Abstract
Purpose: To estimate the strength and shape of the dose–response relationship between sedentary behaviour and all-cause, cardiovascular disease (CVD) and cancer mortality, and incident type 2 diabetes (T2D), adjusted for physical activity (PA). Data Sources: Pubmed, Web of Knowledge, Medline, Embase, Cochrane Library and Google Scholar (through September-2016); reference lists. Study Selection: Prospective studies reporting associations between total daily sedentary time or TV viewing time, and ≥ one outcome of interest. Data Extraction: Two independent reviewers extracted data, study quality was assessed; corresponding authors were approached where needed. Data Synthesis: Thirty-four studies (1,331,468 unique participants; good study quality) covering 8 exposure-outcome combinations were included. For total sedentary behaviour, the PA-adjusted relationship was non-linear for all-cause mortality (RR per 1 h/day: were 1.01 (1.00–1.01) ≤ 8 h/day; 1.04 (1.03–1.05) > 8 h/day of exposure), and for CVD mortality (1.01 (0.99–1.02) ≤ 6 h/day; 1.04 (1.03–1.04) > 6 h/day). The association was linear (1.01 (1.00–1.01)) with T2D and non-significant with cancer mortality. Stronger PA-adjusted associations were found for TV viewing (h/day); non-linear for all-cause mortality (1.03 (1.01–1.04) ≤ 3.5 h/day; 1.06 (1.05–1.08) > 3.5 h/day) and for CVD mortality (1.02 (0.99–1.04) ≤ 4 h/day; 1.08 (1.05–1.12) > 4 h/day). Associations with cancer mortality (1.03 (1.02–1.04)) and T2D were linear (1.09 (1.07–1.12)). Conclusions: Independent of PA, total sitting and TV viewing time are associated with greater risk for several major chronic disease outcomes. For all-cause and CVD mortality, a threshold of 6–8 h/day of total sitting and 3–4 h/day of TV viewing was identified, above which the risk is increased.
Electronic supplementary material
The online version of this article (10.1007/s10654-018-0380-1) contains supplementary material, which is available to authorized users.
Keywords: Sedentary, Prevention, Meta-analysis, Public health, Mortality, Diabetes
Introduction
Since the mid-twentieth century people have spent an increasingly greater amount of their time sedentary [1, 2]. Sedentary behaviours are defined as any waking time activity during which one is in seated, reclined or lying posture, expending low levels of energy [3, 4]. Americans spend 55% of their waking time, or 7.7 h/day, sedentary [5]. Europeans are estimated to spend on average 40% of their leisure time watching TV [6], equal to 2.8 h/day in the UK, which is not declining [7]. Accumulation of sedentary time is independent from lack of accumulation of moderate-to-vigorous physical activity (MVPA), e.g. sufficient levels in MVPA do not preclude relatively high levels of sedentary time and vice versa [8–10]. Moreover, the health effects of sedentary behaviours tend to persist, with some attenuation, after accounting for MVPA [11–13]. One recent meta-analysis including over 1 million adults documented that high levels of sitting time increased premature mortality risk in all but the most physically active individuals who accumulate ≥ 1 h/day of moderate-intensity activity [12].
Previous meta-analyses have attempted to estimate the potential impact of sedentary behaviour on specific health outcomes [9–12, 14–17]. However, they were not without considerable limitations, such as inclusion of non-prospective studies [10, 11] and use of an ambiguous sedentary behaviour exposure, defined by different exposure types across studies (i.e. a mix of total sitting, TV viewing or total leisure sitting time, which show different health associations [10–12]), and/or different exposure units or categories [10, 11, 16, 17]. Most importantly, few meta-analyses have examined dose–response associations, to determine whether there is a marked increase in risk of incident disease or mortality at a specific level on the sedentary time continuum [12, 14–17]. This information is essential to determine whether recommendations, currently only providing guidance to “sit less”, need further quantification. For all-cause mortality, spending > 3 or > 4 h/day of TV viewing and > 7 h/day in any sitting activity have been suggested as detrimental [14–16]. It is currently unknown whether these thresholds (if any) are the same for cardiovascular disease (CVD) and cancer mortality. For type 2 diabetes (T2D), existence of such threshold has only been examined in relation to TV viewing time (based on 3 studies only) [15], which is not reflective of total sitting time. Recent studies reported 3.8–5.9% of all deaths are due to daily sitting time [14, 18]. So far this is unknown for TV viewing time, which shows stronger health associations [12] and may be one of the most amenable types of sedentary behaviour [19].
We therefore aimed to examine the dose–response association between separate types of sedentary behaviour and all-cause, CVD and cancer mortality, as well as incident T2D and CVD, using the current prospective evidence. As PA is known to attenuate sedentary behaviour associations [12], we also aimed to map this attenuating effect across the whole continuous sedentary behaviour dose-spectrum, by comparing dose–response curves with and without adjustment for PA. Finally, in order to demonstrate the population impact of the established dose–response relationships, we calculated the population attributable fraction (PAF) due to TV viewing for these health outcomes in England.
Methods
Data sources and searches
Studies had to have assessed the association between total daily sitting/sedentary, TV viewing or leisure sitting time, and at least one of the outcomes of interest: all-cause, CVD or cancer mortality, incident (fatal and non-fatal) CVD and incident T2D. Time spent sitting/sedentary could be self-reported or objectively measured. Only primary research studies with a prospective design, with at least an abstract in English and investigating non-diseased adults (≥ 18 years) in the general population were included.
Sources included:
Electronic literature databases: Pubmed, Web of Knowledge, Medline, Embase, Cochrane Library and Google Scholar from 1st August 2014 to 30th September 2016. Search terms are listed in Online Appendix Table 1.
Reference lists of existing systematic reviews [9–12, 14–17, 20, 21], examining associations between sedentary behaviours and health outcomes, which together cover up to October 2015.
Authors’ personal literature databases up to 30th September 2016.
Reference lists of included articles.
Study selection
Titles and abstracts were screened by one author (RP) using the inclusion criteria, full reports were assessed where these were met or where there was uncertainty, allowing a final decision on eligibility. Where different articles formed part of the same cohort study, only data from the most recent publication for any given exposure-outcome combination was used. A minimum of 4 different eligible cohorts were required in order to carry out an analysis. Authors were contacted for additional information where needed.
Data extraction and quality assessment
Using a pre-designed data extraction spreadsheet, two authors carried out independent extractions and disagreement was resolved through discussion (RP and EM).
The quality of each study was assessed using these criteria: size of cohort, length of follow-up, description of inclusion criteria and sampling strategy and sample representativeness, based on the Newcastle–Ottawa scale [22]. No overall quality score was assigned for use in analyses, to prevent the scale itself becoming a source of bias [23].
Data synthesis and analysis
Extracted data were harmonized, converting each measure into one of: total sedentary, TV viewing or leisure sedentary time, quantified in h/day. Categories of sedentary time were assigned a dose, either the mid-point, or, in case of open-ended categories, half the width of the adjacent interval from the boundary (Online Appendix Tables 2 and 3). Where the lowest exposure was not the referent category, hazards were recalculated [24, 25].
Estimates of the linear association for each contributing study were calculated using Generalized Least-Squares regression [26, 27]. These were used to perform a random effects meta-analysis within each exposure-outcome combination, for both the most adjusted model without adjustment for PA and the least adjusted model with adjustment for PA [28]. These provided the summary RR per additional hour/day of exposure. Statistical heterogeneity of contributing studies was assessed with the I2 statistic, which was considered low if < 25%, and high if > 75% [29]. To examine publication bias and small study effect, Funnel plots were used, Egger’s tests were derived for each exposure-outcome combination with ≥ 5 contributing studies [30].
Following the estimation of a linear association, a restricted cubic spline transformation was carried out to investigate the shape of the dose–response relationship. Knots were placed at the 10th, 50th and 90th percentiles [31]. A random effects meta-analysis was then carried out to estimate the non-linear relationship between sedentary time and the respective health outcome. Where a change in strength of association was seen at a certain level on the exposure continuum, the RR (per h/day increment in exposure) on either side of this exposure level was calculated as the difference in log(RR) divided by difference in hours of exposure. Results were presented back on the original scale and were based on PA-adjusted analyses.
Sensitivity analyses were carried out to investigate the influence of study characteristics which might lead to risk of bias. Where sufficient data were available these were carried out on the linear PA adjusted associations. Factors investigated include: adiposity adjustment, sex, length of follow-up, age of cohort at baseline and representativeness of cohort.
Population attributable fraction (PAF)
PAF estimates were calculated for TV viewing, indicating the proportional reduction in incidence of the respective outcome if prevalent TV viewing levels were reduced to zero, assuming causality. As we did not have access to TV viewing data worldwide, TV viewing data from the 2012 Health Survey for England (HSE), a nationally representative sample of the English population, were used to carry out a Monte-Carlo micro-simulation as an illustration of the potential magnitude of the impact. Each 17+ year old participant in HSE was probabilistically assigned an RR based on the RR and associated uncertainty from the PA-adjusted dose–response analysis which corresponded to their TV viewing category (0, 0 to < 2, 2 to < 4, 4 to < 6 and 6+ h/day of TV viewing). These assigned RRs, along with the proportional contribution of each individual, according to HSE survey weights, were used to calculate an attributable fraction for each participant, according to the formula below [32].
Equation 1—Population attributable fraction
| 1 |
Pi = proportion of population at exposure level i, Pi′ = proportion of population at counterfactual exposure level, i.e. zero exposure, RR = the relative risk at exposure level i. n = the number of exposure levels.
This procedure was then repeated 5000 times and the final PAF estimate was the median value across the 5000 simulations. A 95% CI was calculated using the 2.5th and 97.5th percentiles of the simulated distribution.
Stata version 14.2, StataCorp, USA was used for the meta-analysis. The PAF calculations were carried out using Analytica Free 101 edition, Lumina Decision Systems Inc., USA.
Role of the funding source
This work was supported by the British Heart Foundation, the Medical Research Council, Cancer Research UK, Economic and Social Research Council, National Institute for Health Research, and the Wellcome Trust. The funders had no role in study design, conduct, or reporting of the results.
Results
Search results
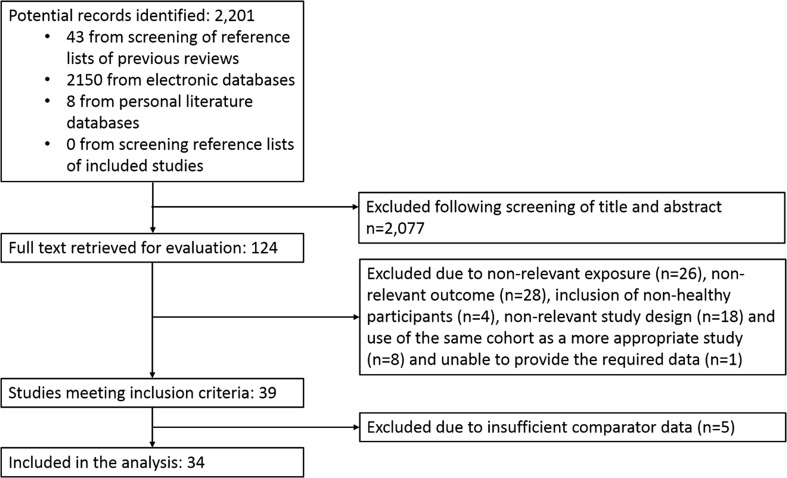
The literature search provided 2201 potential articles. Following screening of titles and abstracts, full text was retrieved for 124 publications, rendering 39 studies for which inclusion criteria were met (Fig. 1). For 5 studies there was an insufficient number of comparator cohorts within the same exposure-outcome combination [33–37], leaving 34 studies, across 8 exposure-outcome combinations, to be included in the analysis [38–71]. An insufficient number of cohorts was identified to allow investigation of associations between leisure sedentary time with any outcome. There were also insufficient studies investigating incident CVD with any exposure. We were therefore unable to carry out the planned analysis on these exposures and outcomes. Additional data were successfully obtained from 11 studies [38–48].
Fig. 1.
Flow diagram for study inclusion
Study characteristics
Data from a total of 1,331,468 unique participants was included. Table 1 and Online Appendix Table 4 summarize the characteristics of the 34 included studies (with additional data from publications describing cohort characteristics [72–85]). The size of included studies ranged from 208 [51] to 240,819 [61] participants with a mean of 39,161. Follow-up was on average 8.9 years and varied from 2 [69] to 31 [46] years. The numbers of cases and participants by outcome are shown in Table 2. Of the 34 studies, 17 were from North America, 9 from Europe, 4 from Australia and 4 from Asia. The dates of publication spanned 2001–2016, although 65% were published in 2013 or later.
Table 1.
Descriptive information on the 34 studies included in the analysis by outcome (studies for each outcome are sorted by the exposures investigated and first author’s name)
| Authors | Country/cohort | Exposures investigated (events) | n at baseline/average follow-up (years) | Average age at baselinea/proportion male | Full list of covariates included in models |
|---|---|---|---|---|---|
| All-cause mortality | |||||
| Chau et al. [40] | Norway/HUNT3 | Total sitting (640), TV viewing (684) |
42,077/3.3 | 53.3/0.45 | Sex, body mass index, education level, meeting PA guidelines, smoking status, general health status, cardio-metabolic disease (CMD) status with age as the time axis |
| Kim et al. [45] | USA/Multiethnic Cohort Study (MEC) | Total sitting (19,143), TV viewing (19,143) |
134,596/13.7 | 59.0/0.46 | Hazard ratios were calculated with age as the time metric, adjusted for age at cohort entry (5-year age groups), education, ethnicity and smoking history [including the following variables: smoking status, average number of cigarettes, average number of cigarettes squared, number of years smoked (time dependent), number of years since quitting (time dependent) and interactions between ethnicity and the smoking variables]. also adjusted for history of hypertension and/or diabetes at enrolment, alcohol consumption, energy intake and physical activity (METs per week for moderate activity, vigorous work and strenuous sports) |
| Pulsford et al. [66] | UK/Whitehall 2 | Total sitting (450), TV viewing (324) |
5132/15.7 | 43.9/0.73 | Age, gender, employment grade and ethnicity, smoking status, alcohol consumption, fruit and vegetable consumption, BMI, physical functioning, daily walking time and MVPA. |
| Ensrud et al. [42] | USA/Osteoporotic Fractures in Men Study (MrOS) | Total sitting—accelerometer measured (409) | 2918/4.5 | 79/1.00 | Age, race, site, season, education, marital status, health status, smoking, comorbidity burden, depressive symptoms, cognitive function, number of instrumental activity of daily living impairments, and % body fat., Physical Activity Scale for the Elderly, Gait Speed, and Time Spent Asleep |
| Fox et al. [51] | UK/OPAL | Total sitting—accelerometer measured (32) | 208/4.2 | 78.1/0.51 | Adjusting for age, gender, educational attainment, IMD, weight status, GP Management System and number of self-reported chronic illnesses at baseline; additionally adjusting for lower limb function |
| Inoue et al. [55] | Japan/Japan Public Health Center-based prospective study group | Total sitting (4564) | 83,034/8.7 | 56.0/0.47 | Age (5-year age categories), area (11 PHC areas), occupation (stratified, full-time agriculture/forestry/fishery, full-time salaried/self-employed/professional, multiple occupations, full-time housework/retired/unemployed), history of diabetes (Y/N), smoking status (never, past, current), alcohol intake status (almost none, occasional, regular), BMI (14 to < 20, 20 to < 27, ≥ 27), total energy intake (quintiles), heavy physical work or strenuous exercise (none, < 1 h, ≥ 1 h), walking or standing hours (< 1 h, 1–3 h, ≥ 3 h), and leisure-time sports or physical exercise (< day/week, 1–2 days/week, ≥ 3–4 days/week) |
| Katzmarzyk et al. [56] | Canada/Canada Fitness Survey | Total sitting (1832) | 17,013/12.0 | 42.0/0.43 | Age, sex (as a continuous variable), smoking (former, current, non-smoker), alcohol consumption (abstainer, < 10 drinks per month, 10–50 drinks per month, > 50 drink per month), leisure time physical activity (as a continuous variable, METh wk−1), and the Physical Activity Readiness Questionnaire (pass/fail/missing) |
| Matthews et al. [60] | USA/Southern Community Cohort Study | Total Sitting (5007) | 64,304/6.4 | 69.9/0.45 | Sex, source of enrollment (community health center or general population), educational level (< 9 years, 9–11 years, high school, some college, or beyond college), household income (< $15,000, $15,000–$24,999, $25,000–$49,999, or ≥ $50,000), cigarette smoking (never; former, < 1 pack/day; former, ≥ 1 pack/day; current, < 1 pack/day; or current, ≥ 1 pack/day), BMI(< 18.5, 18.5–24.9, 25–29.9, 30.0–34.9, or ≥ 35.0), sleep duration (< 7, 7–8, or ≥ 9 h/day), diabetes (Y/N), employment status (Y/N), and overall physical activity level (quartiles). (Age was used as the underlying time metric) |
| Matthews et al. [62] | USA/NIH-AARP Diet and Health Study | Total sitting (12,201) | 154,614/6.8 | 69.8/0.52 | Age, Education (< 12 years, high school graduate, some college, college graduate, unknown), Smoking history (never, stopped 10+, 5–9, 1–4 years ago, stopped < 1 year, current smoker, unknown), Sleep duration (< 4, 4, 5.9, 6, 7.9, 8, 9.9, 10+ h/day, unknown), Overall health (excellent, very good, good, fair, poor, unknown), and BMI (< 25, 25, 29.9, 30+ kg/m2, unknown) additionally adjusted for Overall physical activity (< 1, 1–1.9, 2–2.9, 3–3.9, 4+ h/day) |
| Pavey et al. [63] | Australia/Australian Longitudinal Study on Women’s Health | Total sitting (1364) | 47,53/6.0 | 78.0/0.00 | Age, education, marital status, area, smoking, alcohol consumption, BMI, PA |
| Petersen et al. [64] | Denmark/Danish Health Examination Survey | Total sitting (1074) | 71,363/5.4 | 48.1/0.40 | Age, sex, education, physical activity level in leisure time, smoking habits, body mass index, alcohol consumption, diabetes, and hypertension |
| Schmid et al. [67] | USA/National Health and Nutrition Examination Survey | Total sitting—accelerometer measured (112) | 1677/2.9 | 67.0/0.49 | Age, sex, education, ethnicity, smoking, light physical activity, alcohol consumption, history of diabetes, history of cardiovascular disease (coronary heart disease, congestive heart failure, stroke), history of cancer, and mobility limitations. Plus accelerometer measured MVPA |
| Seguin et al. [68] | USA/Women’s Health Initiative (WHI) Observational Study (OS) and Extension Study (ES). | Total sitting (13,316) | 92,234/12.2 | 64.0/0.00 | Age, race, physical activities, and physical function score adjusted |
| van der Ploeg et al. [71] | Australia/45 and up cohort | Total sitting (4405) | 22,2497/2.8 | 62.1/0.48 | Age, sex, educational level, marital status, urban or rural residence, physical activity, BMI, smoking status, self-rated health, and receiving help with daily tasks for a long-term illness or disability |
| Basterra-Gortari et al. [39] | Spain/The SUN Cohort | TV viewing (97) | 13,284/8.2 | 37/0.38 | Age (continuous), sex, smoking history (never, current, quit), total energy intake, Mediterranean diet adherence (continuous), baseline body mass index (continuous), physical activity (quartiles), computer use (continuous) and driving (continuous) |
| Dunstan et al. [49] | Australia/The Australian Diabetes, Obesity and Lifestyle Study (AusDiab) | TV viewing (284) | 8800/6.6 | 50.47/0.44 | Age, sex, smoking (current or ex-smoker), education (≥ 12 years), total energy intake, alcohol intake, Diet Quality Index, waist circumference, hypertension (blood pressure ≥ 140/90 mm Hg or antihypertensive medication use), total plasma cholesterol (mmol/L), HDL-C (mmol/L), serum triglycerides (mmol/L, log), lipid-lowering medication use, and glucose tolerance status and exercise time |
| Keadle et al. [57] | USA/NIH-AARP Diet and Health Study | TV viewing (36,590) | 221,426/14.1 | 62.5/0.57 | Age (years), sex, race (white, black, other, missing), education (< 12 years, high school graduate, some college, college graduate, missing), smoking history (never; quit, ≤ 20 cigarettes/day; quit, > 20 cigarettes/day; current, ≤ 20 cigarettes/day; current, > 20 cigarettes/day; unknown), MVPA (never or rarely, 1, 1–3, 4–7, Z7 h/week) and diet quality (quintiles), BMI categories (18.5 to < 25, 25 to < 30, 30–35 and > 35 kg/m2) and health status (good, very good, excellent) |
| Matthews et al. [61] | USA/NIH-AARP Diet and Health Study | TV viewing (17,044) | 240,819/8.5 | 62.5/0.56 | Age, sex, race (white, black, other, missing), education (< 12 year, high school graduate, some college, college graduate, missing), smoking history (never; quit, ≤ 20 cigarettes/day; quit, > 20 cigarettes/day; current, ≤ 20 cigarettes/day; current, > 20 cigarettes/day; unknown), diet quality (quintiles) moderate-vigorous physical activity (never/rarely; < 1, 1–3, 4–7, > 7 h/week) |
| Muennig et al. [46] | USA/General Socal Survey | TV viewing (2048) | 9344/b | 44.0/0.42 | Year, race, sex, education status, age, and income |
| Suzuki et al. [70] | Japan/Japan Collaborative cohort study | TV viewing (9880) | 110,792/b | 57.8/0.41 | Age and area of study |
| Wijndaele et al. [48] | UK/EPIC-Norfolk | TV viewing (1270) | 13,197/9.5 | 61.5/0.43 | Age and gender, education level, smoking status, alcohol consumption, medication for hypertension (not in models examining cancer mortality), medication for dyslipidaemia (not in models examining cancer mortality), baseline history of diabetes, family history of CVD and family history of cancer, total PAEE (METh/day) |
| CVD mortality | |||||
| Kim et al. [45] | USA/Multiethnic Cohort Study (MEC) | Total sitting (6535), TV viewing (6535) |
134,596/13.7 | 59.0/0.46 | Hazard ratios were calculated with age as the time metric, adjusted for age at cohort entry (5–year age groups), education, ethnicity and smoking history [including the following variables: smoking status, average number of cigarettes, average number of cigarettes squared, number of years smoked (time dependent), number of years since quitting (time dependent) and interactions between ethnicity and the smoking variables]. also adjusted for history of hypertension and/or diabetes at enrolment, alcohol consumption, energy intake and physical activity (METs per week for moderate activity, vigorous work and strenuous sports) |
| Ensrud et al. [42] | USA/Osteoporotic Fractures in Men Study (MrOS) | Total sitting– accelerometer measured (138) | 2918/4.5 | 79/1.00 | Age, race, site, season, education, marital status, health status, smoking, comorbidity burden, depressive symptoms, cognitive function, number of instrumental activity of daily living impairments, and % body fat |
| Katzmarzyk et al. [56] | Canada/Canada Fitness Survey | Total sitting (759) | 17,013/12.0 | 42.0/0.43 | Age, sex (as a continuous variable), smoking (former, current, non-smoker), alcohol consumption (abstainer, < 10 drinks per month, 10–50 drinks per month, > 50 drink per month), leisure time physical activity (as a continuous variable, METh week−1), and the Physical Activity Readiness Questionnaire (pass/fail/missing) |
| Matthews et al. [60] | USA/Southern Community Cohort Study | Total Sitting (1376) | 64,304/6.4 | 69.9/0.45 | Sex, source of enrollment (community health center or general population), educational level (< 9 years, 9–11 years, high school, some college, or beyond college), household income (< $15,000, $15,000–$24,999, $25,000–$49,999, or ≥ $50,000), cigarette smoking (never; former, < 1 pack/day; former, ≥ 1 pack/day; current, < 1 pack/day; or current, ≥ 1 pack/day), BMI(< 18.5, 18.5–24.9, 25–29.9, 30.0–34.9, or ≥ 35.0), sleep duration (< 7, 7–8, or ≥ 9 h/day), diabetes (Y/N), employment status (Y/N), and overall physical activity level (quartiles) (age was used as the underlying time metric) |
| Matthews et al. [62] | USA/NIH-AARP Diet and Health Study | Total sitting (3339) | 154,614/6.8 | 69.8/0.52 | Age, Education (< 12 years, high school graduate, some college, college graduate, unknown), Smoking history (never, stopped 10+, 5–9, 1–4 years ago, stopped < 1 year, current smoker, unknown), Sleep duration (< 4, 4, 5.9, 6, 7.9, 8, 9.9, 10+ h/day, unknown), Overall health (excellent, very good, good, fair, poor, unknown), and BMI (< 25, 25, 29.9, 30+ kg/m2, unknown) additionally adjusted for Overall physical activity (< 1, 1–1.9, 2–2.9, 3–3.9, 4+ h/day) |
| Seguin et al. [68] | USA/Women’s Health Initiative (WHI) Observational Study (OS) and Extension Study (ES). | Total sitting (3878) | 92,234/12.2 | 64.0/0.00 | Age, race, physical activities, and physical function score adjusted |
| Dunstan et al. [49] | Australia/The Australian Diabetes, Obesity and Lifestyle Study (AusDiab) | TV viewing (87) | 8800/6.6 | 50.47/0.44 | Age, sex, smoking (current or ex-smoker), education (≥ 12 years), total energy intake, alcohol intake, Diet Quality Index, waist circumference, hypertension (blood pressure ≥ 140/90 mm Hg or antihypertensive medication use), total plasma cholesterol (mmol/L), HDL-C (mmol/L), serum triglycerides (mmol/L, log), lipid-lowering medication use, and glucose tolerance status and exercise time |
| Ikehara et al. [54] | Japan/The Japan Collaborative Cohort Study | TV viewing (5835) | 85,899/19.2 | 41.9/0.42 | Age and Sex, BMI, smoking, ethanol intake, education level, hours of sport, hours of walking, sleep duration, perceived mental stress, presence of job, frequency of fresh fish intake and depressive symptoms |
| Matthews et al. [61] | USA/NIH-AARP Diet and Health Study | TV viewing (4684) | 240,819/8.5 | 62.5/0.56 | Age, sex, race (white, black, other, missing), education (< 12 year, high school graduate, some college, college graduate, missing), smoking history (never; quit, ≤ 20 cigarettes/day; quit, > 20 cigarettes/day; current, ≤ 20 cigarettes/day; current, > 20 cigarettes/day; unknown), diet quality (quintiles) moderate-vigorous physical activity (never/rarely; < 1, 1–3, 4–7, > 7 h/week) |
| Warren et al. [47] | USA/ACLS Cohort | TV viewing (377) | 7744/b | 47.1/1.00 | Age, physically inactive, current smoker, alcohol intake (less than one, between one and two, and more than two drinks per day), BMI, family history of CVD, hypertension, diabetes, and hypercholesterolemia |
| Wijndaele et al. [48] | UK/EPIC-Norfolk | TV viewing (373) | 13,197/9.5 | 61.5/0.43 | Age and gender, education level, smoking status, alcohol consumption, medication for hypertension (not in models examining cancer mortality), medication for dyslipidaemia (not in models examining cancer mortality), baseline history of diabetes, family history of CVD and family history of cancer, total PAEE (MET_h/day) |
| Cancer mortality | |||||
| Kim et al. [45] | USA/Multiethnic Cohort Study (MEC) | Total sitting (6697), TV viewing (6697) |
134,596/13.7 | 59.0/0.46 | Hazard ratios were calculated with age as the time metric, adjusted for age at cohort entry (5–year age groups), education, ethnicity and smoking history [including the following variables: smoking status, average number of cigarettes, average number of cigarettes squared, number of years smoked (time dependent), number of years since quitting (time dependent) and interactions between ethnicity and the smoking variables]. also adjusted for history of hypertension and/or diabetes at enrolment, alcohol consumption, energy intake and physical activity (METs per week for moderate activity, vigorous work and strenuous sports) |
| Ensrud et al. [42] | USA/Osteoporotic Fractures in Men Study (MrOS) | Total sitting– accelerometer measured (129) | 2918/4.5 | 79/1.00 | Age, race, site, season, education, marital status, health status, smoking, comorbidity burden, depressive symptoms, cognitive function, number of instrumental activity of daily living impairments, % body fat |
| Katzmarzyk et al. [56] | Canada/Canada Fitness Survey | Total sitting (547) | 17,013/12.0 | 42.0/0.43 | Age, sex (as a continuous variable), smoking (former, current, non-smoker), alcohol consumption (abstainer, < 10 drinks per month, 10–50 drinks per month, > 50 drink per month), leisure time physical activity (as a continuous variable, METh wk−1), and the Physical Activity Readiness Questionnaire (pass/fail/missing) |
| Matthews et al. [60] | USA/Southern Community Cohort Study | Total Sitting (1227) | 64,304/6.4 | 69.9/0.45 | Sex, source of enrollment (community health center or general population), educational level (< 9 years, 9–11 years, high school, some college, or beyond college), household income (< $15,000, $15,000–$24,999, $25,000–$49,999, or ≥ $50,000), cigarette smoking (never; former, < 1 pack/day; former, ≥ 1 pack/day; current, < 1 pack/day; or current, ≥ 1 pack/day), BMI(< 18.5, 18.5–24.9, 25–29.9, 30.0–34.9, or ≥ 35.0), sleep duration (< 7, 7–8, or ≥ 9 h/day), diabetes (Y/N), employment status (Y/N), and overall physical activity level (quartiles). (Age was used as the underlying time metric) |
| Matthews et al. [62] | USA/NIH-AARP Diet and Health Study | Total sitting (4507) | 154,614/6.8 | 69.8/0.52 | Age, Education (< 12 yrs, high school graduate, some college, college graduate, unknown), Smoking history (never, stopped 10+, 5–9, 1–4 years ago, stopped < 1 year, current smoker, unknown), Sleep duration (< 4, 4, 5.9, 6, 7.9, 8, 9.9, 10+ h/day, unknown), Overall health (excellent, very good, good, fair, poor, unknown), and BMI (< 25, 25, 29.9, 30+ kg/m2, unknown) additionally adjusted for Overall physical activity (< 1, 1–1.9, 2–2.9, 3–3.9, 4+ h/day) |
| Seguin et al. [68] | USA/Women’s Health Initiative (WHI) Observational Study (OS) and Extension Study (ES). | Total sitting (4759) | 92,234/12.2 | 64.0/0.00 | Age, race, physical activities, and physical function score adjusted |
| Dunstan et al. [49] | Australia/The Australian Diabetes, Obesity and Lifestyle Study (AusDiab) | TV viewing (125) | 8800/6.6 | 50.47/0.44 | Age, sex, smoking (current or ex-smoker), education (≥ 12 years), total energy intake, alcohol intake, Diet Quality Index, waist circumference, hypertension (blood pressure ≥ 140/90 mm Hg or antihypertensive medication use), total plasma cholesterol (mmol/L), HDL-C (mmol/L), serum triglycerides (mmol/L, log), lipid-lowering medication use, and glucose tolerance status and exercise time |
| Keadle et al. [57] | USA/NIH-AARP Diet and Health Study | TV viewing (15,161) | 221,426/14.1 | 62.5/0.57 | Age (years), sex, race (white, black, other, missing), education (< 12 years, high school graduate, some college, college graduate, missing), smoking history (never; quit, ≤ 20 cigarettes/day; quit, > 20 cigarettes/day; current, ≤ 20 cigarettes/day; current, > 20 cigarettes/day; unknown), MVPA (never or rarely, 1, 1–3, 4–7, Z7 h/week) and diet quality (quintiles), BMI categories (18.5 to < 25, 25 to < 30, 30–35 and > 35 kg/m2) and health status (good, very good, excellent) |
| Matthews et al. [61] | USA/NIH-AARP Diet and Health Study | TV viewing (7652) | 240,819/8.5 | 62.5/0.56 | Age, sex, race (white, black, other, missing), education (< 12 year, high school graduate, some college, college graduate, missing), smoking history (never; quit, ≤ 20 cigarettes/day; quit, > 20 cigarettes/day; current, ≤ 20 cigarettes/day; current, > 20 cigarettes/day; unknown), diet quality (quintiles) moderate-vigorous physical activity (never/rarely; < 1, 1–3, 4–7, > 7 h/week) |
| Suzuki et al. [70] | Japan/Japan Collaborative cohort study | TV viewing (3787) | 110,792/b | 57.8/0.41 | Age and area of study |
| Wijndaele et al. [48] | UK/EPIC-Norfolk | TV viewing (570) | 13,197/9.5 | 61.5/0.43 | Age and gender, education level, smoking status, alcohol consumption, medication for hypertension (not in models examining cancer mortality), medication for dyslipidaemia (not in models examining cancer mortality), baseline history of diabetes, family history of CVD and family history of cancer, total PAEE (MET_h/day) |
| Incident type 2 diabetes | |||||
| Ding et al. [41] | Australia/45 and up | Total sitting (829) | 54,997/3.4 | 45 +/0.46 | Sex, age, quintile of socioeconomic disadvantage, household income categories, country of birth (categorised), highest education, CVD disease (yes/no), high cholesterol (yes/no), family history of T2DM (yes/no), smoking (yes/no), alcohol consumption ≤ drinks, > 14 drinks, total minutes of MVPA (≥ 300 min/150 to < 300 min/< 150 min), fruit intake per day (≥ 2 servings/< 2 servings), vegetable intake per day (≥ 5/< 5 servings), body weight status (healthy weight/underweight/overweight/obese) and psychological distress (none or low or moderate/high or very high) |
| Gibbs et at [43] | USA/The Coronary Artery Risk Development in Young Adults (CARDIA) Study | Total sitting (201) | 1869/5 | 45.3/0.43 | Adjusted for age, center, race, sex, education, income, smoking, alcohol, wear time, and log-transformed MVPA |
| Manini et al. [59] | USA/Women’s Health Initiative (WHI) Observational Study (OS) | Total sitting (7426) | 88,250/11.1 | 63.95/0.00 | Age, ethnicity and race, college education, income less than $35,000 per year, marital status, comorbidity propensity score (feeling depressed, hypertension, hyperlipidemia, osteoarthritis, history of cancer, and CVD), number of immediate family members with history of diabetes, currently smoking, alcohol intake > 7 drinks per week, percent of daily caloric intake as carbohydrate and percent of daily caloric intake as fat, BMI (kg = m2) and minutes performing MVPA |
| Petersen et al. [65] | Denmark/DANHES | Total sitting (1790) | 72,608/4.9 | 48.5/0.40 | Sex, age, education (< 12, 12–14 and 15+ years), smoking habits(never-smoker, ex-smoker, occasional smoker, daily smoker (1–15 g of tobacco/day) and heavy smoker (> 15 g of tobacco/day)), body mass index (continuous), alcohol consumption (number of drinks/week), and hypertension (yes current/yes previously, no) and previous cardiovascular disease (yes current/yes previously, no) and MVPA (summed from 4 domains (work, transport, domestic and leisure time using IPAQ) |
| Anjana et al. [38] | India/Chennai urban rural epidemiology study (CURES-142) | TV viewing (385) | 1376/10 | 38/0.42 | Age, gender, family history of diabetes, physical inactivity, generalized obesity, abdominal obesity, household income, total energy, energy adjusted saturated fatty acid (SFA g/day in quartiles), and dietary fiber (g/day in quartiles) for the selected diet variables. In addition, added sugars (g/day) and meat intake (g/day) were further adjusted for dairy intake |
| Ford et al. [50] | Germany/EPIC–Potsdam | TV viewing (927) | 23,855/7.8 | 49.7/0.38 | Age, sex, educational status, and occupational activity, plus smoking status, alcohol use, and physical activity |
| Hu et al. [52] | USA/The health professional follow-up study (HPFS) | TV viewing (767) | 31,379/8.0 | 55.3/1.00 | Age, length of follow up, smoking, parental history of diabetes, alcohol consumption, and physical activity |
| Hu et al. [53] | USA/Nurses’ Health Study | TV viewing (1515) | 68,497/5.3 | 57.4/0.00 | Age, hormone use, alcohol consumption, smoking, family history of diabetes, and physical activity |
| Joseph et al. [44] | USA/Multi-ethnic study of atherosclerosis (MESA) | TV viewing (655) | 5829/11.1 | 61.8/46.4 | Age, race, gender, education, current occupation status, study site, current smoking, systolic blood pressure, and current hypertension medication usage |
| Krishnan et al. [58] | USA/The Black Women’s Health Study (BWHS) | TV viewing (2928) | 45,668/4.0 | 21–69/0.00 | Age, time period, family history of diabetes, years of education, family income, marital status, smoking, alcohol consumption, energy intake, coffee consumption, vigorous activity television watching and walking |
| Smith et al. [69] | UK/English Longitudinal Study of Aging—ELSA | TV viewing (129) | 5964/2.0 | 64.6/0.44 | Age, sex, physical activity, smoking, alcohol, depressive symptoms, long-standing illness, disability (impairment in activities of daily living/instrumental activities of daily living) |
aWhere a range is presented this was age range of included participants
bInformation not available
Table 2.
Summary linear estimates of associations between sedentary behaviour (hours/day) with major chronic disease outcomes from random-effects meta-analysis
| Outcome (cases/n baseline) | Exposure (number of contributing studies for non-PA adjusted/PA adjusted estimates) | Without PA adjustment | With PA adjustment | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Summary RR (95%CI) | p value RR = 1 | I2 (%) | p value non-linearity | Summary RR (95%CI) | p value RR = 1 | I2 (%) | p value non-linearity | ||
| All-cause mortality (110,395/1,138,042) | Total sedentary behaviour (12/13) | 1.03 (1.02, 1.04) | < 0.001 | 49.7 | < 0.001 | 1.02 (1.01, 1.03) | < 0.001 | 65.6 | < 0.001 |
| TV viewing (7/7) | 1.07 (1.04, 1.09) | < 0.001 | 70.9 | < 0.001 | 1.05 (1.04, 1.05) | < 0.001 | 0 | < 0.001 | |
| CVD mortality (24,042/667,524) | Total sedentary behaviour (6/5) | 1.03 (1.02, 1.03) | < 0.001 | 0 | 0.001 | 1.02 (1.01, 1.03) | 0.004 | 22.7 | 0.010 |
| TV viewing (5/6) | 1.05 (1.02, 1.09) | 0.005 | 90.1 | 0.203 | 1.04 (1.01, 1.08) | 0.028 | 88.8 | < 0.001 | |
| Cancer mortality (35,241/684,673) | Total sedentary behaviour (6/5) | 1.01 (1.00–1.02) | 0.212 | 84.1 | 0.168 | 1.01 (1.00, 1.02) | 0.268 | 77.3 | 0.326 |
| TV viewing (4/4) | 1.03 (1.02, 1.04) | < 0.001 | 0 | 0.642 | 1.02 (1.01, 1.03) | < 0.001 | 0 | 0.648 | |
| Type 2 diabetes (17,552/400,292) | Total sedentary behaviour (4) | [Insufficient number of studies] | 1.01 (1.00, 1.01) | < 0.001 | 0 | 0.529 | |||
| TV viewing (5/6) | 1.12 (1.08, 1.16) | < 0.001 | 65.9 | 0.767 | 1.09 (1.07, 1.12) | < 0.001 | 31.7 | 0.066 | |
Study quality and methods of measurement
The quality of the included studies was generally good (see Table 1 and Online Appendix Table 4). Three of the five smallest cohorts (presenting 32 cases/208 participants [51], 112/1677 [67], and 409/2918 [42]) measured sedentary time objectively. Of 34 studies, 22 had ≥ 10,000 participants [39–41, 45, 48, 50, 52–62, 64, 65, 68, 70, 71]. There were 3 all-male studies [42, 47, 52] and 5 all-female studies [53, 58, 59, 63, 68] (providing data from 4 cohorts). Some articles presented results for multiple exposures and/or outcomes, with 34 publications presenting 57 analyses; the numbers of contributing articles for each exposure/outcome combination are presented in Table 2.
Most studies assessed sedentary behaviour by questionnaire (31 out of 34) with three studies measuring sedentary time objectively using accelerometers worn on the participant’s hip, waist or lower back for up to 7 days (Online Appendix Table 4) [42, 51, 67].
Outcomes were assessed objectively in 27 of the 34 studies (Online Appendix Table 4). This represents those studies with a mortality outcome assessed using death registries, in addition to four studies which used an objective measure to define T2D status [38, 44, 50, 65], the remainder being self-reported T2D.
All included studies reported adjusted effect estimates, with adjustment for PA present in all but four studies [44, 46, 51, 70]. One study presented results for some outcomes with PA adjustment and some without [42]. Adjustment for PA varied in detail; from simply meeting the PA guideline or not, to calculating weight-adjusted energy expenditure across multiple domains of PA (Online Appendix Table 4).
All-cause mortality
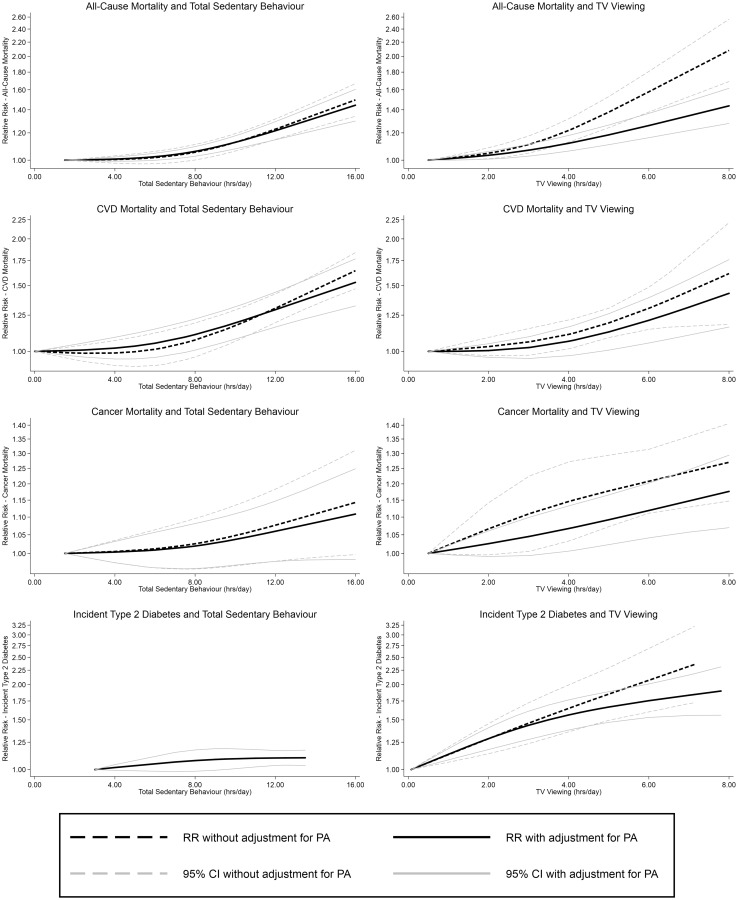
The association between total sedentary behaviour and all-cause mortality appeared to be non-linear, both with and without adjustment for PA (Fig. 2). Testing for non-linearity supported this finding. At lower levels of exposure, there were small increases in risk associated with increasing sedentary behaviour; above approximately 8 h/day of sedentary behaviour, the risk increased more rapidly. In PA adjusted analyses this resulted in an estimated RR of 1.01 (1.00–1.01) for each additional hour of exposure below 8 h/day and 1.04 (1.03–1.05) for each hour above 8 h/day (Online Appendix Table 5).
Fig. 2.
Non-linear associations between sedentary behaviour and health outcomes presented with and without PA adjustment
For TV viewing, the association also appeared to be non-linear (Fig. 2 and Table 2) with a change in gradient at approximately 3.5 h/day in the PA adjusted analyses (Fig. 2). Below this level the RR was 1.03 (1.01–1.04) per hour/day, and greater increases in risk were seen above this level (RR = 1.06 (1.05–1.08) per h/day; Online Appendix Table 5). Due to insufficient availability of data, investigation of the associations between leisure-time SB and mortality could not be undertaken.
CVD mortality
For total sedentary behaviour, non-linearity was seen again for both the non-PA adjusted and PA adjusted models (Fig. 2 and Table 2). In the PA adjusted analysis, the threshold was in the region of 6 h/day of exposure, below which each additional hour was associated with an estimated RR of 1.01 (0.99–1.02) and above which each additional hour was associated with an RR of 1.04 (1.03–1.04) (Online Appendix Table 5).
The PA adjusted non-linear association between CVD mortality and TV viewing showed greater risk increases with every hour above a threshold of approximately 4 h/day (Fig. 2). The estimated RR for each additional hour of TV viewing was 1.02 (0.99–1.04) below 4 h and 1.08 (1.05–1.12) above.
Cancer mortality
The linear association between total sedentary behaviour and cancer mortality was marginally non-significant and unaffected by PA adjustment; the PA adjusted estimate was 1.01 (1.00–1.02). There was no evidence for non-linearity (Table 2).
The linear association between TV viewing and cancer mortality was estimated to be 1.03 (1.02–1.04) in non-PA adjusted models and 1.02 (1.01–1.03) when adjusted for PA. There was no evidence for non-linearity for either PA adjusted or unadjusted associations (Fig. 2 and Table 2).
Type 2 diabetes
The linear association between total sedentary behaviour and T2D was estimated to be 1.01 (1.00, 1.01), which could only be estimated with PA adjustment as there were insufficient studies without PA adjustment (Table 2 and Fig. 2).
PA adjusted analysis of the association between TV viewing and T2D shows some deviation from linearity (Fig. 2) although statistical evidence was equivocal (p = 0.066; Table 2). The PA adjusted linear association was estimated to be 1.09 (1.07, 1.12) However, larger increases in risk were seen with increasing TV viewing below approximately 4 h (1.12 (1.08–1.15) in PA adjusted analysis), above this level increasing TV viewing was associated with lower increases in risk (1.05 (1.03–1.07)). In non PA-adjusted analyses the association appeared linear with an estimated RR of 1.12 (1.08, 1.16) associated with each additional hour of TV viewing.
Across all combinations, PA adjustment appeared to attenuate the effect estimates. The difference between the effect sizes with and without PA adjustment was relatively small when total sedentary time was the exposure, but somewhat greater when TV viewing was examined. Substantial heterogeneity was observed for the pooled effect estimates, ranging from I2 values of 0% for both total sedentary behaviour and CVD, and total sedentary behaviour and cancer mortality, to I2 of 90.1% for TV viewing and CVD mortality. Funnel plots and Egger’s tests, showed no definitive evidence for publication bias (Online Appendix Fig. 5). However, the low numbers of contributing studies for some associations made it difficult to rule out these biases.
Sensitivity analyses are presented in Online Appendix Table 6. The RR of CVD mortality associated with each additional hour of TV viewing was greater when studies of younger participants were excluded, 1.08 (1.06–1.10) compared with 1.04 (1.01–1.08) in the main analysis. All other sensitivity analyses showed no substantive change from the main findings.
Population attributable fractions for TV viewing
For all-cause mortality, 8% (6–10%) was associated with TV-viewing in the English population, when using the PAF method. This estimate was 5% (1–8%) for CVD and 5% (2–7%) for cancer mortality. For T2D 29% (26–32%) of incidence was estimated to be related to TV-viewing.
Discussion
This meta-analysis, incorporating data of 1,331,468 participants, shows an increased risk for all-cause and CVD mortality and incidence of T2D with higher levels of total sitting as well as TV viewing time, independent of PA. For all outcomes, associations with TV viewing were stronger, and the strongest association overall was found between TV viewing and incident of T2D. There was also evidence of an independent association between sedentary behaviour and cancer mortality, although only for a specific type of sedentary behaviour, i.e. TV viewing time.
Most importantly, investigation of the shape of the associations indicated that the increased risk of all-cause and CVD mortality was strongest for sitting time volumes greater than 8 and 6 h/day, respectively, in PA adjusted analyses. For TV viewing time, an increased risk for all-cause and CVD mortality was strongest above levels of about 3–4 h/day. The associations between TV viewing with T2D and cancer mortality appeared to be more linear. In general, PA adjustment resulted in some attenuation of the estimated linear and non-linear associations, which was somewhat stronger for TV viewing compared to total sitting time. Furthermore, we estimated that a sizeable fraction of mortality and incidence of all examined outcomes were associated with TV viewing, ranging from 5% for CVD and cancer mortality, to a substantial 29% for T2D.
Potential mechanisms
Biological mechanisms have been suggested to explain the independent associations of sedentary behaviour, in particular for cardio-metabolic diseases, through independent effects of prolonged sitting on lipid and glucose metabolism in the large skeletal muscles involved in posture (legs and core) and on hemodynamic vascular signalling potentially causing atherogenesis [86–89]. Associations for TV viewing were generally stronger than those for total sitting time with the same outcome which could be explained by several factors. Firstly, TV viewing has been linked to higher intakes of energy and macronutrients along with greater energy from snacks [90]. Poor diet quality and increased total calorie intake have been associated with increased risk of mortality and is a strong determinant of T2D, suggesting an important mediating role for dietary intake which is likely less relevant for total sitting time [91, 92]. Second, a potentially different confounding structure for TV viewing may be more difficult to fully account for. Third, criterion validity of self-reported TV viewing estimates tends to be stronger than those for total sitting time estimates [93, 94]. Lastly, the typical timing of TV viewing, i.e. in the evening following the main meal of the day [95], may exacerbate the repetitive cardiovascular effects of postprandial glucose and lipid excursions following this meal, especially if TV viewing is predominantly accumulated in prolonged bouts of sitting [96, 97].
Limitations of the available evidence
The methods used to measure exposure varied; measurement of sedentary behaviour is still primarily reliant on self-report questionnaires. Heterogeneity in question phrasing, the time period considered and whether a question is single or multipart can all influence validity [93]. Misclassification of sedentary exposure would potentially dilute the association in our analysis, resulting in possible underestimation of effect size. The use of accelerometer measured sedentary time addresses some of the limitations of questionnaires, however this data has its own limitations. For example, some accelerometer methods cannot detect cycling or swimming, or fail to distinguish between sitting/lying and standing still [87]. This substantial heterogeneity in exposure measurement contributed to the high heterogeneity indices (I2) for the pooled estimates which may have influenced our overall findings. It is possible that only some of the constituents of total sitting are detrimental to health, for example sitting while reading is potentially advantageous [98]. That we were unable to investigate leisure sitting time in this meta-analysis due to insufficient studies would indicate that more research is required on the effect of different domains of sedentary behaviour. In addition to the exposure measure, the quality of the measurement of important covariates, such as PA, diet and socio-economic position, varied greatly between studies, if included at all, potentially leading to residual confounding. The low number of studies for some combinations meant that investigation of leisure time sitting could not be carried out. It also led to a lack of statistical power for subgroup or sensitivity analysis and bias assessment. This also meant that meta-regression techniques to investigate the impact of the potential sources of heterogeneity were precluded.
Strengths and limitations of the meta-analysis
This meta-analysis considered total sedentary behaviour and TV viewing time as separate exposures. This is important as they have different associated socio-demographic and/or behavioural patterns (e.g. dietary intake) and therefore different confounding/mediating patterns [6, 90]. Inclusion of emerging research using objectively measured sedentary time is another strength. In addition, we investigated the shape of the dose–response curves, to identify where the greatest risk/benefits lie along the spectrum of exposure for all exposure—outcome combinations. Moreover, to our knowledge, this is the first study to calculate PAF estimates for TV viewing time and all considered health outcomes based on meta-analytical risk estimates when potential non-linearity of associations were taken into account.
However, our meta-analysis also has certain limitations. The use of summary data means heterogeneity of used statistical methods may influence comparability of included studies [29]. In order to investigate the effect of PA adjustment we had to select models which were as similar as possible except for adjustment for PA. However, in some studies additional differences in covariates were seen between these models and this may have resulted in residual confounding of the considered study-specific risk estimates. It was also necessary to make several assumptions during the dose-assignment procedure. Whilst these assumptions may have been crude in studies reporting little detail on the exposure, this approach allowed us to consider the totality of the currently published evidence. Treating the many and heterogeneous conditions that make up cancer as one outcome may have contributed to our mixed findings with these analyses. Investigating separate cancer types may be more informative, where there is enough data [99]. Attempting to reduce reverse causality by only including prospective studies may not have been entirely effective, especially in the case of T2D. An estimated 27% of those with the condition have no formal diagnosis, therefore having the condition may have preceded ascertainment of exposure data [100]. Finally, the calculation of PAFs rests on the assumption of causality, and the use of unbiased estimates with no measurement error.
Public health impact
To calculate the PAF estimates we have used the exposure profile representative of the population of England. These might not be representative for other countries. However, average US TV viewing levels, for example, are similar, 2.6 h on a weekday and 3.3 h on a weekend day compared with 2.7 h and 3.1 h/day respectively in England [101]. The estimated 29% of T2D in England in 2012 that could be prevented or postponed by eliminating TV viewing, assuming causality, reflects the high RRs seen for this association. The linear association and relatively high RR even at lower exposure levels are important contributors, as a large proportion of HSE participants report lower TV viewing levels (75% of participants report < 4 h/day), but only a small proportion reports no TV viewing (3%). The PAF estimates for all-cause mortality (8%) and CVD and cancer mortality (both 5%) also suggest a potentially important burden of preventable disease from current population levels of TV viewing.
The differing nature of the relationships between TV viewing and different outcomes may complicate any prevention strategy. The prevention of T2D would perhaps be best served by reducing TV viewing among the whole population, however, to prevent other outcomes targeting those with highest exposure levels may be more appropriate as these are the individuals for whom any reduction would confer the greatest benefit. Furthermore, the effect of any behaviour change will also be influenced by the nature of the replacement activity [13]. For example, greater reductions in risk may occur when replacing sedentary time with strenuous exercise compared with walking for pleasure. Replacing some sedentary behaviours may confer greater benefits than others, e.g. replacing TV viewing may be more beneficial than replacing general screen time [13].
Another potentially important determinant of the health effects of sedentary behaviour is the extent to which breaks are taken in extended periods of sitting. None of the studies included in this meta-analysis took into account accumulation pattern of sitting and therefore this falls outwith the scope of this study.
Conclusion
This study demonstrates an increasing risk of disease and mortality with increasing total sitting time and TV viewing time. It also revealed a threshold of 6–8 h/day of total sitting and 3–4 h/day of TV viewing, above which risk for several important health outcomes increased more rapidly. This suggests that sedentary behaviour guidelines may need further quantification of sitting time volumes that should be avoided, although for some outcomes such as T2D, any sitting time reductions would be beneficial. With 8% of all mortality and 29% of T2D in the English population associated with certain sedentary behaviours, there is great potential for substantial public health benefits. Improvements in the measurement of sedentary time and a better understanding of its confounding structure are therefore essential to improving future public health and clinical guidelines.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by the British Heart Foundation (Intermediate Basic Science Research Fellowship Grant No. FS/12/58/29709 to KW) and the Medical Research Council (Unit Programme No. MC_UU_12015/3 for SB and KW and MC_UU_12015/1 for SJS). JW’s MT’s contributions were undertaken under the auspices of the Centre for Diet and Activity Research (CEDAR), a UKCRC Public Health Research Centre of Excellence which is funded by the British Heart Foundation, Cancer Research UK, Economic and Social Research Council, Medical Research Council, the National Institute for Health Research, and the Wellcome Trust. RP is funded via a NIHR Research Professorship award to Christopher Millett. AS is supported by a Medical Research Council Doctoral Training Studentship. This manuscript does not reflect the opinions of any of these funding bodies.
Author contributions
JW, SB and KW conceived this study. PE, SS and MT contributed to the design of the study. RP and EMc conducted data extraction with help from TdS. RP conducted the analysis with help from AS, SS and MT. RP and KW wrote the initial draft of the manuscript, all authors contributed to revisions. All authors approved the final manuscript.
Conflict of interest
All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Human and animal rights
This article does not contain any studies with animals performed by any of the authors.
Footnotes
Richard Patterson: Corresponding author and guarantor.
Eoin McNamara and Marko Tainio: Authors listed as joint second authors.
References
- 1.Brownson RC, Boehmer TK, Luke DA. Declining rates of physical activity in the United States: what are the contributors? Annu Rev Public Health. 2005;26:421–443. doi: 10.1146/annurev.publhealth.26.021304.144437. [DOI] [PubMed] [Google Scholar]
- 2.Dunstan DW, Healy GN, Sugiyama T, Owen N. ‘Too much sitting’ and metabolic risk—has modern technology caught up with us? Eur Endocrinol. 2010;6(1):19–23. doi: 10.17925/EE.2010.06.00.19. [DOI] [Google Scholar]
- 3.Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32(9 Suppl):S498–S504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 4.Tremblay MS, Aubert S, Barnes JD, Saunders TJ, Carson V, Latimer-Cheung AE, et al. Sedentary behavior research network (SBRN)—terminology consensus project process and outcome. Int J Behav Nutr Phys Activity. 2017;14(1):75. doi: 10.1186/s12966-017-0525-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matthews CE, Chen KY, Freedson PS, Buchowski MS, Beech BM, Pate RR, et al. Amount of time spent in sedentary behaviors in the United States, 2003–2004. Am J Epidemiol. 2008;167(7):875–881. doi: 10.1093/aje/kwm390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Office for Official Publications of the European Communities. Time use at different stages of life Results from 13 European countries. Luxemburg; 2003.
- 7.Scholes S, Mindell J. Chapter 2—Physical activity in adults. In: Craig R, Mindell J, editors. Health survey for England 2012. Volume 1: Health, social care and lifestyles. Leeds: Health and Social Care Information Centre; 2013. [Google Scholar]
- 8.Owen N, Healy GN, Matthews CE, Dunstan DW. Too much sitting: the population health science of sedentary behavior. Exerc Sport Sci Rev. 2010;38(3):105–113. doi: 10.1097/JES.0b013e3181e373a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ford ES, Caspersen CJ. Sedentary behaviour and cardiovascular disease: a review of prospective studies. Int J Epidemiol. 2012;41(5):1338–1353. doi: 10.1093/ije/dys078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilmot EG, Edwardson CL, Achana FA, Davies MJ, Gorely T, Gray LJ, et al. Sedentary time in adults and the association with diabetes, cardiovascular disease and death: systematic review and meta-analysis. Diabetologia. 2012;55(11):2895–2905. doi: 10.1007/s00125-012-2677-z. [DOI] [PubMed] [Google Scholar]
- 11.Biswas A, Oh PI, Faulkner GE, Bajaj RR, Silver MA, Mitchell MS, et al. Sedentary time and its association with risk for disease incidence, mortality, and hospitalization in adults: a systematic review and meta-analysis. Ann Intern Med. 2015;162(2):123–132. doi: 10.7326/M14-1651. [DOI] [PubMed] [Google Scholar]
- 12.Ekelund U, Steene-Johannessen J, Brown WJ, Fagerland MW, Owen N, Powell KE, et al. Does physical activity attenuate, or even eliminate, the detrimental association of sitting time with mortality? A harmonised meta-analysis of data from more than 1 million men and women. Lancet. 2016;388(10051):1302–1310. doi: 10.1016/S0140-6736(16)30370-1. [DOI] [PubMed] [Google Scholar]
- 13.Wijndaele K, Sharp SJ, Wareham NJ, Brage S. mortality risk reductions from substituting screen time by discretionary activities. Med Sci Sports Exerc. 2017;49(6):1111–1119. doi: 10.1249/MSS.0000000000001206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chau JY, Grunseit AC, Chey T, Stamatakis E, Brown WJ, Matthews CE, et al. Daily sitting time and all-cause mortality: a meta-analysis. PLoS ONE. 2013;8(11):e80000. doi: 10.1371/journal.pone.0080000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grontved A, Hu FB. Television viewing and risk of type 2 diabetes, cardiovascular disease, and all-cause mortality a meta-analysis. J Am Med Assoc (JAMA) 2011;305(23):2448–2455. doi: 10.1001/jama.2011.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun J-W, Zhao L-G, Yang Y, Ma X, Wang Y-Y, Xiang Y-B. Association Between television viewing time and all-cause mortality: a meta-analysis of cohort studies. Am J Epidemiol. 2015;182(11):908–916. doi: 10.1093/aje/kwv164. [DOI] [PubMed] [Google Scholar]
- 17.Pandey A, Salahuddin U, Garg S, Ayers C, Kulinski J, Anand V, et al. Continuous dose–response association between sedentary time and risk for cardiovascular disease: a meta-analysis. JAMA Cardiol. 2016;1(5):575–583. doi: 10.1001/jamacardio.2016.1567. [DOI] [PubMed] [Google Scholar]
- 18.Rezende LFM, Sá TH, Mielke GI, Viscondi JYK, Rey-López JP, Garcia LMT. All-cause mortality attributable to sitting time. Am J Prev Med. 2016;51(2):253–263. doi: 10.1016/j.amepre.2016.01.022. [DOI] [PubMed] [Google Scholar]
- 19.Ramsey Buchanan L, Rooks-Peck CR, Finnie RKC, Wethington HR, Jacob V, Fulton JE, et al. Reducing recreational sedentary screen time. Am J Prev Med. 2016;50(3):402–415. doi: 10.1016/j.amepre.2015.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thorp AA, Owen N, Neuhaus M, Dunstan DW. Sedentary behaviors and subsequent health outcomes in adults a systematic review of longitudinal studies, 1996–2011. Am J Prev Med. 2011;41(2):207–215. doi: 10.1016/j.amepre.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 21.Proper KI, Singh AS, van Mechelen W, Chinapaw MJM. Sedentary behaviors and health outcomes among adults a systematic review of prospective studies. Am J Prev Med. 2011;40(2):174–182. doi: 10.1016/j.amepre.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 22.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of non-randomised studies in meta-analyses. Oxford, UK; 2000.
- 23.Juni P, Witschi A, Bloch R, Egger M. The hazards of scoring the quality of clinical trials for meta-analysis. JAMA. 1999;282(11):1054–1060. doi: 10.1001/jama.282.11.1054. [DOI] [PubMed] [Google Scholar]
- 24.Hamling J, Lee P, Weitkunat R, Ambuhl M. Facilitating meta-analyses by deriving relative effect and precision estimates for alternative comparisons from a set of estimates presented by exposure level or disease category. Stat Med. 2008;27(7):954–970. doi: 10.1002/sim.3013. [DOI] [PubMed] [Google Scholar]
- 25.Orsini N, Li R, Wolk A, Khudyakov P, Spiegelman D. Meta-analysis for linear and nonlinear dose–response relations: examples, an evaluation of approximations, and software. Am J Epidemiol. 2012;175(1):66–73. doi: 10.1093/aje/kwr265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greenland S, Longnecker MP. Methods for trend estimation from summarized dose–response data, with applications to meta-analysis. Am J Epidemiol. 1992;135(11):1301–1309. doi: 10.1093/oxfordjournals.aje.a116237. [DOI] [PubMed] [Google Scholar]
- 27.Orsini N, Bellocco R, Greenland S. Generalized least squares for trend estimation of summarized dose–response data. Stata J. 2006;6(1):40–57. [Google Scholar]
- 28.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 29.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Higgins J, Green S. Cochrane handbook for systematic reviews of interventions. The Cochrane Collaboration. http://handbook.cochrane.org/.
- 31.Harrell FE., Jr . Regression modeling strategies: with applications to linear models, logistic regression, and survival analysis. New York: Springer; 2001. [Google Scholar]
- 32.WHO. Metrics: population attributable fraction (PAF). http://www.who.int/healthinfo/global_burden_disease/metrics_paf/en/. Accessed 15 May 2017.
- 33.Wijndaele K, Brage S, Besson H, Khaw KT, Sharp SJ, Luben R, et al. Television viewing and incident cardiovascular disease: prospective associations and mediation analysis in the EPIC Norfolk Study. PLoS ONE. 2011;6(5):e20058. doi: 10.1371/journal.pone.0020058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patel AV, Bernstein L, Deka A, Feigelson HS, Campbell PT, Gapstur SM, et al. Leisure time spent sitting in relation to total mortality in a prospective cohort of US adults. Am J Epidemiol. 2010;172(4):419–429. doi: 10.1093/aje/kwq155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Herber-Gast GC, Jackson CA, Mishra GD, Brown WJ. Self-reported sitting time is not associated with incidence of cardiovascular disease in a population-based cohort of mid-aged women. Int J Behav Nutr Phys Activity. 2013;10:55. doi: 10.1186/1479-5868-10-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chomistek AK, Manson JE, Stefanick ML, Lu B, Sands-Lincoln M, Going SB, et al. Relationship of sedentary behavior and physical activity to incident cardiovascular disease: results from the Women’s Health Initiative. J Am Coll Cardiol. 2013;61(23):2346–2354. doi: 10.1016/j.jacc.2013.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Borodulin K, Karki A, Laatikainen T, Peltonen M, Luoto R. Daily sedentary time and risk of cardiovascular disease: the National FINRISK 2002 study. J Phys Activity Health. 2014 doi: 10.1123/jpah.2013-0364. [DOI] [PubMed] [Google Scholar]
- 38.Anjana RM, Sudha V, Nair DH, Lakshmipriya N, Deepa M, Pradeepa R, et al. Diabetes in Asian Indians-how much is preventable? Ten-year follow-up of the Chennai Urban Rural Epidemiology Study (CURES-142) Diabetes Res Clin Pract. 2015;109(2):253–261. doi: 10.1016/j.diabres.2015.05.039. [DOI] [PubMed] [Google Scholar]
- 39.Basterra-Gortari FJ, Bes-Rastrollo M, Gea A, Nunez-Cordoba JM, Toledo E, Martinez-Gonzalez MA. Television viewing, computer use, time driving and all-cause mortality: the SUN cohort. J Am Heart Assoc. 2014 doi: 10.1161/JAHA.114.000864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chau JY, Grunseit A, Midthjell K, Holmen J, Holmen TL, Bauman AE, et al. Sedentary behaviour and risk of mortality from all-causes and cardiometabolic diseases in adults: evidence from the HUNT3 population cohort. Br J Sports Med. 2013 doi: 10.1136/bjsports-2012-091974. [DOI] [PubMed] [Google Scholar]
- 41.Ding D, Chong S, Jalaludin B, Comino E, Bauman AE. Risk factors of incident type 2-diabetes mellitus over a 3-year follow-up: results from a large Australian sample. Diabetes Res Clin Pract. 2015;108(2):306–315. doi: 10.1016/j.diabres.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 42.Ensrud KE, Blackwell TL, Cauley JA, Dam TTL, Cawthon PM, Schousboe JT, et al. Objective measures of activity level and mortality in older men. J Am Geriatr Soc. 2014;62(11):2079–2087. doi: 10.1111/jgs.13101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gibbs BB, Gabriel KP, Reis JP, Jakicic JM, Carnethon MR, Sternfeld B. Cross-sectional and longitudinal associations between objectively measured sedentary time and metabolic disease: the coronary artery risk development in young adults (CARDIA) study. Diabetes Care. 2015;38(10):1835–1843. doi: 10.2337/dc15-0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Joseph JJ, Echouffo-Tcheugui JB, Golden SH, Chen H, Jenny NS, Carnethon MR, et al. Physical activity, sedentary behaviors and the incidence of type 2 diabetes mellitus: the Multi-Ethnic Study of Atherosclerosis (MESA) BMJ Open Diabetes Res Care. 2016;4(1):e000185. doi: 10.1136/bmjdrc-2015-000185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim Y, Wilkens LR, Park SY, Goodman MT, Monroe KR, Kolonel LN. Association between various sedentary behaviours and all-cause, cardiovascular disease and cancer mortality: the Multiethnic Cohort Study. Int J Epidemiol. 2013;42(4):1040–1056. doi: 10.1093/ije/dyt108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Muennig P, Rosen Z, Johnson G. Do the psychosocial risks associated with television viewing increase mortality? Evidence from the 2008 General Social Survey-National Death Index dataset. Ann Epidemiol. 2013;23(6):355–360. doi: 10.1016/j.annepidem.2013.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Warren TY, Barry V, Hooker SP, Sui X, Church TS, Blair SN. Sedentary behaviors increase risk of cardiovascular disease mortality in men. Med Sci Sports Exerc. 2010;42(5):879–885. doi: 10.1249/MSS.0b013e3181c3aa7e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wijndaele K, Brage S, Besson H, Khaw KT, Sharp SJ, Luben R, et al. Television viewing time independently predicts all-cause and cardiovascular mortality: the EPIC Norfolk study. Int J Epidemiol. 2011;40(1):150–159. doi: 10.1093/ije/dyq105. [DOI] [PubMed] [Google Scholar]
- 49.Dunstan DW, Barr EL, Healy GN, Salmon J, Shaw JE, Balkau B, et al. Television viewing time and mortality: the Australian Diabetes, Obesity and Lifestyle Study (AusDiab) Circulation. 2010;121(3):384–391. doi: 10.1161/CIRCULATIONAHA.109.894824. [DOI] [PubMed] [Google Scholar]
- 50.Ford ES, Schulze MB, Kroger J, Pischon T, Bergmann MM, Boeing H. Television watching and incident diabetes: findings from the European Prospective Investigation into Cancer and Nutrition-Potsdam Study. J Diabetes. 2010;2(1):23–27. doi: 10.1111/j.1753-0407.2009.00047.x. [DOI] [PubMed] [Google Scholar]
- 51.Fox KR, Ku PW, Hillsdon M, Davis MG, Simmonds BA, Thompson JL, et al. Objectively assessed physical activity and lower limb function and prospective associations with mortality and newly diagnosed disease in UK older adults: an OPAL four-year follow-up study. Age Ageing. 2015;44(2):261–268. doi: 10.1093/ageing/afu168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hu FB, Leitzmann MF, Stampfer MJ, Colditz GA, Willett WC, Rimm EB. Physical activity and television watching in relation to risk for type 2 diabetes mellitus in men. Arch Intern Med. 2001;161(12):1542–1548. doi: 10.1001/archinte.161.12.1542. [DOI] [PubMed] [Google Scholar]
- 53.Hu FB, Li TY, Colditz GA, Willett WC, Manson JE. Television watching and other sedentary behaviors in relation to risk of obesity and type 2 diabetes mellitus in women. JAMA. 2003;289(14):1785–1791. doi: 10.1001/jama.289.14.1785. [DOI] [PubMed] [Google Scholar]
- 54.Ikehara S, Iso H, Wada Y, Tanabe N, Watanabe Y, Kikuchi S, et al. Television viewing time and mortality from stroke and coronary artery disease among Japanese men and women—the Japan Collaborative Cohort Study. Circ J. 2015;79(11):2389–2395. doi: 10.1253/circj.CJ-14-1335. [DOI] [PubMed] [Google Scholar]
- 55.Inoue M, Iso H, Yamamoto S, Kurahashi N, Iwasaki M, Sasazuki S, et al. Daily total physical activity level and premature death in men and women: results from a large-scale population-based cohort study in Japan (JPHC study) Ann Epidemiol. 2008;18(7):522–530. doi: 10.1016/j.annepidem.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 56.Katzmarzyk PT, Church TS, Craig CL, Bouchard C. Sitting time and mortality from all causes, cardiovascular disease, and cancer. Med Sci Sports Exerc. 2009;41(5):998–1005. doi: 10.1249/MSS.0b013e3181930355. [DOI] [PubMed] [Google Scholar]
- 57.Keadle SK, Moore SC, Sampson JN, Xiao Q, Albanes D, Matthews CE. Causes of death associated with prolonged TV viewing: NIH-AARP diet and health study. Am J Prev Med. 2015;49(6):811–821. doi: 10.1016/j.amepre.2015.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Krishnan S, Rosenberg L, Palmer JR. Physical activity and television watching in relation to risk of type 2 diabetes: the Black Women’s Health Study. Am J Epidemiol. 2009;169(4):428–434. doi: 10.1093/aje/kwn344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Manini TM, LaMonte MJ, Seguin RA, Manson JE, Hingle M, Garcia L, et al. Modifying effect of obesity on the association between sitting and incident diabetes in post-menopausal women. Obesity. 2014;22(4):1133–1141. doi: 10.1002/oby.20620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Matthews CE, Cohen SS, Fowke JH, Han X, Xiao Q, Buchowski MS, et al. Physical activity, sedentary behavior, and cause-specific mortality in black and white adults in the Southern Community Cohort Study. Am J Epidemiol. 2014;180(4):394–405. doi: 10.1093/aje/kwu142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Matthews CE, George SM, Moore SC, Bowles HR, Blair A, Park Y, et al. Amount of time spent in sedentary behaviors and cause-specific mortality in US adults. Am J Clin Nutr. 2012;95(2):437–445. doi: 10.3945/ajcn.111.019620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Matthews CE, Moore SC, Sampson J, Blair A, Xiao Q, Keadle SK, et al. Mortality benefits for replacing sitting time with different physical activities. Med Sci Sports Exerc. 2015 doi: 10.1249/MSS.0000000000000621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pavey TG, Peeters GG, Brown WJ. Sitting-time and 9-year all-cause mortality in older women. Br J Sports Med. 2012;49(2):95–99. doi: 10.1136/bjsports-2012-091676. [DOI] [PubMed] [Google Scholar]
- 64.Petersen CB, Bauman A, Gronbaek M, Wulff Helge J, Thygesen LC, Tolstrup JS. Total sitting time and risk of myocardial infarction, coronary heart disease and all-cause mortality in a prospective cohort of Danish adults. Int J Behav Nutr Phys Activity. 2014;11:13. doi: 10.1186/1479-5868-11-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Petersen CB, Bauman A, Tolstrup JS. Total sitting time and the risk of incident diabetes in Danish adults (the DANHES cohort) over 5 years: a prospective study. Br J Sports Med. 2016;50(22):1382–1387. doi: 10.1136/bjsports-2015-095648. [DOI] [PubMed] [Google Scholar]
- 66.Pulsford RM, Stamatakis E, Britton AR, Brunner EJ, Hillsdon M. Associations of sitting behaviours with all-cause mortality over a 16-year follow-up: the Whitehall II study. Int J Epidemiol. 2015;44(6):1909–1916. doi: 10.1093/ije/dyv191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schmid D, Ricci C, Leitzmann MF. Associations of objectively assessed physical activity and sedentary time with all-cause mortality in US adults: the NHANES study. PLoS ONE. 2015;10(3):e0119591. doi: 10.1371/journal.pone.0119591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Seguin R, Buchner DM, Liu J, Allison M, Manini T, Wang CY, et al. Sedentary behavior and mortality in older women: the Women’s Health Initiative. Am J Prev Med. 2014;46(2):122–135. doi: 10.1016/j.amepre.2013.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Smith L, Hamer M. Television viewing time and risk of incident diabetes mellitus: the English Longitudinal Study of Ageing. Diabet Med. 2014;31(12):1572–1576. doi: 10.1111/dme.12544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Suzuki K. Health conditions and mortality in the Japan Collaborative Cohort Study for Evaluation of Cancer (JACC) Asian Pacific J Cancer Prev. 2007;8:25–34. [PubMed] [Google Scholar]
- 71.van der Ploeg HP, Chey T, Korda RJ, Banks E, Bauman A. Sitting time and all-cause mortality risk in 222 497 Australian adults. Arch Intern Med. 2012;172(6):494–500. doi: 10.1001/archinternmed.2011.2174. [DOI] [PubMed] [Google Scholar]
- 72.The Women’s Health Initiative Study Group Design of the Women’s Health Initiative clinical trial and observational study. The Women’s Health Initiative Study Group. Control Clin Trials. 1998;19(1):61–109. doi: 10.1016/S0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 73.Day N, Oakes S, Luben R, Khaw KT, Bingham S, Welch A, et al. EPIC-Norfolk: study design and characteristics of the cohort. European Prospective Investigation of Cancer. Br J Cancer. 1999;80(Suppl 1):95–103. [PubMed] [Google Scholar]
- 74.Kolonel LN, Henderson BE, Hankin JH, Nomura AM, Wilkens LR, Pike MC, et al. A multiethnic cohort in Hawaii and Los Angeles: baseline characteristics. Am J Epidemiol. 2000;151(4):346–357. doi: 10.1093/oxfordjournals.aje.a010213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Krokstad S, Langhammer A, Hveem K, Holmen TL, Midthjell K, Stene TR, et al. Cohort Profile: the HUNT Study, Norway. Int J Epidemiol. 2013;42(4):968–977. doi: 10.1093/ije/dys095. [DOI] [PubMed] [Google Scholar]
- 76.Langer RD, White E, Lewis CE, Kotchen JM, Hendrix SL, Trevisan M. The Women’s Health Initiative Observational Study: baseline characteristics of participants and reliability of baseline measures. Ann Epidemiol. 2003;13(9 Suppl):S107–S121. doi: 10.1016/S1047-2797(03)00047-4. [DOI] [PubMed] [Google Scholar]
- 77.Ohno Y, Tamakoshi A. Japan collaborative cohort study for evaluation of cancer risk sponsored by monbusho (JACC study) J Epidemiol. 2001;11(4):144–150. doi: 10.2188/jea.11.144. [DOI] [PubMed] [Google Scholar]
- 78.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156(9):871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 79.Blank JB, Cawthon PM, Carrion-Petersen ML, Harper L, Johnson JP, Mitson E, et al. Overview of recruitment for the osteoporotic fractures in men study (MrOS) Contemp Clin Trials. 2005;26(5):557–568. doi: 10.1016/j.cct.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 80.Boeing H, Wahrendorf J, Becker N. EPIC-Germany—a source for studies into diet and risk of chronic diseases. European Investigation into Cancer and Nutrition. Ann Nutr Metab. 1999;43(4):195–204. doi: 10.1159/000012786. [DOI] [PubMed] [Google Scholar]
- 81.Eriksen L, Gronbaek M, Helge JW, Tolstrup JS, Curtis T. The Danish Health Examination Survey 2007–2008 (DANHES 2007–2008) Scand J Public Health. 2011;39(2):203–211. doi: 10.1177/1403494810393557. [DOI] [PubMed] [Google Scholar]
- 82.Friedman GD, Cutter GR, Donahue RP, Hughes GH, Hulley SB, Jacobs DR, Jr, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41(11):1105–1116. doi: 10.1016/0895-4356(88)90080-7. [DOI] [PubMed] [Google Scholar]
- 83.Schatzkin A, Subar AF, Thompson FE, Harlan LC, Tangrea J, Hollenbeck AR, et al. Design and serendipity in establishing a large cohort with wide dietary intake distributions: the National Institutes of Health-American Association of Retired Persons Diet and Health Study. Am J Epidemiol. 2001;154(12):1119–1125. doi: 10.1093/aje/154.12.1119. [DOI] [PubMed] [Google Scholar]
- 84.Segui-Gomez M, de la Fuente C, Vazquez Z, de Irala J, Martinez-Gonzalez MA. Cohort profile: the ‘Seguimiento Universidad de Navarra’ (SUN) study. Int J Epidemiol. 2006;35(6):1417–1422. doi: 10.1093/ije/dyl223. [DOI] [PubMed] [Google Scholar]
- 85.Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40(1):181–188. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- 86.Hamilton MT, Hamilton DG, Zderic TW. Exercise physiology versus inactivity physiology: an essential concept for understanding lipoprotein lipase regulation. Exerc Sport Sci Rev. 2004;32(4):161–166. doi: 10.1097/00003677-200410000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tremblay MS, Colley RC, Saunders TJ, Healy GN, Owen N. Physiological and health implications of a sedentary lifestyle. Appl Physiol Nutr Metab. 2010;35(6):725–740. doi: 10.1139/H10-079. [DOI] [PubMed] [Google Scholar]
- 88.Hamilton MT, Hamilton DG, Zderic TW. Role of low energy expenditure and sitting in obesity, metabolic syndrome, type 2 diabetes, and cardiovascular disease. Diabetes. 2007;56(11):2655–2667. doi: 10.2337/db07-0882. [DOI] [PubMed] [Google Scholar]
- 89.Young DR, Hivert MF, Alhassan S, Camhi SM, Ferguson JF, Katzmarzyk PT, et al. Sedentary behavior and cardiovascular morbidity and mortality: a science advisory from the American Heart Association. Circulation. 2016;134(13):e262–e279. doi: 10.1161/CIR.0000000000000440. [DOI] [PubMed] [Google Scholar]
- 90.Bowman SA. Television-viewing characteristics of adults: correlations to eating practices and overweight and health status. Prev Chron Dis. 2006;3(2):A38. [PMC free article] [PubMed] [Google Scholar]
- 91.Alderman MH, Cohen H, Madhavan S. Dietary sodium intake and mortality: the National Health and Nutrition Examination Survey (NHANES I) Lancet. 1998;351(9105):781–785. doi: 10.1016/S0140-6736(97)09092-2. [DOI] [PubMed] [Google Scholar]
- 92.Kant AK, Schatzkin A, Graubard BI, Schairer C. A prospective study of diet quality and mortality in women. JAMA. 2000;283(16):2109–2115. doi: 10.1001/jama.283.16.2109. [DOI] [PubMed] [Google Scholar]
- 93.Healy GN, Clark BK, Winkler EA, Gardiner PA, Brown WJ, Matthews CE. Measurement of adults’ sedentary time in population-based studies. Am J Prev Med. 2011;41(2):216–227. doi: 10.1016/j.amepre.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wijndaele K, Bourdeaudhuij ID, Godino JG, Lynch BM, Griffin SJ, Westgate K, et al. Reliability and validity of a domain-specific last 7-d sedentary time questionnaire. Med Sci Sports Exerc. 2014;46(6):1248–1260. doi: 10.1249/MSS.0000000000000214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Almoosawi S, Winter J, Prynne CJ, Hardy R, Stephen AM. Daily profiles of energy and nutrient intakes: are eating profiles changing over time? Eur J Clin Nutr. 2012;66(6):678–686. doi: 10.1038/ejcn.2011.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dunstan DW, Kingwell BA, Larsen R, Healy GN, Cerin E, Hamilton MT, et al. Breaking up prolonged sitting reduces postprandial glucose and insulin responses. Diabetes Care. 2012;35(5):976–983. doi: 10.2337/dc11-1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Peddie MC, Bone JL, Rehrer NJ, Skeaff CM, Gray AR, Perry TL. Breaking prolonged sitting reduces postprandial glycemia in healthy, normal-weight adults: a randomized crossover trial. Am J Clin Nutr. 2013;98(2):358–366. doi: 10.3945/ajcn.112.051763. [DOI] [PubMed] [Google Scholar]
- 98.Bavishi A, Slade MD, Levy BR. A chapter a day: association of book reading with longevity. Soc Sci Med. 2016;164:44–48. doi: 10.1016/j.socscimed.2016.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lynch B. Sedentary Behavior and cancer: a systematic review of the literature and proposed biological mechanisms. Cancer Epidemiol Biomarkers Prev. 2010;19(11):2691–2709. doi: 10.1158/1055-9965.EPI-10-0815. [DOI] [PubMed] [Google Scholar]
- 100.Holman N, Forouhi NG, Goyder E, Wild SH. The Association of Public Health Observatories (APHO) diabetes prevalence model: estimates of total diabetes prevalence for England, 2010–2030. Diabet Med. 2011;28(5):575–582. doi: 10.1111/j.1464-5491.2010.03216.x. [DOI] [PubMed] [Google Scholar]
- 101.Beurau of Labour Statistics. American time use survey summary. United States Department of Labor. https://www.bls.gov/news.release/atus.nr0.htm (2016). Accessed 23 Jan 2017.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.