Abstract
Enteroviruses (EVs) are among the most commonly detected viruses infecting humans worldwide. Although the prevalence of EVs is widely studied, the status of EV prevalence in sub-Saharan Africa remains largely unknown. The objective of our present study was therefore to increase our knowledge on EV circulation in sub-Saharan Africa. We obtained 749 fecal samples from a cross-sectional study conducted on Malawian children aged 6 to 60 months. We tested the samples for the presence of EVs using real time PCR, and typed the positive samples based on partial viral protein 1 (VP1) sequences. A large proportion of the samples was EV positive (89.9%). 12.9% of the typed samples belonged to EV species A (EV-A), 48.6% to species B (EV-B) and 38.5% to species C (EV-C). More than half of the EV-C strains (53%) belonged to subgroup C containing, among others, Poliovirus (PV) 1-3. The serotype most frequently isolated in our study was CVA-13, followed by EV-C99. The strains of CVA-13 showed a vast genetic diversity, possibly representing a new cluster, ‘F’. The majority of the EV-C99 strains grouped together as cluster B. In conclusion, this study showed a vast circulation of EVs among Malawian children, with an EV prevalence of 89.9%. Identification of prevalences for species EV-C comparable to our study (38.5%) have only previously been reported in sub-Saharan Africa, and EV-C is rarely found outside of this region. The data found in this study are an important contribution to our current knowledge of EV epidemiology within sub-Saharan Africa.
Introduction
Genus Enterovirus is a member of the family of Picornaviridae and consists of 13 species of which 7 classify as viruses which infect humans, i.e. Enterovirus A-D and Rhinovirus A-C [1]. Enteroviruses (EV’s) are known to cause a wide variety of clinical symptoms, ranging from mild respiratory infections to invasive disease such as meningitis, encephalitis and acute flaccid paralysis (AFP). Systematic surveillance programs have led to an increased knowledge on EV circulation in large parts of the world. In Europe and the United States, EVs are found in 5-12% of clinical samples [2–6]. Most of the EV strains found in Europe and the USA belong to species B, while species A is dominant in Asia [2, 3, 5, 7–12]. Regular outbreaks of EVs causing severe disease and complications, such as EV-A71 and EV-D68, have been reported in various countries in North-America, Europe and Asia, as well as in Australia [13, 14]. The status of EV prevalence in sub-Saharan Africa remains largely unknown, due to incomplete sampling or data collection. The few data available report a high EV prevalence – up to 50% – with an EV-C proportion of up to 76% amongst circulating EV strains [15–28].
Poliovirus (PV) is the most well-known EV causing AFP [29, 30]. Global vaccination programs have significantly reduced the incidence of PV infections, with PV now being endemic in only three countries (Afghanistan, Pakistan and Nigeria) [31]. PV belongs to species EV-C, and attenuated PV, as administered in the oral polio vaccine (OPV), can recombine with other strains belonging to EV-C to form vaccine-derived poliovirus (VDPV) [32–35]. Outbreaks of such VDPVs causing polio-like symptoms have been reported in the Philippines, Madagascar, the Dominican Republic, Haiti, Cambodia, Nigeria and Egypt [32, 36–40]. A high prevalence of EV-C in sub-Saharan Africa could increase the chances of VDPV’s arising in this continent.
The aim of this study was to provide further insights into the prevalence of EVs in children in sub-Saharan Africa, to assess the distribution of species EV-A, -B and –C, and finally to examine the genetic variability within the circulating species and genotypes. For this, we used fecal samples obtained in a case-control study conducted on children in Malawi. We report a high frequency of EVs classifiable as subgroup C of species C, a group that also contains PV.
Materials and methods
Patients and samples
A total of 749 fecal samples obtained from children included in the case-control SevAna (Severe Anemia) study in Southern Malawi between 2002 and 2004 were included in this study. The SevAna study was ethically approved and has been described in detail previously [41]. The samples used in our analyses were obtained from patients with: severe anemia (hemoglobin < 5 g/dl), hospital controls without severe anemia and randomly selected community controls. All included participants were between 6 and 60 months of age. A questionnaire, including date of birth, sex, date of recruitment and discharge and clinical symptoms, was completed for each participant. Fecal samples were stored at -20 °C and shipped to Leiden University Medical Center, the Netherlands. After storage for 10 years, the remaining samples were brought to the Academic Medical Center, Amsterdam for continued storage at -20 °C.
Virus isolation and detection
The Boom nucleic acid extraction method was used to isolate RNA from each sample [42]. RT-PCR was performed as described previously using primers EV-1 and EV-2 to determine presence of EV in the samples (Table 1) [43]. Samples with a Ct-value < 40 were considered to be EV positive. Samples with a Ct-value < 30 were included for sequencing.
Table 1.
Primers and probes used for RT-PCR (primers EV-1, EV-2 and probe WT-MGB), semi-nested PCR (primers 224, 222, AN89 and AN88) and sequencing (primers AN89 and AN88)
| Primer/probe | Sequence 5’-3’ | Polarity | Gene | Genomic location |
|---|---|---|---|---|
| 224 | GCIATGYTIGGIACICAYRT | Forward | VP1 | 1977-1996 |
| 222 | CICCIGGIGGIAYRWACAT | Reverse | VP1 | 2969-2951 |
| AN89 | CCAGCACTGACAGCAGYNGARAYNGG | Forward | VP1 | 2602-2627 |
| AN88 | TACTGGACCACCTGGNGGNAYRWACAT | Reverse | VP1 | 2977-2951 |
| EV-1 | GGCCCTGAATGCGGCTAAT | Forward | 5’UTR | 450-468 |
| EV-2 | GGGATTGTCACCATAAGCAGCC | Reverse | 5’UTR | 600-579 |
| WT-MGB (probe) | CGGAACCGACTACTTTGGGT | 5’UTR | 532-551 |
Enterovirus typing and phylogenetic analysis
A sensitive, semi-nested PCR amplification of VP1 sequences was performed, including primers 224 and 222 for the first PCR and primers AN89 and AN88 for the second PCR, as described previously (Table 1) [44]. The size of the PCR fragments was analyzed by gel electrophoresis. Positive samples with a PCR fragment size of ~ 350 to 400 base pairs (bp) were selected and sequenced using the BigDye Terminator kit, together with primers AN89 and AN88. CodonCode Aligner was used to assemble the obtained sequences. The sequences were typed using the online RIVM enterovirus genotyping tool (National Institute for Public Health and the Environment, http://www.rivm.nl/mpf/typingtool/enterovirus/ accessed 1st December 2014) and by comparison with reference strains in GenBank using BLAST (NCBI, https://blast.ncbi.nlm.nih.gov/ accessed 1st December 2014). The VP1 sequences obtained in this study were aligned with GenBank reference strains for respective genotypes, using ClustalX2 software. Neighbor-joining trees were constructed of study strains and reference strains, using the p-distance model implemented in MEGA 6 Software. One thousand bootstrap replicates were used to test the support for branches within the tree.
The nucleotide sequence data reported in this paper will appear in the DDBJ/EMBL/GenBank nucleotide sequence databases with the accession numbers MG793383-MG793425.
Statistical analysis
Baseline characteristics were calculated as frequencies and percentages for categorical variables, and as median and interquartile range (IQR) for numerical variables. We examined associations between EV positivity and the variables sex, inclusion group and age by Fisher’s exact test and Mann-Whitney-U test. Within the community control group, we examined the association between possible EV-related symptoms (i.e. gastro-intestinal symptoms, respiratory symptoms, central nervous system symptoms and fever) and EV infection using logistic regression analysis, and corrected for sex and age. Since 38.2% of the community control group was diagnosed with malaria, we also corrected for diagnosis of malaria. All statistical analyses were performed using IBM SPSS Statistics 24. Correlations were considered to be significant at an alfa-level of 0.05 or lower.
Results
EV prevalence in Malawian children
The baseline characteristics of the study participants are shown in Table 2. Baseline characteristics, sex and median age were comparable among the three inclusion groups.
Table 2.
Baseline characteristics
| Cases with severe anemia, n = 227 | Hospital controls, n = 261 | Community controls, n = 249 | Totala (n = 749) | |
|---|---|---|---|---|
| Patient characteristics | ||||
| Male sex, n (%) | 107 (47) | 133 (51) | 120 (48) | 371 (50) |
| Age in years, median (IQR) | 1.30 (0.85-2.16) | 1.76 (1.06-2.39) | 2.00 (1.20-3.01) | 1.64 (1.02-2.60) |
| EV-positive, n (%) | 198 (87) | 234 (90) | 229 (92) | 673 (90) |
| Sequenced, n (%)b | 77/198 (39%) | 104/234 (44%) | 99/229 (43%) | 283/673 (42%) |
| EV-A, n (%) | 15 (19) | 8 (8) | 15 (15) | 38 (13) |
| EV-B, n (%) | 37 (48) | 58 (56) | 40 (40) | 137(48) |
| EV-C, n (%) | 25 (32) | 38 (37) | 44 (44) | 108 (38) |
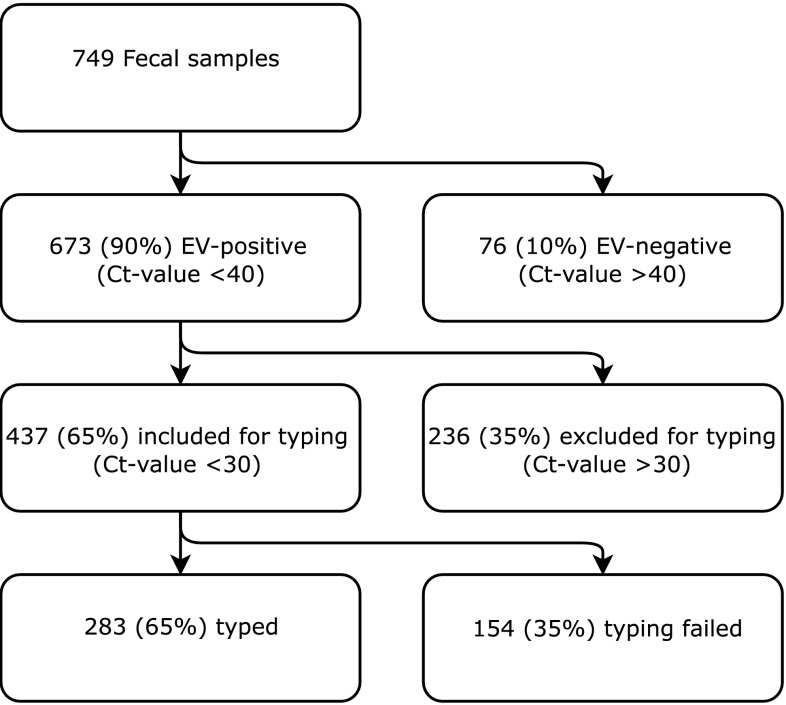
Overall, 673 of the 749 fecal samples (89.9%) tested positive for EV targeting the 5’UTR by qPCR. Of 437 samples included for genotyping, good quality sequences could be retrieved from 283/437 (65%) of samples (Figure 1). In total, we found 59 different genotypes (Table 3). Enterovirus B was the most frequently detected species, followed by Enterovirus C and Enterovirus A (53%, 34% and 13%, respectively) (Table 3). No Enterovirus D was detected.
Fig. 1.
Flowchart for all the included samples
Table 3.
List of all typed strains
| No. of typed strains | % of all typed strains | |
|---|---|---|
| Species and type | ||
| HEV-A | ||
| CVA-2 | 2 | 0.7% |
| CVA-4 | 2 | 0.7% |
| CVA-5 | 4 | 1.4% |
| CVA-6 | 5 | 1.7% |
| CVA-7 | 1 | 0.3% |
| CVA-8 | 2 | 0.7% |
| CVA-10 | 2 | 0.7% |
| CVA-14 | 1 | 0.3% |
| CVA-16 | 2 | 0.7% |
| EV-A76 | 3 | 1.0% |
| EV-A89 | 2 | 0.7% |
| EV-A119 | 8 | 2.8% |
| EV-A120 | 3 | 1.0% |
| All HEV-A | 37 | 12.9% |
| HEV-B | ||
| CVA-9 | 9 | 3,1% |
| CVB-2 | 1 | 0.3% |
| CVB-4 | 1 | 0.3% |
| CVB-5 | 1 | 0.3% |
| E1 | 7 | 2.4% |
| E2 | 2 | 0.7% |
| E5 | 5 | 1.7% |
| E6 | 10 | 3.5% |
| E7 | 4 | 1.4% |
| E9 | 1 | 0.3% |
| E11 | 4 | 1.4% |
| E12 | 1 | 0.3% |
| E13 | 7 | 2.4% |
| E14 | 9 | 3.1% |
| E15 | 12 | 4.2% |
| E18 | 6 | 2.1% |
| E19 | 5 | 1.7% |
| E20 | 3 | 1.0% |
| E21 | 4 | 1.4% |
| E24 | 1 | 0.3% |
| E25 | 3 | 1.0% |
| 5 | 1.7% | |
| E29 | 4 | 1.4% |
| E33 | 4 | 1.4% |
| EV-B69 | 1 | 0.3% |
| EV-B73 | 1 | 0.3% |
| EV-B75 | 5 | 1.7% |
| EV-B78 | 4 | 1.4% |
| EV-B79 | 2 | 0.7% |
| EV-B82 | 4 | 1.4% |
| EV-B80 | 8 | 2.8% |
| EV-B88 | 2 | 0.7% |
| EV-B97 | 1 | 0.3% |
| EV-B100 | 2 | 0.7% |
| All HEV-B | 139 | 48.6% |
| HEV-C | ||
| CVA-1 | 9 | 3,1% |
| CVA-11 | 7 | 2.4% |
| CVA-13 | 34 | 11.9% |
| CVA-17 | 3 | 1.0% |
| CVA-19 | 1 | 0.3% |
| CVA-20 | 12 | 4.2% |
| CVA-24 | 8 | 2.8% |
| EV-C99 | 31 | 10.8% |
| EV-C116 | 3 | 1.0% |
| PV2 (Sabin-like) | 1 | 0.3% |
| PV3 (Sabin-like) | 1 | 0.3% |
| All HEV-C | 110 | 38.5% |
| All typed strains | 286 | 100% |
Within EV-B, the most frequently detected genotypes were echovirus 6 (10/286, 3.5%) and echovirus 15 (12/286, 4.2%), while several of the higher numbered EV-B genotypes were also detected (EV-B69-100). In EV-C, CV-A13 (34/286, 11.9%) and EV-C99 (31/286 10.8%) were the most frequently detected genotypes. EV-A119 (8/286, 2.8%) and CVA6 (5/286, 1.7%) were the genotypes most frequently detected within EV-A (Table 1). One PV2-strain and one PV3-strain were identified, both of which were shown to have a ≥ 98.6% match to their respective reference Sabin strains in GenBank, indicating that these are vaccine-like polioviruses.
Enterovirus C has previously been divided into subgroups A, B and C [45]. Of all the typed EV-C strains in our study, 53% belonged to subgroup C, 35% to subgroup B and 12% to subgroup A.
EV prevalence was not associated with age (p = 0.882), sex (p = 0.629) or study group (p = 0.250). Within the community control group, the reporting of possible EV-related symptoms was not correlated with EV infection (p = 0.531).
Genetic diversity of EV-C strains
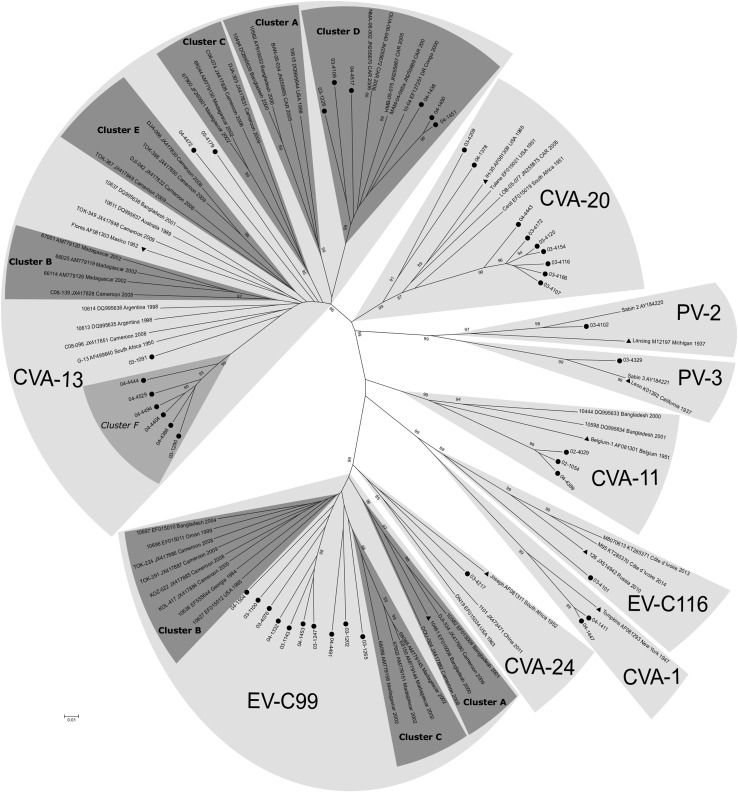
Figure 2 shows the genetic relationship of our Malawian EV-C strains with respective reference strains obtained from GenBank.
Fig. 2.
Phylogenetic relationships, based on the VP1 3’ terminal nucleotide sequences, for Malawian field strains and reference strains available in GenBank. Supportive percentage bootstrap replicates ≥ 85% are shown. Studied strains are indicated by circles. For reference strains, the location and year of isolation are indicated (DR Congo, Democratic Republic of the Congo; CAR, Central African Republic). The prototype strains are indicated by triangles. Cluster F is marked in light grey to indicate that this is a potential new cluster, based on the nt and aa percentage similarity to the other clusters
All strains grouped with their corresponding reference strains into type-specific clusters with strong bootstrap support (Figure 2). The nucleotide (nt) and amino acid (aa) identity within the serotypes was ≥ 75% and ≥ 88% respectively for all types except for CVA-13. For CVA-13 it was ≥ 70.0% and ≥ 84.3%, respectively.
As reported previously, the CVA-13 sequences grouped into clusters A to D, with ≥ 79.2% and ≥ 91.7% nt and aa identity within each of these clusters (Table 4) [27, 46]. Five of the Malawian CVA-13 sequences clustered with strains from the Central African Republic and the Democratic Republic of the Congo in CVA-13 Cluster D (bootstrap value 100%). The nt and aa identity within this cluster was ≥ 81.5% and ≥ 91.5%, respectively (Table 4). The nt and aa identity of cluster D compared to the other clusters was 69.3%-73.3% and 79.7%-89.2%, respectively, whereas the identity scores between clusters A, B, C and E were 71.3%-77.3% for nt, and 81.2%-94.0% for aa identity (Table 4).
Table 4.
Nucleotide (Nt) and amino acid (Aa) identity scores; identity scores are given within each CVA-13 cluster (minimum identity scores), and in comparison between CVA-13 clusters
| Nt identity | Aa identity | |
|---|---|---|
| Within CVA-13 clusters | ||
| Cluster A | ≥ 80.6% | ≥ 93.1% |
| Cluster B | ≥ 79.4% | ≥ 96.9% |
| Cluster C | ≥ 78.5% | ≥ 93.7% |
| Cluster D | ≥ 81.5% | ≥ 91.5% |
| Cluster E | ≥ 78.8% | ≥ 91.8% |
| Cluster F | ≥ 89.1% | ≥ 95.3% |
| Between CVA-13 clusters | ||
| Between cluster A, B, C and E | 72.3%-77.3% | 86.4%-94.0% |
| Cluster D compared to cluster A, B, C and E | 69.0%-73.3% | 79.7%-89.2% |
| Cluster F compared to cluster A, B, C, D and E | 69.0%-77.0% | 82.4%-94.6% |
(range of minimum through maximum identity scores)
Furthermore, seven Malawian CVA-13 strains, as well as several reference strains, did not belong to a known cluster. Of these, six of our sequences clustered together, supported by a bootstrap value of 99%, suggesting a new cluster, ‘F’ (Figure 2). The nt and aa identity within this cluster was 89.1%% and 95.3% respectively, while the nt and aa identity compared to the other clusters was 69.0%-77.0% and 82.4%-94.6% respectively (Table 4).
EV-C99 is known to consist of three clusters, i.e. A, B and C [27, 46], supported by bootstrap values of 96%, 5% and 97% respectively in our phylogram. Our strains did not group with any of the clusters. Three of our strains (03-1265, 03-1202 and 04-4491) were most similar to cluster C (≥ 80.8% nt identity and ≥ 92.6% aa identity). The remaining seven strains were most closely related to cluster B (≥ 80.8% nt identity and ≥ 94.9% aa identity).
Discussion
Our study contains new information about EV epidemiology and genetic diversity within sub-Saharan Africa, which contributes to our knowledge on EV-C circulation. Since epidemiological data from sub-Saharan Africa are scarce and the circulation of EV-C is focused upon in light of the PV eradication campaign, data from older cohorts like ours are still highly relevant. We detected EV in 89.9% of fecal samples collected from children between 2002 and 2004 in two hospitals in southern Malawi. This EV frequency is higher than in previous studies from sub-Saharan Africa, that reported EV prevalence numbers ranging from 1.5% to 50% [15–28]. Furthermore, it exceeds the 50% EV prevalence that has previously been reported in Malawi [26]. This difference might be partially explained by several factors. Firstly, in our study, we used real time PCR for detection of EV in fecal samples, whereas until recently, cell culture and virus isolation was the method most often used to detect EVs. 5’UTR PCR has been shown to detect EV from clinical specimens with a higher sensitivity than cell culture, resulting in higher yields especially for the non-B viruses [4, 6, 47]. Secondly, we hypothesize that our high EV prevalence is further explained by our relatively young population. While other studies often focus on a broader age group, EV’s are more prevalent in young age groups, when compared to older children and adults [15, 19]. Thirdly, the inclusion criteria of the SevAna study led to a higher number of participants included during the rainy season, in which malaria, a well-known cause of anemia, is highly prevalent. Possible seasonal variation in EV prevalence, much like in the Western world, might therefore have led to a higher detected prevalence in our study.
Interestingly, while we included one sample for each study participant, high EV incidence numbers have been found in studies that included multiple samples from children followed over a longer time-period. One study in Kenya showed that in a group of HIV-positive children 92% had at least one EV positive fecal sample during a 1-year study period [21]. A study conducted in Norway found that 90% of healthy children had shed EV at least once during a two-year follow-up [48].
In our study, 34% of the typed EV strains belonged to species EV-C. Although EV-C is a rather rare species in most of the world [3, 7, 8, 49–51], it accounts for up to 76% of typed EV strains found in African populations [18, 20, 22, 25–28]. The high proportion of EV-C subgroup C as found in our study is in accordance with findings in Cameroon and Madagascar. Furthermore, the types within EV-C that were most frequently detected in our study (CVA-13, CVA-20, EV-C99 and CVA-24) are found in approximately the same proportions in Cameroon and Madagascar [27, 52].
We saw a vast genetic diversity within EV-C subgroup C, especially within serotype CVA-13. It has been reported by others that CVA-13 strains group together in clusters (A-E) [27, 46]. In our phylogram, we could see this clustering, although cluster B and E were supported by low bootstrap values (73% and 33% respectively). Cluster D seems genetically distinct from the other clusters, with the maximum nt identity percentage compared to the other clusters falling below 75% (73.3%, table 4). Furthermore, several of the CVA-13 strains in our study did not fall within any of the clusters. Four of those strains grouped together, possibly forming a new cluster ‘F’.
For EV-C99, cluster B in our phylogenetic tree is merely supported by a bootstrap value of 5%. This is most likely a result of several of our strains grouping close to cluster B. Even so, the joint group of cluster B and our strains is supported by a bootstrap value of merely 25%.
We found PV in two of our samples (one strain PV-2 and one strain PV-3). Since the oral polio vaccine is administered at birth, this low prevalence is in accordance with our participants being between 6 and 60 months of age. The PV strains found in our study are most likely derived from children who had received a boost dose, or by secondary spread of the vaccine.
We found several strains of recently discovered genotypes EV-A119, EV-A120 and EV-C116. The prototype strains of these genotypes are derived from samples obtained years after the collection date of samples analyzed in our study [53–55]. We found eight strains of EV-A119, whereas the oldest known reference strain dates back to 2008 [52]. EV-A119 has only been detected in three children in Cameroon, Côte d’Ivoire and Nigeria [16, 18, 56]. The large proportion of EV-A119 in our database is therefore remarkable. In contrast, EV-A71, circulating widely in Asia and Europe, and also reported in several studies in sub-Saharan Africa, was not detected in our population [7, 27, 57]. Furthermore, we found several EV-B genotypes – Echo 1, Echo 15 and types EV-B69-100 – that are rarely found in Asia, Europe and the US, but seem to be rather prevalent in sub-Saharan Africa [7, 18, 27, 28, 57].
The major limitation of our study is the sample collection taking place between 2002 and 2004. Over time, the circulation and distribution of genotypes might have changed. However, the high diversity within CVA-13 found in our study and the repeated isolation of this type throughout the whole study period is interesting and suggests continuous circulation. Furthermore, making use of sequence based typing, which was not available at the time of sample collection, gives a unique insight into an older sample set, e.g. revealing circulation of EV-A119 before the first strain was even identified.
In conclusion, we found high rates of EV prevalence in young children in Malawi and high rates of EV-C – specifically of subgroup C within EV-C. High EV-C circulation is worrying, as strains belonging to this species are able to recombine with PV, giving rise to virulent VDPV strains. Furthermore, we saw a vast genetic diversity within CVA-13. Further studies using full length sequences of our study strains should reveal whether and to what scale recombined EV-C strains containing PV fragments are circulating within our population. Moreover, these future studies will also show the exact genetic diversity within CVA-13 – focusing on the genetic variety of cluster D when compared to the other clusters, as well as the genetic diversity of cluster F.
Abbreviations
- EV
Enterovirus
- PV
Poliovirus
- CV
Coxsackievirus
- OPV
Oral polio vaccine
- VDPV
Vaccine-derived poliovirus
- (q)PCR
(Quantitative) polymerase chain reaction
- Ct-value
Threshold cycle value
- bp
Base pairs
- UTR
Untranslated region
- VP1
Virus protein 1
- nt
Nucleotide
- aa
Amino acid
Funding
The SevAna study was supported by a grant (064722) from the Wellcome Trust. Our study, using the SevAna study samples, did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Ethical approval
The SeVana study was ethically approved by the Ethics Committees of the College of Medicine, University of Malawi, and the Liverpool School of Tropical Medicine, United Kingdom. For our present study, no ethical approval was required.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.International Committee on Taxonomy of Viruses https://talk.ictvonline.org/taxonomy/. Accessed January 2018
- 2.van der Sanden SM, Koopmans MP, van der Avoort HG. Detection of human enteroviruses and parechoviruses as part of the national enterovirus surveillance in the Netherlands, 1996–2011. Eur J Clin Microbiol Infect Dis. 2013;32(12):1525–1531. doi: 10.1007/s10096-013-1906-9. [DOI] [PubMed] [Google Scholar]
- 3.Trallero G, Avellon A, Otero A, De Miguel T, Perez C, Rabella N, Rubio G, Echevarria JE, Cabrerizo M. Enteroviruses in Spain over the decade 1998–2007: virological and epidemiological studies. J Clin Virol. 2010;47(2):170–176. doi: 10.1016/j.jcv.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 4.Abedi GR, Watson JT, Pham H, Nix WA, Oberste MS, Gerber SI. Enterovirus and human parechovirus surveillance—United States, 2009–2013. MMWR Morb Mortal Wkly Rep. 2015;64(34):940–943. doi: 10.15585/mmwr.mm6434a3. [DOI] [PubMed] [Google Scholar]
- 5.Harvala H, Calvert J, Van Nguyen D, Clasper L, Gadsby N, Molyneaux P, Templeton K, McWilliams Leitch C, Simmonds P. Comparison of diagnostic clinical samples and environmental sampling for enterovirus and parechovirus surveillance in Scotland, 2010 to 2012. Euro Surveill. 2014;19(15):20772. doi: 10.2807/1560-7917.ES2014.19.15.20772. [DOI] [PubMed] [Google Scholar]
- 6.Benschop K, Minnaar R, Koen G, van Eijk H, Dijkman K, Westerhuis B, Molenkamp R, Wolthers K. Detection of human enterovirus and human parechovirus (HPeV) genotypes from clinical stool samples: polymerase chain reaction and direct molecular typing, culture characteristics, and serotyping. Diagn Microbiol Infect Dis. 2010;68(2):166–173. doi: 10.1016/j.diagmicrobio.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 7.Janes VA, Minnaar R, Koen G, van Eijk H, Dijkman-de Haan K, Pajkrt D, Wolthers KC, Benschop KS. Presence of human non-polio enterovirus and parechovirus genotypes in an Amsterdam hospital in 2007 to 2011 compared to national and international published surveillance data: a comprehensive review. Euro Surveill. 2014;19(46):20964. doi: 10.2807/1560-7917.ES2014.19.46.20964. [DOI] [PubMed] [Google Scholar]
- 8.Logotheti M, Pogka V, Horefti E, Papadakos K, Giannaki M, Pangalis A, Sgouras D, Mentis A. Laboratory investigation and phylogenetic analysis of enteroviruses involved in an aseptic meningitis outbreak in Greece during the summer of 2007. J Clin Virol. 2009;46(3):270–274. doi: 10.1016/j.jcv.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 9.Chung WY, Chiang PS, Luo ST, Lin TY, Tsao KC, Lee MS. A molecular approach applied to enteroviruses surveillance in Northern Taiwan, 2008–2012. PLoS One. 2016;11(12):e0167532. doi: 10.1371/journal.pone.0167532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Momoki ST. Surveillance of enterovirus infections in Yokohama city from 2004 to 2008. Jpn J Infect Dis. 2009;62(6):471–473. [PubMed] [Google Scholar]
- 11.Tsao KC, Huang CG, Huang YL, Chen FC, Huang PN, Huang YC, Lin TY, Shih SR, Chang SC. Epidemiologic features and virus isolation of enteroviruses in Northern Taiwan during 2000–2008. J Virol Methods. 2010;165(2):330–332. doi: 10.1016/j.jviromet.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 12.Tseng FC, Huang HC, Chi CY, Lin TL, Liu CC, Jian JW, Hsu LC, Wu HS, Yang JY, Chang YW, Wang HC, Hsu YW, Su IJ, Wang JR, Laboratories CD-TVR, Sentinel Physician N. Epidemiological survey of enterovirus infections occurring in Taiwan between 2000 and 2005: analysis of sentinel physician surveillance data. J Med Virol. 2007;79(12):1850–1860. doi: 10.1002/jmv.21006. [DOI] [PubMed] [Google Scholar]
- 13.Chang PC, Chen SC, Chen KT. The current status of the disease caused by enterovirus 71 infections: epidemiology, pathogenesis, molecular epidemiology, and vaccine development. Int J Environ Res Public Health. 2016;13(9):842. doi: 10.3390/ijerph13090842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gong YN, Yang SL, Shih SR, Huang YC, Chang PY, Huang CG, Kao KC, Hu HC, Liu YC, Tsao KC. Molecular evolution and the global reemergence of enterovirus D68 by genome-wide analysis. Medicine (Baltimore) 2016;95(31):e4416. doi: 10.1097/MD.0000000000004416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Attoh J, Obodai E, Adiku T, Odoom JK. Prevalence of human enteroviruses among apparently healthy nursery school children in Accra. Pan Afr Med J. 2014;18:66. doi: 10.11604/pamj.2014.18.66.3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ayukekbong J, Kabayiza JC, Lindh M, Nkuo-Akenji T, Tah F, Bergstrom T, Norder H. Shift of Enterovirus species among children in Cameroon—identification of a new enterovirus, EV-A119. J Clin Virol. 2013;58(1):227–232. doi: 10.1016/j.jcv.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 17.Baba MM, Oderinde BS, Patrick PZ, Jarmai MM. Sabin and wild polioviruses from apparently healthy primary school children in northeastern Nigeria. J Med Virol. 2012;84(2):358–364. doi: 10.1002/jmv.23184. [DOI] [PubMed] [Google Scholar]
- 18.Cristanziano VD, Bottcher S, Diedrich S, Timmen-Wego M, Knops E, Lubke N, Kaiser R, Pfister H, Kabore Y, D’Alfonso R. Detection and characterization of enteroviruses and parechoviruses in healthy people living in the South of Cote d’Ivoire. J Clin Virol. 2015;71:40–43. doi: 10.1016/j.jcv.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 19.Fall A, Dia N, Kebe O, Sarr FD, Kiori DE, el Cisse HA, Sy S, Goudiaby D, Richard V, Diop OM, Niang MN. Enteroviruses and rhinoviruses: molecular epidemiology of the most influenza-like illness associated viruses in Senegal. Am J Trop Med Hyg. 2016;95(2):339–347. doi: 10.4269/ajtmh.15-0799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hellferscee O, Tempia S, Walaza S, Variava E, Dawood H, Wolter N, Madhi SA, du Plessis M, Cohen C, Treurnicht FK. Enterovirus genotypes among patients with severe acute respiratory illness, influenza-like illness, and asymptomatic individuals in South Africa, 2012–2014. J Med Virol. 2017;89(10):1759–1767. doi: 10.1002/jmv.24869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khetsuriani N, Helfand R, Pallansch M, Kew O, Fowlkes A, Oberste MS, Tukei P, Muli J, Makokha E, Gary H. Limited duration of vaccine poliovirus and other enterovirus excretion among human immunodeficiency virus infected children in Kenya. BMC Infect Dis. 2009;9:136. doi: 10.1186/1471-2334-9-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.L’Huillier AG, Kaiser L, Petty TJ, Kilowoko M, Kyungu E, Hongoa P, Vieille G, Turin L, Genton B, D’Acremont V, Tapparel C. Molecular epidemiology of human rhinoviruses and enteroviruses highlights their diversity in Sub-Saharan Africa. Viruses. 2015;7(12):6412–6423. doi: 10.3390/v7122948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nasir IA, Shehu MS, Thairu Y. Absence of poliovirus in apparently healthy school children in Bauchi state, Nigeria. J Infect Dev Ctries. 2016;10(8):824–828. doi: 10.3855/jidc.7602. [DOI] [PubMed] [Google Scholar]
- 24.Ouedraogo S, Traore B, Nene Bi ZA, Yonli FT, Kima D, Bonane P, Congo L, Traore RO, Ye D, Marguet C, Plantier JC, Vabret A, Gueudin M. Viral etiology of respiratory tract infections in children at the pediatric hospital in Ouagadougou (Burkina Faso) PLoS One. 2014;9(10):e110435. doi: 10.1371/journal.pone.0110435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rakoto-Andrianarivelo M, Guillot S, Iber J, Balanant J, Blondel B, Riquet F, Martin J, Kew O, Randriamanalina B, Razafinimpiasa L, Rousset D, Delpeyroux F. Co-circulation and evolution of polioviruses and species C enteroviruses in a district of Madagascar. PLoS Pathog. 2007;3(12):e191. doi: 10.1371/journal.ppat.0030191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodriguez-Diaz J, Mira-Pascual L, Collado MC, Endo A, Hyoty H, Mangani C, Maleta K, Ashorn P, Salminen S. Presence of human enteric viruses in the stools of healthy Malawian 6-month-old infants. J Pediatr Gastroenterol Nutr. 2014;58(4):502–504. doi: 10.1097/MPG.0000000000000215. [DOI] [PubMed] [Google Scholar]
- 27.Sadeuh-Mba SA, Bessaud M, Massenet D, Joffret ML, Endegue MC, Njouom R, Reynes JM, Rousset D, Delpeyroux F. High frequency and diversity of species C enteroviruses in Cameroon and neighboring countries. J Clin Microbiol. 2013;51(3):759–770. doi: 10.1128/JCM.02119-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Silva PA, Stark K, Mockenhaupt FP, Reither K, Weitzel T, Ignatius R, Saad E, Seidu-Korkor A, Bienzle U, Schreier E. Molecular characterization of enteric viral agents from children in northern region of Ghana. J Med Virol. 2008;80(10):1790–1798. doi: 10.1002/jmv.21231. [DOI] [PubMed] [Google Scholar]
- 29.Sawyer MH. Enterovirus infections: diagnosis and treatment. Curr Opin Pediatr. 2001;13(1):65–69. doi: 10.1097/00008480-200102000-00012. [DOI] [PubMed] [Google Scholar]
- 30.Abzug MJ. Presentation, diagnosis, and management of enterovirus infections in neonates. Paediatr Drugs. 2004;6(1):1–10. doi: 10.2165/00148581-200406010-00001. [DOI] [PubMed] [Google Scholar]
- 31.World Health Organiation factsheet on poliomyelitis http://www.who.int/mediacentre/factsheets/fs114/en/. Accessed January 2018
- 32.Arita M, Zhu SL, Yoshida H, Yoneyama T, Miyamura T, Shimizu H. A Sabin 3-derived poliovirus recombinant contained a sequence homologous with indigenous human enterovirus species C in the viral polymerase coding region. J Virol. 2005;79(20):12650–12657. doi: 10.1128/JVI.79.20.12650-12657.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bessaud M, Joffret ML, Blondel B, Delpeyroux F. Exchanges of genomic domains between poliovirus and other cocirculating species C enteroviruses reveal a high degree of plasticity. Sci Rep. 2016;6:38831. doi: 10.1038/srep38831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Joffret ML, Jegouic S, Bessaud M, Balanant J, Tran C, Caro V, Holmblat B, Razafindratsimandresy R, Reynes JM, Rakoto-Andrianarivelo M, Delpeyroux F. Common and diverse features of cocirculating type 2 and 3 recombinant vaccine-derived polioviruses isolated from patients with poliomyelitis and healthy children. J Infect Dis. 2012;205(9):1363–1373. doi: 10.1093/infdis/jis204. [DOI] [PubMed] [Google Scholar]
- 35.Jiang P, Faase JA, Toyoda H, Paul A, Wimmer E, Gorbalenya AE. Evidence for emergence of diverse polioviruses from C-cluster coxsackie A viruses and implications for global poliovirus eradication. Proc Natl Acad Sci USA. 2007;104(22):9457–9462. doi: 10.1073/pnas.0700451104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rousset D, Rakoto-Andrianarivelo M, Razafindratsimandresy R, Randriamanalina B, Guillot S, Balanant J, Mauclere P, Delpeyroux F. Recombinant vaccine-derived poliovirus in Madagascar. Emerg Infect Dis. 2003;9(7):885–887. doi: 10.3201/eid0907.020692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adeniji JA, Faleye TO. Enterovirus C strains circulating in Nigeria and their contribution to the emergence of recombinant circulating vaccine-derived polioviruses. Arch Virol. 2015;160(3):675–683. doi: 10.1007/s00705-014-2322-x. [DOI] [PubMed] [Google Scholar]
- 38.Yang CF, Naguib T, Yang SJ, Nasr E, Jorba J, Ahmed N, Campagnoli R, van der Avoort H, Shimizu H, Yoneyama T, Miyamura T, Pallansch M, Kew O. Circulation of endemic type 2 vaccine-derived poliovirus in Egypt from 1983 to 1993. J Virol. 2003;77(15):8366–8377. doi: 10.1128/JVI.77.15.8366-8377.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Centers for Disease Control and Prevention Acute flaccid paralysis associated with circulating vaccine-derived poliovirus—Philippines, 2001. MMWR Morb Mortal Wkly Rep. 2001;50(40):874–875. [PubMed] [Google Scholar]
- 40.Kew O, Morris-Glasgow V, Landaverde M, Burns C, Shaw J, Garib Z, Andre J, Blackman E, Freeman CJ, Jorba J, Sutter R, Tambini G, Venczel L, Pedreira C, Laender F, Shimizu H, Yoneyama T, Miyamura T, van Der Avoort H, Oberste MS, Kilpatrick D, Cochi S, Pallansch M, de Quadros C. Outbreak of poliomyelitis in Hispaniola associated with circulating type 1 vaccine-derived poliovirus. Science. 2002;296(5566):356–359. doi: 10.1126/science.1068284. [DOI] [PubMed] [Google Scholar]
- 41.Jonker FA, Calis JC, Phiri K, Brienen EA, Khoffi H, Brabin BJ, Verweij JJ, van Hensbroek MB, van Lieshout L. Real-time PCR demonstrates Ancylostoma duodenale is a key factor in the etiology of severe anemia and iron deficiency in Malawian pre-school children. PLoS Negl Trop Dis. 2012;6(3):e1555. doi: 10.1371/journal.pntd.0001555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boom R, Sol CJ, Salimans MM, Jansen CL, Wertheim-van Dillen PM, van der Noordaa J. Rapid and simple method for purification of nucleic acids. J Clin Microbiol. 1990;28(3):495–503. doi: 10.1128/jcm.28.3.495-503.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beld M, Minnaar R, Weel J, Sol C, Damen M, van der Avoort H, Wertheim-van Dillen P, van Breda A, Boom R. Highly sensitive assay for detection of enterovirus in clinical specimens by reverse transcription-PCR with an armored RNA internal control. J Clin Microbiol. 2004;42(7):3059–3064. doi: 10.1128/JCM.42.7.3059-3064.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nix WA, Oberste MS, Pallansch MA. Sensitive, seminested PCR amplification of VP1 sequences for direct identification of all enterovirus serotypes from original clinical specimens. J Clin Microbiol. 2006;44(8):2698–2704. doi: 10.1128/JCM.00542-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smura T, Blomqvist S, Vuorinen T, Ivanova O, Samoilovich E, Al-Hello H, Savolainen-Kopra C, Hovi T, Roivainen M. Recombination in the evolution of enterovirus C species sub-group that contains types CVA-21, CVA-24, EV-C95, EV-C96 and EV-C99. PLoS One. 2014;9(4):e94579. doi: 10.1371/journal.pone.0094579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bessaud M, Joffret ML, Holmblat B, Razafindratsimandresy R, Delpeyroux F. Genetic relationship between cocirculating human enteroviruses species C. PLoS One. 2011;6(9):e24823. doi: 10.1371/journal.pone.0024823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shoja ZO, Tabatabie H, Shahmahmoudi S, Nategh R. Comparison of cell culture with RT-PCR for enterovirus detection in stool specimens from patients with acute flaccid paralysis. J Clin Lab Anal. 2007;21(4):232–236. doi: 10.1002/jcla.20171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Witso E, Palacios G, Cinek O, Stene LC, Grinde B, Janowitz D, Lipkin WI, Ronningen KS. High prevalence of human enterovirus a infections in natural circulation of human enteroviruses. J Clin Microbiol. 2006;44(11):4095–4100. doi: 10.1128/JCM.00653-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bingjun T, Yoshida H, Yan W, Lin L, Tsuji T, Shimizu H, Miyamura T. Molecular typing and epidemiology of non-polio enteroviruses isolated from Yunnan Province, the People’s Republic of China. J Med Virol. 2008;80(4):670–679. doi: 10.1002/jmv.21122. [DOI] [PubMed] [Google Scholar]
- 50.Bahri O, Rezig D, Nejma-Oueslati BB, Yahia AB, Sassi JB, Hogga N, Sadraoui A, Triki H. Enteroviruses in Tunisia: virological surveillance over 12 years (1992–2003) J Med Microbiol. 2005;54(Pt 1):63–69. doi: 10.1099/jmm.0.45695-0. [DOI] [PubMed] [Google Scholar]
- 51.Khetsuriani N, Lamonte-Fowlkes A, Oberst S, Pallansch MA, Centers for Disease Control and Prevention Enterovirus surveillance—United States, 1970–2005. MMWR Surveill Summ. 2006;55(8):1–20. [PubMed] [Google Scholar]
- 52.Rakoto-Andrianarivelo M, Rousset D, Razafindratsimandresy R, Chevaliez S, Guillot S, Balanant J, Delpeyroux F. High frequency of human enterovirus species C circulation in Madagascar. J Clin Microbiol. 2005;43(1):242–249. doi: 10.1128/JCM.43.1.242-249.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sadeuh-Mba SA, Bessaud M, Joffret ML, Endegue Zanga MC, Balanant J, Mpoudi Ngole E, Njouom R, Reynes JM, Delpeyroux F, Rousset D. Characterization of Enteroviruses from non-human primates in cameroon revealed virus types widespread in humans along with candidate new types and species. PLoS Negl Trop Dis. 2014;8(7):e3052. doi: 10.1371/journal.pntd.0003052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lukashev AN, Drexler JF, Kotova VO, Amjaga EN, Reznik VI, Gmyl AP, Grard G, Taty Taty R, Trotsenko OE, Leroy EM, Drosten C. Novel serotypes 105 and 116 are members of distinct subgroups of human enterovirus C. J Gen Virol. 2012;93(Pt 11):2357–2362. doi: 10.1099/vir.0.043216-0. [DOI] [PubMed] [Google Scholar]
- 55.Razafindratsimandresy R, Joffret ML, Delpeyroux F, Heraud JM. First full genome sequence of a human enterovirus a120, isolated in Madagascar. Genome Announc. 2014;2(3):e00568–14. doi: 10.1128/genomeA.00568-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Adeniji JA (2016) Enterovirus A119 in a child with Acute Flaccid Paralysis, Nigeria. bioRxiv
- 57.Bessaud M, Pillet S, Ibrahim W, Joffret ML, Pozzetto B, Delpeyroux F, Gouandjika-Vasilache I. Molecular characterization of human enteroviruses in the Central African Republic: uncovering wide diversity and identification of a new human enterovirus A71 genogroup. J Clin Microbiol. 2012;50(5):1650–1658. doi: 10.1128/JCM.06657-11. [DOI] [PMC free article] [PubMed] [Google Scholar]