017. April;41(4):813-22. Epub 2017 Feb 21. [DOI] [PubMed] [Google Scholar]
- 8.Lee MJ, Pottinger PS, Butler-Wu S, Bumgarner RE, Russ SM, Matsen FA., 3rd Propionibacterium persists in the skin despite standard surgical preparation. J Bone Joint Surg Am. 2014. September 3;96(17):1447-50. [DOI] [PubMed] [Google Scholar]
- 9.Matsen FA, 3rd, Russ SM, Bertelsen A, Butler-Wu S, Pottinger PS. Propionibacterium can be isolated from deep cultures obtained at primary arthroplasty despite intravenous antimicrobial prophylaxis. J Shoulder Elbow Surg. 2015. June;24(6):844-7. Epub 2014 Dec 26. [DOI] [PubMed] [Google Scholar]
- 10.Namdari S, Nicholson T, Parvizi J, Ramsey M. Preoperative doxycycline does not decolonize Propionibacterium acnes from the skin of the shoulder: a randomized controlled trial. J Shoulder Elbow Surg. 2017. September;26(9):1495-9. Epub 2017 Jul 19. [DOI] [PubMed] [Google Scholar]
- 11.Phadnis J, Gordon D, Krishnan J, Bain GI. Frequent isolation of Propionibacterium acnes from the shoulder dermis despite skin preparation and prophylactic antibiotics. J Shoulder Elbow Surg. 2016. February;25(2):304-10. Epub 2015 Oct 9. [DOI] [PubMed] [Google Scholar]
- 12.McGoldrick E, McElvany MD, Butler-Wu S, Pottinger PS, Matsen FA., 3rd Substantial cultures of Propionibacterium can be found in apparently aseptic shoulders revised three years or more after the index arthroplasty. J Shoulder Elbow Surg. 2015. January;24(1):31-5. Epub 2014 Jul 9. [DOI] [PubMed] [Google Scholar]
- 13.Ahsan ZS, Somerson JS, Matsen FA., 3rd Characterizing the Propionibacterium load in revision shoulder arthroplasty: a study of 137 culture-positive cases. J Bone Joint Surg Am. 2017. January 18;99(2):150-4. [DOI] [PubMed] [Google Scholar]
- 14.Firth D. Bias reduction of maximum likelihood estimates. Biometrika. 1993. March;80(1):27-38. [Google Scholar]
- 15.Dizay HH, Lau DG, Nottage WM. Benzoyl peroxide and clindamycin topical skin preparation decreases Propionibacterium acnes colonization in shoulder arthroscopy. J Shoulder Elbow Surg. 2017. July;26(7):1190-5. Epub 2017 May 4. [DOI] [PubMed] [Google Scholar]
- 16.Saltzman MD, Nuber GW, Gryzlo SM, Marecek GS, Koh JL. Efficacy of surgical preparation solutions in shoulder surgery. J Bone Joint Surg Am. 2009. August;91(8):1949-53. [DOI] [PubMed] [Google Scholar]
- 17.Mook WR, Klement MR, Green CL, Hazen KC, Garrigues GE. The incidence of Propionibacterium acnes in open shoulder surgery: a controlled diagnostic study. J Bone Joint Surg Am. 2015. June 17;97(12):957-63. [DOI] [PubMed] [Google Scholar]
- 18.Sabetta JR, Rana VP, Vadasdi KB, Greene RT, Cunningham JG, Miller SR, Sethi PM. Efficacy of topical benzoyl peroxide on the reduction of Propionibacterium acnes during shoulder surgery. J Shoulder Elbow Surg. 2015. July;24(7):995-1004. [DOI] [PubMed] [Google Scholar]
- 19.Horneff JG, 3rd, Hsu JE, Voleti PB, O’Donnell J, Huffman GR. Propionibacterium acnes infection in shoulder arthroscopy patients with postoperative pain. J Shoulder Elbow Surg. 2015. June;24(6):838-43. [DOI] [PubMed] [Google Scholar]
- 20.Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011. December 21;93(24):2249-54. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.





