Abstract
Objective
Imaging studies in diffuse low-grade gliomas (DLGG) vary across centers. In order to establish a minimal core of imaging necessary for further investigations and clinical trials in the field of DLGG, we aimed to establish the status quo within specialized European centers.
Methods
An online survey composed of 46 items was sent out to members of the European Low-Grade Glioma Network, the European Association of Neurosurgical Societies, the German Society of Neurosurgery and the Austrian Society of Neurosurgery.
Results
A total of 128 fully completed surveys were received and analyzed. Most centers (n = 96, 75%) were academic and half of the centers (n = 64, 50%) adhered to a dedicated treatment program for DLGG. There were national differences regarding the sequences enclosed in MRI imaging and use of PET, however most included T1 (without and with contrast, 100%), T2 (100%) and TIRM or FLAIR (20, 98%). DWI is performed by 80% of centers and 61% of centers regularly performed PWI.
Conclusion
A minimal core of imaging composed of T1 (w/wo contrast), T2, TIRM/FLAIR, PWI and DWI could be identified. All morphologic images should be obtained in a slice thickness of ≤ 3 mm. No common standard could be obtained regarding advanced MRI protocols and PET.
Importance of the study
We believe that our study makes a significant contribution to the literature because we were able to determine similarities in numerous aspects of LGG imaging. Using the proposed “minimal core of imaging” in clinical routine will facilitate future cooperative studies.
Electronic supplementary material
The online version of this article (10.1007/s11060-018-2916-3) contains supplementary material, which is available to authorized users.
Keywords: Low-grade glioma, Imaging in LGG, Minimal core of imaging, Response criteria
Introduction
Despite published standards and guidelines on treatment and follow-up of diffuse low-grade glioma (DLGG) patients, daily practice frequently demonstrates the inconsistency of imaging studies among centers [1, 2]. As the volume of DLGG publications increases, the usefulness of single-center studies will become more limited, as these may be difficult to replicate. It has been argued that evidence-based practice in the field of DLGG cannot be derived from the standard methodology of oncological randomized clinical trials [3, 4]. Considering the low prevalence of DLGG [5] and the long survival of patients [6], sufficient data might be better collected by networks of centers working together collaboratively. Numerous demographic parameters, oncomolecular features and imaging data (including imaging DLGG growth rates of follow-up MRIs) will be required. Rigorous evaluation of care is additionally needed, for example to prove that maximum safe resection is not only key to oncological outcome, but also to establish and maintain a best possible quality of life [7, 8]. The expectation that randomized oncological studies could add knowledge on these two questions is vanishing [3]. We are convinced that databases dedicated to DLGG research are required, which could include both retro- and prospective data.
The European Low-Grade Glioma Network (ELGGN) gathers surgical and neuroscience specialists from centers with dedicated teams treating DLGG patients. The network was founded 11 years ago to establish the link between all subspecialties involved in the field: neurosurgeons, neuro-oncologists, radiation therapists, neuropathologists, oncomolecular biologists, neuroradiologists, anaesthesiologists, speech therapists, neuropsychologists, and neuroscientists involved in functional brain mapping. Several collaborative studies have been previously published [3, 9–11].
The ELGGN is a powerful platform to address major issues in the management of DLGG. A survey [3] has been created in preparation of the 2015 Annual Meeting, which met the goal to identify points of consensus in patient management. Thus, the network should allow to highlight relevant questions for future studies and establish landmark projects in the interdisciplinary treatment of DLGG.
Following the initial survey, we now aimed to establish a comprehensive imaging survey, in order to investigate the consistency of DLGG imaging in specialized centers across Europe and to identify a “minimal core of imaging” to facilitate cooperative imaging projects within the network.
Methods
An online survey was created by a group of experts in imaging and treatment of DLGG. The use of published imaging guidelines [1, 12, 13] was emphasized and local availability and usage of advanced imaging modalities was added to the survey. The survey was formatted on Survey Grid (EvaSys, Electric Paper Evaluationssysteme GmbH Lüneburg, Germany) and sent to all members of the ELGGN, the European Association of Neurosurgical Societies (EANS) plus the German (DGNC) and Austrian Society of Neurosurgery (ÖGNC). All recipients were members of the respective societies and it was specified that the questionnaire should be filled out by a multidisciplinary team. Participants were not asked to detail how any disagreements were adjudicated, precluding analysis of response heterogeneity at the center level. The survey contained 45 items (see Supplement 1), divided in descriptive data (i.e. amount of DLGG treated per year), radiological details (i.e. MRI sequences that are routinely performed) and questions regarding advanced imaging techniques (i.e. diffusion weighted imaging (DWI)).
In order to distinguish between dynamic susceptibility contrast perfusion imaging (DSC, named PWI in our survey) and dynamic contrast-enhanced MR perfusion (DCE, named Perfusion in our survey), two perfusion modalities were included in the survey, however, the question was answered inappropriately, showing that the nomenclature used for this particular question was misleading. Technical data on MRI scanner manufacturers, acquisition and recovery times, magnetic field strength and contrast agent dosage were not acquired. Further, the survey did not evaluate scientific justification of imaging protocols, it depicted a common denominator across many centers.
Further sections of the survey inquired the routine use of non-invasive mapping (i.e. resting state functional MRI (rs-fMRI) or navigated transcranial magnetic stimulation (nTMS)) and follow-up imaging protocols. The survey consisted of 28 single-choice, 7 multiple-choice and 10 items for free text answers.
In total, 148 data sets were received for detailed analysis. Data from outside Europe’s geographical extension (n = 14) and incomplete surveys (n = 6) were excluded. Overall, 128 fully completed surveys were analyzed descriptively for this study. For additional information and geographical details of the respondents see Table 1.
Table 1.
Distribution of centers per country and average number of treated DLGG
| Number of centers responded | % | Avg. no of LGG per center and year | Range | |
|---|---|---|---|---|
| Country of practice | ||||
| Germany | 40 | 31 | 20 | 5–50 |
| Italy | 14 | 11 | 10 | 5–90 |
| France | 9 | 6 | 25 | 3–100 |
| Switzerland | 8 | 7 | 20 | 5–100 |
| United Kingdom | 8 | 6 | 30 | 10–60 |
| Austria | 7 | 5 | 20 | 6–40 |
| Spain | 7 | 5 | 5 | 3–20 |
| Netherlands | 6 | 5 | 25 | 15–40 |
| Portugal | 4 | 3 | 20 | 2–20 |
| Belgium | 3 | 2 | 15 | 15–30 |
| Greece | 3 | 2 | 25 | 10–30 |
| Poland | 3 | 2 | 25 | 20–30 |
| Czech Republic | 2 | 2 | 15 | 15 |
| Russian Federation | 2 | 2 | 55 | 50–60 |
| Serbia | 2 | 2 | 15 | 10–20 |
| Sweden | 2 | 2 | 20 | 20 |
| Bulgaria | 1 | < 1 | 10 | 10 |
| Denmark | 1 | < 1 | 20 | 20 |
| Hungary | 1 | < 1 | n/a | n/a |
| Lithuania | 1 | < 1 | 30 | 30 |
| Norway | 1 | < 1 | 30 | 30 |
| Romania | 1 | < 1 | 10 | 10 |
| Turkey | 1 | < 1 | 20 | 20 |
| Ukraine | 1 | < 1 | 5 | 5 |
| Use of 3T imaging | 128 | |||
| Always | 29 | 22.8 | ||
| If available | 59 | 46.5 | ||
| Only 1.5T | 39 | 30.7 | ||
| Identical MR scanner | ||||
| Yes | 25 | 20.0 | ||
| No | 24 | 19.2 | ||
| Mostly yes | 72 | 57.6 | ||
| Mostly no | 4 | 3.2 | ||
| Total number of patients | ||||
|---|---|---|---|---|
| Slice thickness of T1 imaging (mm) | ||||
| < 1.5 | 66 | 53.7 | – | 1585 |
| 1.6–3 | 44 | 35.8 | – | 781 |
| > 3 | 13 | 10.6 | – | 356 |
| Slice thickness of T2 imaging (mm) | ||||
| < 1.5 | 40 | 32.8 | – | 1027 |
| 1.6–3 | 54 | 44.3 | – | 1133 |
| > 3 | 28 | 23.0 | – | 542 |
| Imaging intervals in relation to the amount of residual disease | ||||
| No remnant (weeks) | ||||
| < 12 | 3 | 2.4 | ||
| 12–24 | 87 | 68.5 | ||
| > 24 | 15 | 11.8 | ||
| 52 | 4 | 3.1 | ||
| < 10 ml remnant (weeks) | ||||
| < 12 | 4 | 3.1 | ||
| 12–24 | 91 | 71.7 | ||
| > 24 | 12 | 9.4 | ||
| 52 | 0 | 0.0 | ||
| 11–15 ml remnant (weeks) | ||||
| < 12 | 4 | 3.1 | ||
| 12–24 | 95 | 74.8 | ||
| > 24 | 9 | 7.1 | ||
| 52 | 0 | 0.0 | ||
| > 15 ml remnant (weeks) | ||||
| < 12 | 7 | 5.5 | ||
| 12–24 | 97 | 76.4 | ||
| > 24 | 4 | 3.1 | ||
| 52 | 0 | 0.0 | ||
| Unresectable LGG (weeks) | ||||
| < 12 | 1 | 0.8 | ||
| 12–24 | 97 | 76.4 | ||
| > 24 | 6 | 4.7 | ||
| 52 | 3 | 2.4 | ||
Use of imaging infrastructure and average slice thickness. Imaging intervals with respect to the amount of residual disease
Results
Basic information
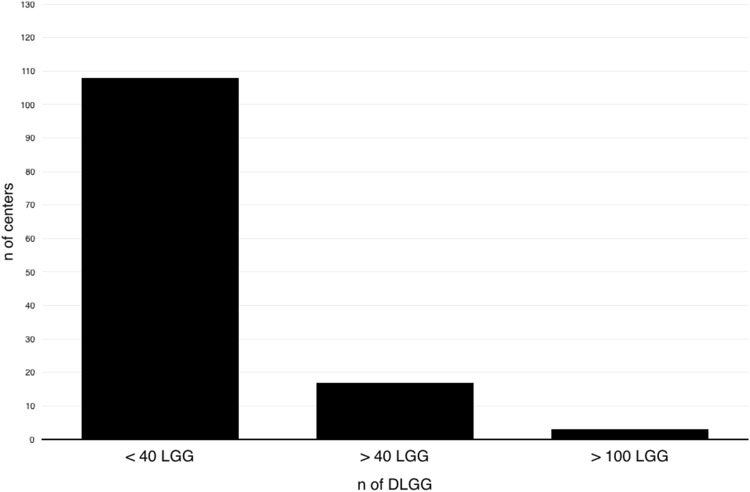
The majority of responding teams worked in an academic hospital (n = 96, 75%), 19% (n = 25) were based in community hospitals, and 6% (n = 8) were located in a private hospital environment. Half of the centers (64, 50%) adhered to a dedicated MRI protocol for DLGG, while the others (64, 50%) did not. There was a broad range in the reported operative activity for each center (2–100 cases/year, average 25). The majority of centers treated less than 40 DLGG per year (n = 108, 85%), whereas only 17 centers (13%) reported to treat more than 40. Three centers (2%) can be considered very high volume centers with 100 DLGG per year (see Fig. 1). A total of 3032 DLGG are managed annually by the responding centers, the majority (1714, 62%) of which are treated in centers with dedicated DLGG programs compared to 1068 (38%) in centers without. Of the centers treating 40 or more DLGG per year 65% used a dedicated DLGG protocol.
Fig. 1.
Distribution of annual DLGG throughout participating centers. Most centers treat less than 40 DLGG per year
Physicians involved in treatment of DLGG
The survey showed that there is a variation in the composition of the multidisciplinary teams involved in DLGG management. The majority of centers (84%) discussed their patients either before (59, 47%) or after surgical treatment (47, 37%). Interestingly, 20 centers (16%) refrained from presenting every surgically treated DLGG in a multi-disciplinary tumor board, and 16 of these only discussed them if adjuvant treatment was advocated. Multi-disciplinary tumor boards consisted of several specialties: neurosurgeons were present in 99%, followed by neuroradiologists (90%), and radiation oncologists (87%). Medical oncologists participated in 80% of neuro-oncological tumor boards, an additional 64% included a specialized neuro-oncologist. Nuclear medicine specialists, however, were available at the tumor board in only 32% of centers.
Imaging
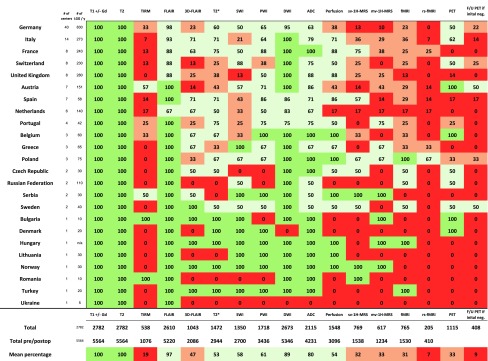
More than half (52%) of the centers routinely used any recent MR imaging for treatment decisions and surgical treatment, without performing an MRI according to their own dedicated protocol. The imaging had to be carried out in a specialized neuroradiology unit (i.e. in a university hospital) in 17% of centers and 31% of centers always scanned their own dedicated protocol. The particular sequences applied by the center are summarized in Table 2. T1 imaging without and with Gadolinium contrast (T1 wo/w) and T2 weighted imaging was obtained in every center (100%), whereas TIRM (turbo inversion resonance magnitude) and FLAIR (fluid attenuation inversion recovery) are performed in 20 and 98%, respectively. Centers used a slice thickness < 1.5 mm in 52% of T1 and 32% of T2 images and a slice thickness of ≤ 3 mm in 89% of T1 and 78% of T2 images. Most of the centers (89%) obtained DWI in every patient with an additional 80% obtaining apparent diffusion coefficient (ADC) maps routinely. Almost two-thirds of the respondents (61%) applied perfusion weighted imaging (PWI) in daily routine.
Table 2.
Availability of MR-sequences in %
T1+/−Gd T1 weighted imaging with and without Gadolinium contrast, TIRM turbo inversion resonance magnitude, FLAIR fluid attenuated inversion recovery, 3D-FLAIR multiplanar reconstruction of FLAIR, T2* gradient-echo T2 with susceptibility, SWI susceptibility weighted imaging, PWI perfusion weighted imaging, DWI diffusion weighted imaging, ADC automated diffusion coefficient, sv-1H-MRS single-voxel Proton magnetic resonance spectroscopy, mv-1H-MRS multi-voxel Proton magnetic resonance spectroscopy, fMRI functional MRI, rs-fMRI resting-state functional MRI, PET positron emission tomography
Follow-up imaging of DLGG always included volumetric analysis (segmentation and approximation) in 45 centers (35%) and linear measurement (3 axes on MRI) in 58 centers (45%), and 26 centers (20%) evaluated the deformation of present lesions or changes in shape to identify progression or regression. All measurements were performed by neuroradiologists in 59% opposed to 24% by neurosurgeons and 3% by neurooncologists. In 14% of centers, all members of the team performed measurements.
For interpretation of the response in DLGG, the RANO criteria [13] were used “always” in 15% of centers and “most of the time” in 46% of centers. 17% used the published criteria “hardly ever” and 22% refused to utilize them.
Although 81% of centers specified to adjust their imaging intervals according to the tumor’s previous growth rate, more than 75% of centers perform follow-up imaging in intervals of 12–24 weeks (see Table 1) in all cases presented, regardless of the amount of residual tumor.
Advanced imaging
Additional advanced sequences, like MR spectroscopy (MRS) were handled variably throughout the centers. One-third of centers routinely obtained data from single-voxel spectroscopy, another third applied a multi-voxel spectroscopy in their MRI protocol.
The question “Do you perform amino acid PET in suspected low-grade glioma?” was answered positively in 33% of all respondents. However, centers tend to discard PET imaging in case of initially negative PET scans. Under these circumstances only 9% would repeat PET imaging later. Interestingly, some countries like Austria and Belgium had a 100% rate of initial PET imaging, whereas French centers performed no PET. Assessment of treatment response and progression was mostly (80%) done with MRI (T2/FLAIR for response, T1wo/w for progression), whereas 14% relied on the combination of MRI and amino-acid PET (aaPET; if initially positive). Six percent of centers performed MRI and aaPET regardless of initial PET presentation. The survey investigated which imaging the centers would rely on to decide whether the tumor has undergone anaplastic transformation. Unsurprisingly, there is no consensus on the imaging modalities used for detection of anaplastic transformation. Most centers used combinations of either T1wo/w with MRS and PWI or T1wo/w with FET-PET (see Table 3).
Table 3.
Choice of imaging modalities for detection of malignant transformation
| n | % | |
|---|---|---|
| T1 + PWI + MRS | 23 | 18 |
| T1 + FET-PET | 20 | 16 |
| T1 + PWI | 20 | 16 |
| T1 | 19 | 15 |
| T1 + PWI + FET-PET | 10 | 8 |
| T1 + PWI + MRS + FET-PET | 8 | 6 |
| T1 + MRS | 4 | 3 |
| T1 + MRS + FET-PET | 3 | 2 |
| T1 + PWI + MRS + other | 3 | 2 |
| FET-PET | 3 | 2 |
| PWI + MRS | 3 | 2 |
| T1 + PWI + other | 2 | 2 |
| T1 + other | 2 | 2 |
| other | 8 | 6 |
| Other | ||
| Biopsy/resection | 7 | 6 |
| Evaluate changes in growth rate/volumetric expansion | 3 | 2 |
| F-DOPA PET | 1 | 1 |
| Arterial-spin labelling MRI | 1 | 1 |
| DWI/ADC | 1 | 1 |
The number of MRI studies that would be available for further investigation was calculated for every MR sequence based on the number of patients treated annually in all responding centers. Up to 2800 studies should be available for analysis every year (see Table 2).
Functional MRI and non-invasive brain mapping
The survey demonstrated a wide heterogeneity regarding the use of functional MRI (fMRI) for patients with DLGG.
Thirty-one percent routinely used fMRI for every patient, while resting-state fMRI is acquired in only 7%. Half of the centers (50%) used fMRI for both, clinical and research purposes, 42% exclusively clinical, and 6% only for research. A minority of 2% used fMRI for didactic purposes in training of students or residents.
The final part of the survey evaluated brain mapping and the respective technique of choice: fMRI, nTMS, or intraoperative direct electrical cortical and subcortical stimulation. 102 centers (80%) preferred invasive intraoperative direct electrical cortical and subcortical stimulation over noninvasive procedures. 8 centers (6%), however, would have chosen nTMS, whereas 18 centers (14%) preferred fMRI. The number of centers using magnetoencephalography (MEG) was low, which fits the distribution of the technique. 87% of centers don’t use MEG or do not own one, although 71% recognized the scientific possibilities of MEG or thought it would be nice to have.
Discussion
Our survey revealed a high level of homogeneity in DLGG imaging workup throughout Europe. Nonetheless, we were able to identify heterogeneities that need to be highlighted. Of note, questions were not designed as detailed individual cases and we acknowledge that this method might have provoked heterogeneity of responses.
Minimal core of imaging
Ellingson et al. [1] proposed a standardized brain imaging protocol for tumor patients. We support the principle of creating standardized protocols for DLGG patients. This should include minimum imaging datasets, recommended imaging frequency and recommendations about additional sequences. The goal of establishing a minimal core of imaging would be to allow further investigations with uniform imaging throughout different countries. This would enable to include numerous patients in future prospective cohorts, ensuring a sufficient volume of data to perform big data analysis [14].
The minimal core of imaging in DLGG needs to take regional and national differences regarding technical standards and reimbursement into account. Therefore, the following imaging algorithm is proposed: MR imaging should incorporate sequences for morphologic descriptive analysis and those focusing on potential malignant transformation. T1wo/w and T2 images were used by all centers and therefore represent the cornerstone of morphologic imaging. In addition, TIRM or FLAIR sequences should be obtained and can also be used for volumetric assessment of DLGG. Although several recommendations set the minimum level of slice thickness in T2 and TIRM/FLAIR imaging to ≤ 4 mm [1], it is known that slice thickness is key for accurate volumetric and treatment response assessment [15–17]. Perfusion weighted imaging (PWI) is used frequently for assessment of treatment response and detection of anaplastic transformation [18, 19]. In our survey, response and transformation were predominantly determined with MRI. T1 (with contrast) and PWI were used in 87 and 55%, respectively. Diffusion weighted imaging (DWI), and especially the computed ADC has been used to investigate malignant transformation [20] and is applied in up to 90% of centers.
We therefore recommend that the minimal MRI sequence dataset should consist of T1wo/w, T2 and TIRM/FLAIR, all in low slice thickness (≤ 3 mm) to facilitate volumetric assessment and further include PWI and DWI to predict malignant transformation. Based on this survey, we cannot make any recommendation regarding the use of additional advanced imaging techniques (spectroscopy, fMRI, PET).
Imaging intervals
More than 75% adhered to follow-up imaging intervals of 12 to 24 weeks and 81% adjusted their imaging intervals depending on the initial growth rate of the tumor [21], which is in accordance with current guidelines [2]. It has been shown that the velocity of tumor expansion is a strong predictor of the patient’s prognosis [21]. However, no studies have addressed the question of how often MRI should be obtained during follow-up, especially with regard to the heterogeneity of DLGG. Although early postoperative FLAIR is known to overestimate the volume of residual tumor [22], most authors recommend an early postoperative MRI within 72 h [23–25] to determine the extent of resection and visualize possible postoperative complications. Follow-up imaging intervals of 12–24 weeks are recommended with longer intervals for cases of “less aggressive” tumours [1, 13, 26]. The definition of “aggressiveness”, however, varies in the literature.
Standardized response assessment protocols
Only 15% of the centers reported to use RANO criteria for low-grade gliomas [13] thoroughly in all their DLGG cases for the interpretation of treatment response, while 46% of centers do so “most of the time”. Thus, 39% of centers do not routinely apply these criteria. RANO criteria include T1wo/w, T2/FLAIR, development of new lesions, clinical status, and steroid use in order to categorize treatment effects in complete response, partial response, stable disease, and progressive disease. Since DLGG constitutes a slowly progressive disease, these criteria were defined in order to achieve a standardized common ground for the definitions of treatment responses and endpoints in clinical trials. Measurement of tumor diameter has various shortcomings, not only the high intra- and interobserver variability [27], but also the known problem of head positioning during acquisition of the MRI [16, 17]. Then, assuming that many centers are performing volumetric assessment of tumors, how should treatment response be defined? Translating volume to diameter (D = (2 × V)^(1/3)) is a fundamental step. In contrast to the assessment of tumor diameters, the concept of volume-derived mean diameter overcomes the above mentioned confounding factors. Moreover, the curve showing evolution of mean diameter with time can be easily analyzed by applying a linear fit. Following the curve of the diameter as a function of time is a more sensitive way to monitor treatment response than applying RANO criteria [21, 28, 29]. Indeed, the major concern about RANO criteria is that pretreatment dynamics is not integrated in the definition of the different response categories. However, it seems obvious that putting down the tumor growth rate to 0 mm/year with chemotherapy, while its pretreatment value was 6 mm/year, should be interpreted as a response, whereas RANO criteria would interpret this as “stable disease”.
In contrast to this highly standardized protocol, our survey focused more on the clinical routine in an attempt to accurately reflect every day practice in the treatment of DLGG across Europe. Although the highly standardized approach provided by the RANO criteria is not systematically applied, our data demonstrate that at least the imaging studies, comply in most centers with the RANO-defined protocols.
Additionally, other factors should also be interpreted into our decision making, such as cognitive testing, psychological burden of disease, and seizure activity.
Malignant transformation
There is no consensus regarding the radiological malignant transformation in DLGG throughout Europe. Newly apparent contrast enhancement indicating breakdown of the blood brain barrier represents the classical sign of malignant transformation (MT) of these tumors [30], but preceding changes in advanced MRI investigations may allow identification of patients at risk up to 12 months earlier [31]. Simple measurement of growth rate [19, 32, 33] and integration of MR spetroscopy [18, 19, 34] are used routinely in most of the centers as predictors of tumor transformation. Both, proton- and phosphorus spectroscopy, available in numerous centers, have been proven to correlate with Ki67 and IDH1 mutation [35]. Perfusion measurements, and in particular the determination of relative cerebral blood volume (rCBV), seems to correlate with the vascularity determined at histopathological examination [31]. Arising of lactate resonance is predictive of the increase of rCBV up to 1.75, this parameter is predictive for a dramatic decrease of overall survival [36–38], and are indicators of MT that may be identified months before apparent contrast enhancement. Likewise, ADC can be used for discrimination of tumor subtypes and raising suspicion of malignization [20]. Nevertheless, both techniques are only supported by a low level of evidence [20].
Positron emission tomography (PET)
The use of amino acid PET (aaPET) was surprisingly variable between centers and countries. While Austria and Belgium performed PET in 100% of cases, none of the Dutch or French centers used PET at all. In contrast to patients with WHO grades III/IV gliomas, the evidence for aaPET to monitor patients with WHO grade II gliomas is limited [39, 40]. Most WHO grade II gliomas are nonenhancing with infiltrating tumor borders, and so several studies demonstrated the usefulness of aaPET in defining tumor extent or malignant transformation [41–45]. This has been demonstrated and validated in series for 11C-MET, 18F-FET, and 18F-FDOPA PET [46]. Although MRI is the standard of care in following DLGG patients, its reliability in distinguishing tumor tissue from treatment effects is limited [47]. Transient blood–brain barrier alteration with contrast enhancement after radiotherapy with or without concomitant Temozolomide, for example, can mimic tumor progression. There are numerous reasons for the restricted application of PET: (1) While 18F-FDG is used routinely, access to aaPET is limited [46]. (2) Another obstacle to withhold patients and healthcare professionals from PET is limited reimbursement. In our survey, 9% of centers repeated aaPET scans during follow-up, even if they were initially negative. However, there are several groups advocating to perform aaPET in all cases of DLGG [48], notwithstanding the initial uptake behavior.
fMRI and noninvasive neurophysiological imaging
fMRI has repeatedly been shown to harbor a low specificity and sensitivity for any presurgical evaluation [49–53]. Since the tumor itself impairs oxygenation levels in its surrounding fMRI is not a reliable surrogate marker for neuronal activity in DLGG patients [50, 51, 54]. Nevertheless, eloquence is thoroughly based on fMRI in as much as 16% of centers.
Yet, 80% of European centers prefer invasive intraoperative direct electrical cortical and subcortical stimulation over noninvasive procedures for the determination of eloquent cortex, which reflects commonly agreed practice and level of evidence [55–57].
With the still small but increasing distribution of nTMS, some centers chose this technique for surgical decision making, which also illustrates the highly specialized nature of the enrolled centers. Although nTMS is a noninvasive modality, several reports proved not only the accuracy but also the feasibility of using it as a highly reliable tool for presurgical planning and intraoperative navigation [23, 58, 59] of primary motor functions. While non-invasive mapping can be reliably performed for primary motor functions (task-based fMRI, nTMS), this is currently not true for higher order functions (movement coordination, language, spatial consciousness, mentalizing, …) [60].
MEG was also rarely used. Nonetheless, despite its limited availability and high costs, its usefulness for presurgical planning and follow-up has repeatedly been reported [61–63].
Irrespective of the used modality, noninvasive evaluation of eloquent function, especially if adjacent to or within the tumor is mandatory in order to identify the optimal time point for re-resection. This is even more relevant in the contrast of tumor-induced cortical reorganization potentially allowing gross total resection of previously unresectable tumors [64–68].
In the near future, ongoing developments might be capable to achieve a change in imaging practice. Alongside with huge developments of machine learning using conventional MRI, which suppose the robustness of standardized sequences; an increased interest of metabolic multinuclear MR imaging and integration of multiparametric data into realistic metabolic-dynamic mathematical models is noticed.
Strengths and limitations
The survey solely focused on imaging modalities for preoperative workup and follow up investigations of DLGG patients. The large number of centers involved and the high conformity of surgical treatment within participants of the ELGGN represent the major strength of this study. Up to 2800 MRIs per year would be available in this network. Online surveys are limited by the accuracy of the given statements and their representation of a multidisciplinary team cannot be guaranteed in online survey, which limits the reliability of the results. Questions were not designed as detailed individual cases and we acknowledge that this method might have provoked heterogeneity of responses. Additionally, the study did not aim to investigate the outcome of DLGG treatment.
Conclusion
It appears mandatory to standardize the initial management and follow-up of DLGG in order to maximize the number of included patients in future multicentric studies. If a certain proximity of imaging protocol throughout Europe is already present, this work emphasizes the need to clarify important questions such as assessment of treatment response or detection of DLGG malignant transformation. ELGGN can help to resolve important issues and to promote a better care for patients suffering from DLGG.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Open access funding provided by University of Innsbruck and Medical University of Innsbruck.
Compliance with ethical standards
Conflict of interest
On behalf of all authors we declare no conflict of interest.
References
- 1.Ellingson BM, Bendszus M, Boxerman J, Barboriak D, Erickson BJ, Smits M, Nelson SJ, Gerstner E, Alexander B, Goldmacher G, Wick W, Vogelbaum M, Weller M, Galanis E, Kalpathy-Cramer J, Shankar L, Jacobs P, Pope WB, Yang D, Chung C, Knopp MV, Cha S, van den Bent MJ, Chang S, Yung WK, Cloughesy TF, Wen PY, Gilbert MR, Jumpstarting Brain Tumor Drug Development Coalition Imaging Standardization Steering C Consensus recommendations for a standardized Brain Tumor Imaging Protocol in clinical trials. Neuro Oncol. 2015;17:1188–1198. doi: 10.1093/neuonc/nov225.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weller M, van den Bent M, Tonn JC, Stupp R, Preusser M, Cohen-Jonathan-Moyal E, Henriksson R, Le Rhun E, Balana C, Chinot O, Bendszus M, Reijneveld JC, Dhermain F, French P, Marosi C, Watts C, Oberg I, Pilkington G, Baumert BG, Taphoorn MJB, Hegi M, Westphal M, Reifenberger G, Soffietti R, Wick W, European Association for Neuro-Oncology Task Force on G European Association for Neuro-Oncology (EANO) guideline on the diagnosis and treatment of adult astrocytic and oligodendroglial gliomas. Lancet Oncol. 2017;18:e315-e329. doi: 10.1016/S1470-2045(17)30517-X. [DOI] [PubMed] [Google Scholar]
- 3.Mandonnet E, Wager M, Almairac F, Baron M-H, Blonski M, Freyschlag CF, Barone F, Fontaine D, Pallud J, Hegi M, Viegas C, Zetterling M, Spena G, Goodden J, Rutten G-J, Taillandier L, Foroglu N, Darlix A, Skrap M, Martino J, von Campe G, Madadaki C, Gayat E, de Witt Hamer P, Gil Robles S, Sarubbo S, Santorius T, Bello L, Forster M-T, Duffau H (2017) Survey on current practice within the European Low-Grade Glioma Network: where do we stand and what is the next step? Neuro-Oncol Pract. 10.1093/nop/npw031 [DOI] [PMC free article] [PubMed]
- 4.Duffau H, Mandonnet E, Pallud J, Krieg SM, Gil Robles S, De Witt Hamer P, Schucht P, Zetterling M, Santarius T, Colle H, Ryan M, Smits A, von Campe G, Bello L, Forster MT, Sarubbo S, Spena G, Baron MH, Yordanova YN, Darlix A, Viegas C, Almairac F, Martino J, Goodden J, Chumas P, Freyschlag CF, Robe P, Wager M, Polivka M, Giakoumettis D, Robert E, Guillevin R, Grivas A, Fontaine D, van Greemen K, Lubrano V, Rutten GJ, Barone F, Rofes A, Rech F, Rigau V, Wagemakers M, Papagno C, Aerts A, Visch-Brink E, Tate M, Garg N, Klein M, Satoer D, De Witte E, van Brussel L, Reyes A, van Ierschot F, Veenstra W, Snijders T, Taillandier L, Blonski M. Evidenced-based medicine in glioma: molecular biology is only part of the story. Lancet Oncol. 2017 [Google Scholar]
- 5.Wohrer A, Waldhor T, Heinzl H, Hackl M, Feichtinger J, Gruber-Mosenbacher U, Kiefer A, Maier H, Motz R, Reiner-Concin A, Richling B, Idriceanu C, Scarpatetti M, Sedivy R, Bankl HC, Stiglbauer W, Preusser M, Rossler K, Hainfellner JA. The Austrian Brain Tumour Registry: a cooperative way to establish a population-based brain tumour registry. J Neurooncol. 2009;95:401–411. doi: 10.1007/s11060-009-9938-9. [DOI] [PubMed] [Google Scholar]
- 6.Duffau H, Taillandier L. New concepts in the management of diffuse low-grade glioma: proposal of a multistage and individualized therapeutic approach. Neuro Oncol. 2015;17:332–342. doi: 10.1093/neuonc/nov204.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jakola AS, Unsgard G, Myrmel KS, Kloster R, Torp SH, Lindal S, Solheim O. Low grade gliomas in eloquent locations—implications for surgical strategy, survival and long term quality of life. PLoS ONE. 2012;7:e51450. doi: 10.1371/journal.pone.0051450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moritz-Gasser S, Herbet G, Maldonado IL, Duffau H. Lexical access speed is significantly correlated with the return to professional activities after awake surgery for low-grade gliomas. J Neurooncol. 2012;107:633–641. doi: 10.1007/s11060-011-0789-9. [DOI] [PubMed] [Google Scholar]
- 9.Beez T, Boge K, Wager M, Whittle I, Fontaine D, Spena G, Braun S, Szelenyi A, Bello L, Duffau H, Sabel M, European Low Grade Glioma N Tolerance of awake surgery for glioma: a prospective European Low Grade Glioma Network multicenter study. Acta Neurochir (Wien) 2013;155:1301–1308. doi: 10.1007/s00701-013-1759-0. [DOI] [PubMed] [Google Scholar]
- 10.Szelenyi A, Bello L, Duffau H, Fava E, Feigl GC, Galanda M, Neuloh G, Signorelli F, Sala F, Workgroup for Intraoperative Management in Low-Grade Glioma Surgery within the European Low-Grade Glioma Intraoperative electrical stimulation in awake craniotomy: methodological aspects of current practice. Neurosurg Focus. 2010;28:E7. doi: 10.3171/2009.12.FOCUS09237. [DOI] [PubMed] [Google Scholar]
- 11.De Witt Hamer PC, Hendriks EJ, Mandonnet E, Barkhof F, Zwinderman AH, Duffau H. Resection probability maps for quality assessment of glioma surgery without brain location bias. PLoS ONE. 2013;8:e73353. doi: 10.1371/journal.pone.0073353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, Degroot J, Wick W, Gilbert MR, Lassman AB, Tsien C, Mikkelsen T, Wong ET, Chamberlain MC, Stupp R, Lamborn KR, Vogelbaum MA, van den Bent MJ, Chang SM. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28:1963–1972. doi: 10.1200/JCO.2009.26.3541. [DOI] [PubMed] [Google Scholar]
- 13.van den Bent MJ, Wefel JS, Schiff D, Taphoorn MJB, Jaeckle K, Junck L, Armstrong T, Choucair A, Waldman AD, Gorlia T, Chamberlain M, Baumert BG, Vogelbaum MA, Macdonald DR, Reardon DA, Wen PY, Chang SM, Jacobs AH. Response assessment in neuro-oncology (a report of the RANO group): assessment of outcome in trials of diffuse low-grade gliomas. Lancet Oncol. 2011;12:583–593. doi: 10.1016/S1470-2045(11)70057-2. [DOI] [PubMed] [Google Scholar]
- 14.Ostrom QT, Bauchet L, Davis FG, Deltour I, Fisher JL, Langer CE, Pekmezci M, Schwartzbaum JA, Turner MC, Walsh KM, Wrensch MR, Barnholtz-Sloan JS. The epidemiology of glioma in adults: a “state of the science” review. Neuro Oncol. 2014;16:896–913. doi: 10.1093/neuonc/nou087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bauknecht HC, Romano VC, Rogalla P, Klingebiel R, Wolf C, Bornemann L, Hamm B, Hein PA. Intra- and interobserver variability of linear and volumetric measurements of brain metastases using contrast-enhanced magnetic resonance imaging. Invest Radiol. 2010;45:49–56. doi: 10.1097/RLI.0b013e3181c02ed5. [DOI] [PubMed] [Google Scholar]
- 16.Reuter M, Gerstner ER, Rapalino O, Batchelor TT, Rosen B, Fischl B. Impact of MRI head placement on glioma response assessment. J Neurooncol. 2014;118:123–129. doi: 10.1007/s11060-014-1403-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmitt P, Mandonnet E, Perdreau A, Angelini ED. Effects of slice thickness and head rotation when measuring glioma sizes on MRI: in support of volume segmentation versus two largest diameters methods. J Neurooncol. 2013;112:165–172. doi: 10.1007/s11060-013-1051-4. [DOI] [PubMed] [Google Scholar]
- 18.Bobek-Billewicz B, Stasik-Pres G, Hebda A, Majchrzak K, Kaspera W, Jurkowski M. Anaplastic transformation of low-grade gliomas (WHO II) on magnetic resonance imaging. Folia Neuropathol. 2014;52:128–140. doi: 10.5114/fn.2014.43784. [DOI] [PubMed] [Google Scholar]
- 19.Hlaihel C, Guilloton L, Guyotat J, Streichenberger N, Honnorat J, Cotton F. Predictive value of multimodality MRI using conventional, perfusion, and spectroscopy MR in anaplastic transformation of low-grade oligodendrogliomas. J Neurooncol. 2010;97:73–80. doi: 10.1007/s11060-009-9991-4. [DOI] [PubMed] [Google Scholar]
- 20.Fouke SJ, Benzinger T, Gibson D, Ryken TC, Kalkanis SN, Olson JJ. The role of imaging in the management of adults with diffuse low grade glioma: a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2015;125:457–479. doi: 10.1007/s11060-015-1908-9. [DOI] [PubMed] [Google Scholar]
- 21.Pallud J, Blonski M, Mandonnet E, Audureau E, Fontaine D, Sanai N, Bauchet L, Peruzzi P, Frenay M, Colin P, Guillevin R, Bernier V, Baron MH, Guyotat J, Duffau H, Taillandier L, Capelle L. Velocity of tumor spontaneous expansion predicts long-term outcomes for diffuse low-grade gliomas. Neuro Oncol. 2013;15:595–606. doi: 10.1093/neuonc/nos331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Belhawi SM, Hoefnagels FW, Baaijen JC, Aliaga ES, Reijneveld JC, Heimans JJ, Barkhof F, Vandertop WP, Hamer PC. Early postoperative MRI overestimates residual tumour after resection of gliomas with no or minimal enhancement. Eur Radiol. 2011;21:1526–1534. doi: 10.1007/s00330-011-2081-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takahashi S, Jussen D, Vajkoczy P, Picht T. Plastic relocation of motor cortex in a patient with LGG (low grade glioma) confirmed by NBS (navigated brain stimulation) Acta Neurochir (Wien) 2012;154:2003–2008. doi: 10.1007/s00701-012-1492-0. [DOI] [PubMed] [Google Scholar]
- 24.Bette S, Kaesmacher J, Huber T, Delbridge C, Ringel F, Boeckh-Behrens T, Meyer B, Zimmer C, Kirschke JS, Gempt J. Value of early postoperative FLAIR volume dynamic in glioma with no or minimal enhancement. World Neurosurg. 2016;91:548–559.e541. doi: 10.1016/j.wneu.2016.03.034. [DOI] [PubMed] [Google Scholar]
- 25.Albert FK, Forsting M, Sartor K, Adams HP, Kunze S. Early postoperative magnetic resonance imaging after resection of malignant glioma: objective evaluation of residual tumor and its influence on regrowth and prognosis. Neurosurgery. 1994;34:45–60. doi: 10.1097/00006123-199401000-00008. [DOI] [PubMed] [Google Scholar]
- 26.Chang EF, Clark A, Jensen RL, Bernstein M, Guha A, Carrabba G, Mukhopadhyay D, Kim W, Liau LM, Chang SM, Smith JS, Berger MS, McDermott MW. Multiinstitutional validation of the University of California at San Francisco low-grade glioma prognostic scoring system. J Neurosurg. 2009;111:203–210. doi: 10.3171/2009.2.JNS081101. [DOI] [PubMed] [Google Scholar]
- 27.Sorensen AG, Patel S, Harmath C, Bridges S, Synnott J, Sievers A, Yoon YH, Lee EJ, Yang MC, Lewis RF, Harris GJ, Lev M, Schaefer PW, Buchbinder BR, Barest G, Yamada K, Ponzo J, Kwon HY, Gemmete J, Farkas J, Tievsky AL, Ziegler RB, Salhus MR, Weisskoff R. Comparison of diameter and perimeter methods for tumor volume calculation. J Clin Oncol. 2001;19:551–557. doi: 10.1200/JCO.2001.19.2.551. [DOI] [PubMed] [Google Scholar]
- 28.Pallud J, Mandonnet E, Duffau H, Kujas M, Guillevin R, Galanaud D, Taillandier L, Capelle L. Prognostic value of initial magnetic resonance imaging growth rates for World Health Organization grade II gliomas. Ann Neurol. 2006;60:380–383. doi: 10.1002/ana.20946. [DOI] [PubMed] [Google Scholar]
- 29.Izquierdo C, Alentorn A, Idbaih A, Simo M, Kaloshi G, Ricard D, Barritault M, Meyronet D, Bruna J, Honnorat J, Delattre JY, Ducray F. Long-term impact of temozolomide on 1p/19q-codeleted low-grade glioma growth kinetics. J Neurooncol. 2017 doi: 10.1007/s11060-017-2677-4. [DOI] [PubMed] [Google Scholar]
- 30.Pallud J, Capelle L, Taillandier L, Fontaine D, Mandonnet E, Guillevin R, Bauchet L, Peruzzi P, Laigle-Donadey F, Kujas M, Guyotat J, Baron MH, Mokhtari K, Duffau H. Prognostic significance of imaging contrast enhancement for WHO grade II gliomas. Neuro Oncol. 2009;11:176–182. doi: 10.1215/15228517-2008-066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Danchaivijitr N, Waldman AD, Tozer DJ, Benton CE, Brasil Caseiras G, Tofts PS, Rees JH, Jager HR. Low-grade gliomas: do changes in rCBV measurements at longitudinal perfusion-weighted MR imaging predict malignant transformation? Radiology. 2008;247:170–178. doi: 10.1148/radiol.2471062089. [DOI] [PubMed] [Google Scholar]
- 32.Rees J, Watt H, Jager HR, Benton C, Tozer D, Tofts P, Waldman A. Volumes and growth rates of untreated adult low-grade gliomas indicate risk of early malignant transformation. Eur J Radiol. 2009;72:54–64. doi: 10.1016/j.ejrad.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 33.Cochereau J, Herbet G, Rigau V, Duffau H. Acute progression of untreated incidental WHO Grade II glioma to glioblastoma in an asymptomatic patient. J Neurosurg. 2016;124:141–145. doi: 10.3171/2014.12.JNS141851. [DOI] [PubMed] [Google Scholar]
- 34.Jalbert LE, Neill E, Phillips JJ, Lupo JM, Olson MP, Molinaro AM, Berger MS, Chang SM, Nelson SJ. Magnetic resonance analysis of malignant transformation in recurrent glioma. Neuro Oncol. 2016;18:1169–1179. doi: 10.1093/neuonc/now008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guillevin R, Menuel C, Duffau H, Kujas M, Capelle L, Aubert A, Taillibert S, Idbaih A, Pallud J, Demarco G, Costalat R, Hoang-Xuan K, Chiras J, Vallee JN. Proton magnetic resonance spectroscopy predicts proliferative activity in diffuse low-grade gliomas. J Neurooncol. 2008;87:181–187. doi: 10.1007/s11060-007-9508-y. [DOI] [PubMed] [Google Scholar]
- 36.Shiroishi MS, Habibi M, Rajderkar D, Yurko C, Go JL, Lerner A, Mogensen MA, Kim PE, Boyko OB, Zee CS, Law M. Perfusion and permeability MR imaging of gliomas. Technol Cancer Res Treat. 2011;10:59–71. doi: 10.7785/tcrt.2012.500180. [DOI] [PubMed] [Google Scholar]
- 37.Essig M, Anzalone N, Combs SE, Dorfler A, Lee SK, Picozzi P, Rovira A, Weller M, Law M. MR imaging of neoplastic central nervous system lesions: review and recommendations for current practice. AJNR Am J Neuroradiol. 2012;33:803–817. doi: 10.3174/ajnr.A2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Essig M, Nguyen TB, Shiroishi MS, Saake M, Provenzale JM, Enterline DS, Anzalone N, Dorfler A, Rovira A, Wintermark M, Law M. Perfusion MRI: the five most frequently asked clinical questions. AJR Am J Roentgenol. 2013;201:W495-510. doi: 10.2214/AJR.12.9544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Voges J, Herholz K, Holzer T, Wurker M, Bauer B, Pietrzyk U, Treuer H, Schroder R, Sturm V, Heiss WD. 11C-methionine and 18F-2-fluorodeoxyglucose positron emission tomography: a tool for diagnosis of cerebral glioma and monitoring after brachytherapy with 125I seeds. Stereotact Funct Neurosurg. 1997;69:129–135. doi: 10.1159/000099864. [DOI] [PubMed] [Google Scholar]
- 40.Wyss M, Hofer S, Bruehlmeier M, Hefti M, Uhlmann C, Bartschi E, Buettner UW, Roelcke U. Early metabolic responses in temozolomide treated low-grade glioma patients. J Neurooncol. 2009;95:87–93. doi: 10.1007/s11060-009-9896-2. [DOI] [PubMed] [Google Scholar]
- 41.Dunet V, Rossier C, Buck A, Stupp R, Prior JO. Performance of 18F-fluoro-ethyl-tyrosine (18F-FET) PET for the differential diagnosis of primary brain tumor: a systematic review and Metaanalysis. J Nucl Med. 2012;53:207–214. doi: 10.2967/jnumed.111.096859. [DOI] [PubMed] [Google Scholar]
- 42.Gempt J, Bette S, Ryang YM, Buchmann N, Peschke P, Pyka T, Wester HJ, Forster S, Meyer B, Ringel F. 18F-fluoro-ethyl-tyrosine positron emission tomography for grading and estimation of prognosis in patients with intracranial gliomas. Eur J Radiol. 2015;84:955–962. doi: 10.1016/j.ejrad.2015.01.022. [DOI] [PubMed] [Google Scholar]
- 43.Jansen NL, Suchorska B, Wenter V, Eigenbrod S, Schmid-Tannwald C, Zwergal A, Niyazi M, Drexler M, Bartenstein P, Schnell O, Tonn JC, Thon N, Kreth FW, la Fougere C. Dynamic 18F-FET PET in newly diagnosed astrocytic low-grade glioma identifies high-risk patients. J Nucl Med. 2014;55:198–203. doi: 10.2967/jnumed.113.122333. [DOI] [PubMed] [Google Scholar]
- 44.Jansen NL, Suchorska B, Wenter V, Schmid-Tannwald C, Todica A, Eigenbrod S, Niyazi M, Tonn JC, Bartenstein P, Kreth FW, la Fougere C. Prognostic significance of dynamic 18F-FET PET in newly diagnosed astrocytic high-grade glioma. J Nucl Med. 2015;56:9–15. doi: 10.2967/jnumed.114.144675. [DOI] [PubMed] [Google Scholar]
- 45.Villani V, Carapella CM, Chiaravalloti A, Terrenato I, Piludu F, Vidiri A, Schillaci O, Floris R, Marzi S, Fabi A, Pace A. The Role of PET [18F]FDOPA in Evaluating Low-grade Glioma. Anticancer Res. 2015;35:5117–5122. [PubMed] [Google Scholar]
- 46.Albert NL, Weller M, Suchorska B, Galldiks N, Soffietti R, Kim MM, la Fougere C, Pope W, Law I, Arbizu J, Chamberlain MC, Vogelbaum M, Ellingson BM, Tonn JC. Response Assessment in Neuro-Oncology working group and European Association for Neuro-Oncology recommendations for the clinical use of PET imaging in gliomas. Neuro Oncol. 2016;18:1199–1208. doi: 10.1093/neuonc/now058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kumar AJ, Leeds NE, Fuller GN, Van Tassel P, Maor MH, Sawaya RE, Levin VA. Malignant gliomas: MR imaging spectrum of radiation therapy- and chemotherapy-induced necrosis of the brain after treatment. Radiology. 2000;217:377–384. doi: 10.1148/radiology.217.2.r00nv36377. [DOI] [PubMed] [Google Scholar]
- 48.Unterrainer M, Schweisthal F, Suchorska B, Wenter V, Schmid-Tannwald C, Fendler WP, Schuller U, Bartenstein P, Tonn JC, Albert NL. Serial 18F-FET PET imaging of primarily 18F-FET-negative glioma: does it make sense? J Nucl Med. 2016;57:1177–1182. doi: 10.2967/jnumed.115.171033. [DOI] [PubMed] [Google Scholar]
- 49.Bartos R, Jech R, Vymazal J, Petrovicky P, Vachata P, Hejcl A, Zolal A, Sames M. Validity of primary motor area localization with fMRI versus electric cortical stimulation: a comparative study. Acta Neurochir (Wien) 2009;151:1071–1080. doi: 10.1007/s00701-009-0368-4. [DOI] [PubMed] [Google Scholar]
- 50.Giussani C, Roux FE, Ojemann J, Sganzerla EP, Pirillo D, Papagno C. Is preoperative functional magnetic resonance imaging reliable for language areas mapping in brain tumor surgery? Review of language functional magnetic resonance imaging and direct cortical stimulation correlation studies. Neurosurgery. 2010;66:113–120. doi: 10.1227/01.NEU.0000360392.15450.C9. [DOI] [PubMed] [Google Scholar]
- 51.Kuchcinski G, Mellerio C, Pallud J, Dezamis E, Turc G, Rigaux-Viode O, Malherbe C, Roca P, Leclerc X, Varlet P, Chretien F, Devaux B, Meder JF, Oppenheim C. Three-tesla functional MR language mapping: comparison with direct cortical stimulation in gliomas. Neurology. 2015;84:560–568. doi: 10.1212/WNL.0000000000001226. [DOI] [PubMed] [Google Scholar]
- 52.Morrison MA, Tam F, Garavaglia MM, Hare GM, Cusimano MD, Schweizer TA, Das S, Graham SJ. Sources of variation influencing concordance between functional MRI and direct cortical stimulation in brain tumor surgery. Front Neurosci. 2016;10:461. doi: 10.3389/fnins.2016.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stevens MT, Clarke DB, Stroink G, Beyea SD, D’Arcy RC. Improving fMRI reliability in presurgical mapping for brain tumours. J Neurol Neurosurg Psychiatry. 2016;87:267–274. doi: 10.1136/jnnp-2015-310307. [DOI] [PubMed] [Google Scholar]
- 54.Ille S, Sollmann N, Hauck T, Maurer S, Tanigawa N, Obermueller T, Negwer C, Droese D, Boeckh-Behrens T, Meyer B, Ringel F, Krieg SM. Impairment of preoperative language mapping by lesion location: a functional magnetic resonance imaging, navigated transcranial magnetic stimulation, and direct cortical stimulation study. J Neurosurg. 2015;123:314–324. doi: 10.3171/2014.10.JNS141582. [DOI] [PubMed] [Google Scholar]
- 55.Duffau H, Lopes M, Arthuis F, Bitar A, Sichez JP, Van Effenterre R, Capelle L. Contribution of intraoperative electrical stimulations in surgery of low grade gliomas: a comparative study between two series without (1985-96) and with (1996–2003) functional mapping in the same institution. J Neurol Neurosurg Psychiatry. 2005;76:845–851. doi: 10.1136/jnnp.2004.048520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.De Witt Hamer PC, Robles SG, Zwinderman AH, Duffau H, Berger MS. Impact of intraoperative stimulation brain mapping on glioma surgery outcome: a meta-analysis. J Clin Oncol. 2012;30:2559–2565. doi: 10.1200/JCO.2011.38.4818. [DOI] [PubMed] [Google Scholar]
- 57.Rofes A, Mandonnet E, Godden J, Baron MH, Colle H, Darlix A, de Aguiar V, Duffau H, Herbet G, Klein M, Lubrano V, Martino J, Mathew R, Miceli G, Moritz-Gasser S, Pallud J, Papagno C, Rech F, Robert E, Rutten GJ, Santarius T, Satoer D, Sierpowska J, Smits A, Skrap M, Spena G, Visch E, De Witte E, Zetterling M, Wager M. Survey on current cognitive practices within the European Low-Grade Glioma Network: towards a European assessment protocol. Acta Neurochir (Wien) 2017;159:1167–1178. doi: 10.1007/s00701-017-3192-2. [DOI] [PubMed] [Google Scholar]
- 58.Ille S, Sollmann N, Butenschoen VM, Meyer B, Ringel F, Krieg SM. Resection of highly language-eloquent brain lesions based purely on rTMS language mapping without awake surgery. Acta Neurochir (Wien) 2016;158:2265–2275. doi: 10.1007/s00701-016-2968-0. [DOI] [PubMed] [Google Scholar]
- 59.Kawashima A, Krieg SM, Faust K, Schneider H, Vajkoczy P, Picht T. Plastic reshaping of cortical language areas evaluated by navigated transcranial magnetic stimulation in a surgical case of glioblastoma multiforme. Clin Neurol Neurosurg. 2013;115:2226–2229. doi: 10.1016/j.clineuro.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 60.Krieg SM, Lioumis P, Makela JP, Wilenius J, Karhu J, Hannula H, Savolainen P, Lucas CW, Seidel K, Laakso A, Islam M, Vaalto S, Lehtinen H, Vitikainen AM, Tarapore PE, Picht T. Protocol for motor and language mapping by navigated TMS in patients and healthy volunteers; workshop report. Acta Neurochir (Wien) 2017;159:1187–1195. doi: 10.1007/s00701-017-3187-z. [DOI] [PubMed] [Google Scholar]
- 61.Tarapore PE, Findlay AM, Honma SM, Mizuiri D, Houde JF, Berger MS, Nagarajan SS. Language mapping with navigated repetitive TMS: proof of technique and validation. Neuroimage. 2013;82:260–272. doi: 10.1016/j.neuroimage.2013.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tarapore PE, Martino J, Guggisberg AG, Owen J, Honma SM, Findlay A, Berger MS, Kirsch HE, Nagarajan SS. Magnetoencephalographic imaging of resting-state functional connectivity predicts postsurgical neurological outcome in brain gliomas. Neurosurgery. 2012;71:1012–1022. doi: 10.1227/NEU.0b013e31826d2b78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tarapore PE, Tate MC, Findlay AM, Honma SM, Mizuiri D, Berger MS, Nagarajan SS. Preoperative multimodal motor mapping: a comparison of magnetoencephalography imaging, navigated transcranial magnetic stimulation, and direct cortical stimulation. J Neurosurg. 2012;117:354–362. doi: 10.3171/2012.5.JNS112124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Robles SG, Gatignol P, Lehericy S, Duffau H. Long-term brain plasticity allowing a multistage surgical approach to World Health Organization Grade II gliomas in eloquent areas. J Neurosurg. 2008;109:615–624. doi: 10.3171/JNS/2008/109/10/0615. [DOI] [PubMed] [Google Scholar]
- 65.Ius T, Angelini E, Thiebaut de Schotten M, Mandonnet E, Duffau H. Evidence for potentials and limitations of brain plasticity using an atlas of functional resectability of WHO grade II gliomas: towards a “minimal common brain”. Neuroimage. 2011;56:992–1000. doi: 10.1016/j.neuroimage.2011.03.022. [DOI] [PubMed] [Google Scholar]
- 66.Ille S, Kulchytska N, Sollmann N, Wittig R, Beurskens E, Butenschoen VM, Ringel F, Vajkoczy P, Meyer B, Picht T, Krieg SM. Hemispheric language dominance measured by repetitive navigated transcranial magnetic stimulation and postoperative course of language function in brain tumor patients. Neuropsychologia. 2016;91:50–60. doi: 10.1016/j.neuropsychologia.2016.07.025. [DOI] [PubMed] [Google Scholar]
- 67.Southwell DG, Hervey-Jumper SL, Perry DW, Berger MS. Intraoperative mapping during repeat awake craniotomy reveals the functional plasticity of adult cortex. J Neurosurg. 2016;124:1460–1469. doi: 10.3171/2015.5.JNS142833. [DOI] [PubMed] [Google Scholar]
- 68.Conway N, Wildschuetz N, Moser T, Bulubas L, Sollmann N, Tanigawa N, Meyer B, Krieg SM. Cortical plasticity of motor-eloquent areas measured by navigated transcranial magnetic stimulation in patients with glioma. J Neurosurg. 2017;127:981–991. doi: 10.3171/2016.9.JNS161595. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.