Abstract
Gastritis is an inflammation of the gastric mucosa. In this study, we investigated the efficacy of a medical device, Esoxx®, based on hyaluronic acid and chondroitin sulfate on gastritis-related upper abdominal pain/discomfort and endoscopic features. Fifty patients, affected by gastritis, were randomised to receive the medical device or placebo. The primary endpoint was the medical device efficacy on upper abdominal pain/discomfort associated with gastritis and measured by Visual Analogue Scale (VAS). The secondary endpoints were the efficacy of the medical device on gastritis-related mucosal erosions, blood oozing, and hyperemia (redness)/edema, as assessed by endoscopy, and the patients’ rating of their compliance with the treatments. A significant reduction in VAS pain was observed in the treatment group after a 5-week treatment, if compared with placebo (p < 0.001). In summary, administration of a medical device, based on hyaluronic acid and chondroitin sulfate, improves gastritis-related upper abdominal pain/discomfort and decreases mucosal erosions, blood oozing, and hyperemia (redness)/edema at 5-week follow-up in patients affected by gastritis.
Electronic supplementary material
The online version of this article (10.1007/s13346-018-0531-7) contains supplementary material, which is available to authorized users.
Keywords: Gastritis, Chondroitin sulfate, Hyaluronic acid, Endoscopy, Pain, Discomfort
Introduction
Gastritis is commonly defined as a histologically confirmed inflammation of the gastric mucosa and affects up to 50% of the population worldwide [1]. Gastritis can be triggered by multiple factors including Helicobacter pylori infection, biliary reflux into the stomach, use of non-steroidal anti-inflammatory drugs, unbalanced diet, chemical injuries such as alcohol and acids, and long-term physical and mental stress [1, 2]. This inflammatory condition can result in mucosal erosions, blood oozing, and hyperemia (redness)/edema with inflammatory cell infiltration of the gastric layers [3–5]. The symptomatic treatment of gastritis can be managed by proton pump inhibitors to reduce acid output and buffering products that can counteract the hydrogen ion-induced damage to the mucosa. Glycosaminoglycans, including chondroitin sulfate (CS) and hyaluronic acid (HA), are expressed in human gastric tissue [6]. Sulfated glycosaminoglycans, chondroitin 4,6-sulfate, dermatan sulfate and heparan sulfate have been observed in two gastric regions, the antrum and the body of the stomach, in patients affected by chronic superficial gastritis [7].
CS is a member of the glycosaminoglycan family and, in vertebrates, consists of repeating sulfate-substituted GalNAcβ4GlcAβ3 disaccharide units polymerised into long chains [8]. CS molecular structure was identified by Babkin and Komarov [9] as an effective inhibitor of pepsin-induced damage to the gastroduodenal mucosa. Pepsin, together with mucoitinsulfate, is a key chemical component of the mucous that is spontaneously secreted by the parietal cells. CS has been extensively used for treatment of symptomatic knee osteoarthritis improving pain and overall mobility and has showed structure-modifying effects in knee and finger osteoarthritis [10]. Furthermore, CS has shown good tolerability and safety in the clinical setting [10] and potent anti-inflammatory properties in animal models of arthritis [11]. HA is a non-sulfated, naturally occurring glycosaminoglycan consisting of alternately repeating D-glucuronic acid and N-acetylglucosamine units [12]. HA interacts with several cell surface receptors such as cluster determinant 44 (CD44) and the receptor for hyaluronate-mediated motility (RHAMM), which have been associated with malignant transformation of gastric mucosa, although their expression has also been reported in non-malignant mucosa [13–15]. HA is also involved in innate immune response and inflammation since it participates in leukocyte recruitment via interaction with CD44, activating inflammatory cells, such as macrophages, through CD44-dependent signaling. HA also induces dendritic cell maturation and promotes cytokine release by dendritic cells and endothelial cells through toll-like receptor 4 [16]. Further studies have also shown that HA possesses antibacterial, antifungal [17], and antiviral activities [18]. Due to its biological properties, HA has been extensively used in experimental and clinical osteoarthritis [19–21], lower-leg telangiectasia [22], premature ejaculation [23], and restorative and esthetic surgery [24–26]. However, to date, no clinical study has shown an effectiveness of a compound based on HA and CS on gastritis-related upper abdominal pain/discomfort and endoscopic features. In the present study, we hypothesized that HA and CS would steadily coat the epithelial surface of the gastric mucosa stimulating the healing process in a subset of patients affected by gastritis characterized by upper abdominal pain/discomfort and mucosal erosions, blood oozing, and hyperemia (redness)/edema.
Materials and methods
Patients and study design
This retrospective, anecdotal, double-blind randomised placebo-controlled study was conducted in accordance with the Declaration of Helsinki and institutional review board rules.
Fifty patients (females = 18; males = 32; body mass index = 18.5–24.9) aged between 6 and 87 years [50.2 ± 2.3, mean ± standard error of the mean (SEM)] who had appealed to our “Second Opinion Medical Network” (Modena, Italy) between 2016 and 2017 due to gastritis symptoms were included in this study. The concept of “Second Opinion Medical Consulting Network” has been reviewed elsewhere [27–31]. Before the beginning of the study, the patients underwent a complete physical examination. A gastrointestinal endoscopy (criteria for diagnosis of gastritis were bleeding, vascular pattern [congestion], and excess mucous secretion) and gastric biopsy (from antrum or pyloric areas in all forms of gastritis and mainly from the gastric body for chemical gastritis) were carried out to confirm the diagnosis of gastritis. Eighteen patients presented non-atrophic gastritis (possibly due to unbalanced diet and lifestyle), 8 patients were affected by atrophic gastritis and 24 by chemical gastritis (bile reflux observed by endoscopy) based on the classification by Dixon and coworkers [32]. The inclusion criteria for gastritis were epigastric burning, bloatedness, nausea, meteorism, and belching, accompanied by mucosal erosions, blood oozing, hyperemia (redness)/edema, and upper abdominal pain/discomfort ≥ 40 mm, as measured by Visual Analogue Scale (VAS). The patients included in this study also had dyspepsia. All the symptoms described above were present in all the types of gastritis previously mentioned. Patients were divided into two groups made up of 9 females and 16 males each and were randomised to receive either the medical device or placebo. The patients were instructed to stop previous medical prescriptions for treatment of gastritis including proton pump inhibitors, other buffering and gastroprotective agents, and digestive enzymes 7 days before the beginning of the study. Exclusion criteria for the present study were presence of ulcers at any gastric segment, pyloric stenosis, Helicobacter pylori infection (ruled out by breath test), esophageal stricture or intestinal obstruction, previous gastrointestinal surgery, and a known hypersensitivity to the compounds object of the present study. Patients who received prolonged non-steroidal anti-inflammatory drug therapy in the year preceding this study were also excluded.
Treatment
The medical device (Esoxx®, Alfa Wassermann, Bologna, Italy) is based on a mixture of hyaluronic acid and chondroitin sulfate in a bioadhesive carrier Lutrol® F 127 (poloxamer 407; BASF, Milan, Italy) that acts as a buffering agent to form a barrier and prolong the action on the esophageal mucosa [33]. The formulation also contains polyvinylpyrrolidone, xylitol C, sodium benzoate, potassium sorbate, aromas, and demineralized water. Esoxx® has been proposed for the treatment of the symptoms of gastroesophageal reflux disease and produces a persistent mucosal barrier, as shown by ex vivo studies performed in the swine model [34].
The placebo composition was as follows: 10% Vaseline oil/water emulsion, viscosity enhancer, preservatives, aroma, and water. The used formulations were manufactured by Alfa Wasserman Spa (Bologna, Italy). Ten milliliters of the placebo or medical device was administered four times a day (prior to breakfast, lunch, dinner, and bedtime) for 2 weeks. The 2-week treatment was followed by a week without any medication. Afterwards, the patients underwent 2-week further treatment according to the above-described protocol. We used a 5-week design as we hypothesized that this timecourse was necessary to allow long-lasting protection of the mucosa from gastric acid and promote the repair of histological lesions.
Assessment of upper abdominal pain/discomfort
VAS, a scoring system from 0 (minimum pain) to 100 mm (severe pain), was used to rate the primary endpoint, i.e., improvement in gastritis-related upper abdominal pain/discomfort at 5-week follow-up. In regard to the two children involved in this investigation, their parents were allowed to stay to give their support in relation to the VAS pain scoring. A month after the end of the study, a phone interview was used to determine if the patients’ improvement in gastritis-related upper abdominal pain/discomfort was still persisting.
Assessment of gastritis-related mucosal erosions, blood oozing, and hyperemia (redness)/edema
The secondary endpoints were evaluation of the effect of the medical device on gastritis-related mucosal erosions, blood oozing, and hyperemia (redness)/edema, compared to placebo, as assessed by photographic endoscopy evaluation performed by two blinded pathologists at 5-week follow-up. The pathologists gave a judgment according to the following ranges: (1) 1–30% = poor improvement, (2) 30–60% = moderate improvement, and (3) 60–100% = good improvement.
Patients’ compliance and medical device tolerability
The patients were also asked to rate their compliance/tolerability related to viscosity, taste, and difficulty to swallow the treatments as “poor,” “fair,” “good,” or “very good.”
Statistical analysis
VAS data were analyzed using a two-way analysis of variance (ANOVA) followed by Bonferroni post hoc test using GraphPad Prism (GraphPad Software Inc., San Diego, CA, USA). All data are presented as the means ± SEM. A p value ˂ 0.05 was considered significant.
Results
Upper abdominal pain/discomfort
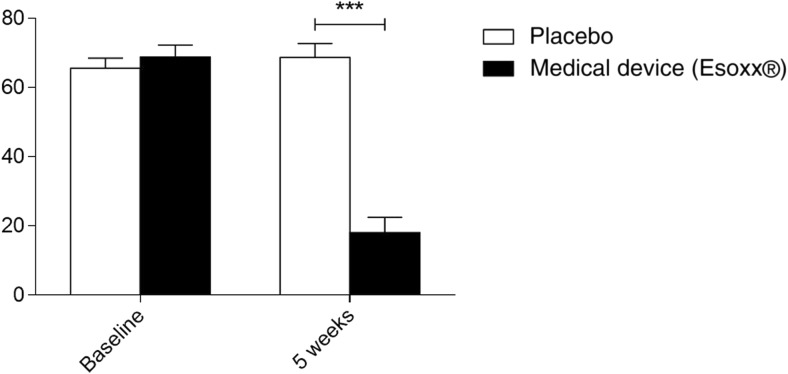
At baseline, upper abdominal pain/discomfort was 65.56 ± 2.9 mm for the placebo group and 68.8 ± 3.4 mm for the treatment group. A significant reduction in upper abdominal pain/discomfort was observed in the treatment group, if compared with placebo at 5-week follow-up (Fig. 1). Among the patients who underwent medical device treatment, 16 patients, including the 2 children, reported high relief from upper abdominal pain/discomfort (post-treatment VAS range = 0–25 mm), 7 reported a moderate reduction in upper abdominal pain/discomfort (post-treatment VAS range = 25–50 mm), and 2 patients presented only a slight reduction in upper abdominal pain/discomfort (post-treatment VAS range = 50–100 mm). All patients in the placebo group fell in the 50–100 mm range showing an improvement in upper abdominal pain/discomfort up to 15%.
Fig. 1.
Comparison of gastritis-related upper abdominal pain/discomfort between patients treated with the medical device (n = 25) and patients receiving placebo (n = 25), as assessed by VAS at 5-week follow-up. Data are reported as the means ± SEM. ***p ˂ 0.001 VAS Visual Analogue Scale, SEM, standard error of the mean
At the phone interview, improvement in upper abdominal pain/discomfort was persistent in 23 patients from the medical device group. The 2 patients from this group who did not experience amelioration in upper abdominal pain/discomfort at 5-week follow-up started a different therapy. No amelioration in upper abdominal pain/discomfort in all patients receiving placebo was observed at the phone interview.
Gastritis-related mucosal erosions, blood oozing, and hyperemia (redness)/edema
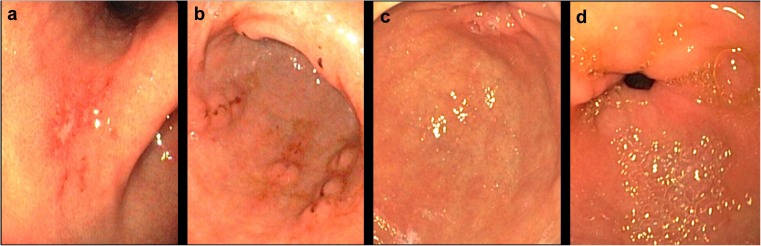
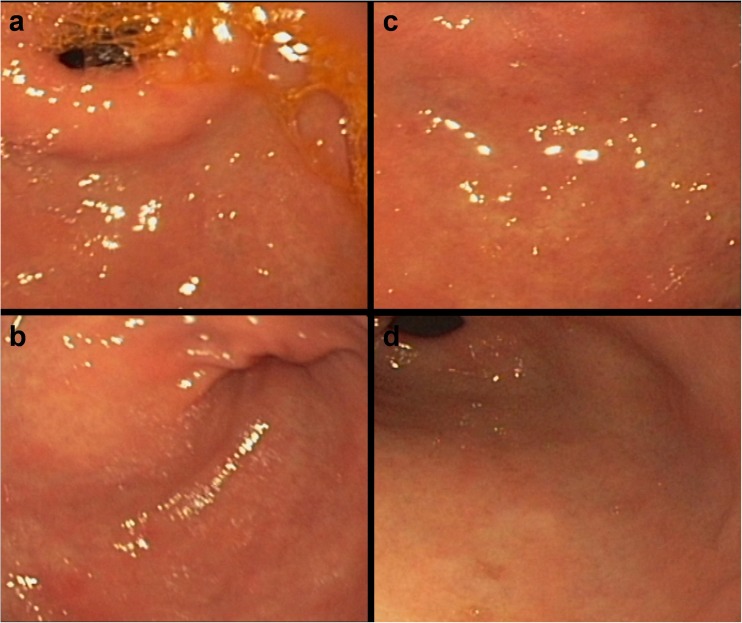
Endoscopic assessment at baseline was compared to the endoscopy performed at 5-week follow-up in terms of erosions, blood oozing, and hyperemia (redness)/edema in the active treatment (Fig. 2) and placebo (Fig. 3) groups. Among the 25 patients who underwent medical device treatment, 17 showed good endoscopic healing according to the above-mentioned parameters, as judged by the two pathologists, 6 showed moderate improvement, and 2 patients showed a poor improvement (these were the same patients who showed only slight improvement in upper abdominal pain/discomfort). The improvement in these parameters was also consistent with amelioration in the dyspeptic symptoms observed at baseline. All patients in the placebo group showed poor improvement in all endoscopic features analysed in this study.
Fig. 2.
Gastritis at baseline (a and b) and 5 weeks following medical device administration (c and d). Gastric erosions with fibrin streaks are visible in (a). Gastric erosions with hematin pigments are visible in (b). Definite improvement is observed after treatment with the medical device (c and d)
Fig. 3.
Gastritis at baseline (a) and 5 weeks following placebo administration (c). Gastritis with reddening and swelling at baseline (b) and 5 weeks following placebo administration (d). No improvement in gastritis can be observed at the 5-week follow-up (c and d)
Patients’ compliance and medical device tolerability
The patients treated with the medical device rated their compliance/tolerability related to viscosity, taste, and difficulty to swallow as good (n = 6) and very good (n = 19). Twenty-two patients in the placebo group rated their compliance/tolerability as very good, while 3 rated their compliance as good. No adverse effects were observed in both groups.
Discussion and conclusions
The present study included 50 patients affected by gastritis characterized by upper abdominal pain/discomfort of at least 40 mm, as measured by VAS. A 5-week administration of a medical device based on HA and CS reduced gastritis-related upper abdominal pain/discomfort in 23 patients at 5-week follow-up, if compared with placebo. This improvement persisted at the phone interview performed a month after the end of the study. The reduction in VAS score was coupled with amelioration in blood oozing, hyperemia (redness)/edema, and mucosal erosions, as assessed by two pathologists by photographic endoscopic examination at 5-week follow-up. A first-in-man attempt to treat gastroduodenal diseases by oral administration of CS was performed by Crandall and Roberts [35] on 22 patients affected by duodenal peptic ulcer with a 45% improvement in symptoms. In line with these results, CS promoted healing of skin ulcer in the rat [36]. Furthermore, Harrison and colleagues [37] showed that CS is an excellent coating for intraocular lens implantation in order to avoid damage to the corneal epithelium. According to this study, CS surpassed the protective qualities of other compounds, while albumin was second best and HA third. Furthermore, CS was the most efficacious protective agent with an effect lasting 40 hours, if compared with sodium hyaluronate. The concept of a protective layer made by CS upon the surface of mucosal lesions is very appealing and can be achieved due to the high affinity of the compound for the injured surfaces leading to a very effective and robust protection [38–41]. Another clinical study reported the anti-inflammatory and healing properties of CS showing that intravesical instillation of 0.2% highly purified CS solution (molecular weight = 20–40 Da) in patients with interstitial cystitis showed a favorable symptomatic outcome in this muscular-epithelial contractile organ [42].
The results from the present study strongly support the hypothesis that HA may cover the submucosal connective tissue inducing epithelial cell shifting and increasing cell motility. In turn, this tissue becomes softer and hydrophilic because of HA availability beneath the mucosa containing fibrin and mucous allowing the repair of the damaged gastric mucosa. At the same time, CS may act synergistically to promote, together with HA and the added adhesive biopolymer, the healing of ulcers and erosions. We speculate that the use of this composition may also be extended to manage the symptoms related to esophagitis, gastrointestinal reflux, and other gastroduodenal diseases although this will need to be proven by future clinical studies. Our investigation presents limitations such as the small number of patients and the lack of a long-term endoscopic follow-up to assess the effect of this treatment in the long run. Furthermore, patients presenting H. pylori-related gastritis have not been taken into account. In conclusion, we speculate that the effectiveness of Esoxx® may rely on HA and CS ability to coat the gastric epithelium, inhibiting gastric fluid acidity and pepsin-induced mucosal erosion. Further studies, involving larger cohorts of patients, are necessary to establish the long-term efficacy of Esoxx® and its underlying mechanism.
Electronic supplementary material
(DOCX 2745 kb)
Acknowledgements
This article was not supported by grants. JCMM acknowledges CONACyT-Mexico for membership.
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
Written informed consent was obtained from the patients for publication of the data included in this manuscript.
References
- 1.Rugge M, Pennelli G, Pilozzi E, Fassan M, Ingravallo G, Russo VM, Di Mario F. Gastritis: the histology report. Dig Liver Dis. 2011;43(Suppl 4):S373–S384. doi: 10.1016/S1590-8658(11)60593-8. [DOI] [PubMed] [Google Scholar]
- 2.Marcial G, Rodríguez C, Medici M, de Valdez GF. New approaches in gastritis treatment, gastritis and gastric cancer. In: Tonino P, editor. New insights in gastroprotection, diagnosis and treatments. Croatia - EUROPEAN UNION: InTech Publisher; 2011. pp. 153–76.
- 3.Cheli R, Giacosa A. Chronic atrophic gastritis and gastric mucosal atrophy—one and the same. Gastrointest Endosc. 1983;29(1):23–25. doi: 10.1016/S0016-5107(83)72493-4. [DOI] [PubMed] [Google Scholar]
- 4.Dewan B, Balasubramanian A. Troxipide in the management of gastritis: a randomized comparative trial in general practice. Gastroenterol Res Pract. 2010;2010:758397. doi: 10.1155/2010/758397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sugawa C, Lucas CE, Rosenberg BF, Riddle JM, Walt AJ. Differential topography of acute erosive gastritis due to trauma or sepsis, ethanol and aspirin. Gastrointest Endosc. 1973;19(3):127–130. doi: 10.1016/S0016-5107(73)73979-1. [DOI] [PubMed] [Google Scholar]
- 6.Sekino T, Murata K, Saito Y, Tsubura K. A study of acidic glycosaminoglycans in human gastric tissue. Digestion. 1977;16(1–2):28–39. doi: 10.1159/000198052. [DOI] [PubMed] [Google Scholar]
- 7.Geocze S, Nader HB, Mincis M, Novo NF, Paiva ER. Sulfated glycosaminoglycan composition of human gastric mucosa: effect of aging, chronic superficial gastritis and adenocarcinoma. Braz J Med Biol Res. 1985;18(4):487–492. [PubMed] [Google Scholar]
- 8.Esko JD, Kimata K, Lindahl U. Proteoglycans and sulfated glycosaminoglycans, Essentials of glycobiology. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 2009. pp. 229–248. [PubMed] [Google Scholar]
- 9.Babkin BP, Komarov SA. The influence of gastric mucus on peptic digestion. Can Med Assoc J. 1932;27(5):463–469. [PMC free article] [PubMed] [Google Scholar]
- 10.Uebelhart D. Clinical review of chondroitin sulfate in osteoarthritis. Osteoarthr Cartil. 2008;16(Suppl 3):S19–S21. doi: 10.1016/j.joca.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 11.Volpi N. Anti-inflammatory activity of chondroitin sulphate: new functions from an old natural macromolecule. Inflammopharmacology. 2011;19(6):299–306. doi: 10.1007/s10787-011-0098-0. [DOI] [PubMed] [Google Scholar]
- 12.Goa KL, Benfield P. Hyaluronic acid. A review of its pharmacology and use as a surgical aid in ophthalmology, and its therapeutic potential in joint disease and wound healing. Drugs. 1994;47(3):536–566. doi: 10.2165/00003495-199447030-00009. [DOI] [PubMed] [Google Scholar]
- 13.Li H, Guo L, Li J, Liu N, Liu J. Alternative splicing of RHAMM gene in Chinese gastric cancers and its in vitro regulation. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2000;17(5):343–347. [PubMed] [Google Scholar]
- 14.Li H, Li J, Guo L. Characteristics of expression of CD44v and receptor for HA-mediated motility (RHAMM) in multi-step gastrocarcinogenesis. Zhonghua Zhong Liu Za Zhi. 1999;21(5):329–331. [PubMed] [Google Scholar]
- 15.Toole BP. Hyaluronan: from extracellular glue to pericellular cue. Nat Rev Cancer. 2004;4(7):528–539. doi: 10.1038/nrc1391. [DOI] [PubMed] [Google Scholar]
- 16.Taylor KR, Gallo RL. Glycosaminoglycans and their proteoglycans: host-associated molecular patterns for initiation and modulation of inflammation. FASEB J. 2006;20(1):9–22. doi: 10.1096/fj.05-4682rev. [DOI] [PubMed] [Google Scholar]
- 17.Ardizzoni A, Neglia RG, Baschieri MC, Cermelli C, Caratozzolo M, Righi E, Palmieri B, Blasi E. Influence of hyaluronic acid on bacterial and fungal species, including clinically relevant opportunistic pathogens. J Mater Sci Mater Med. 2011;22(10):2329–2338. doi: 10.1007/s10856-011-4408-2. [DOI] [PubMed] [Google Scholar]
- 18.Cermelli C, Cuoghi A, Scuri M, Bettua C, Neglia RG, Ardizzoni A, Blasi E, Iannitti T, Palmieri B. In vitro evaluation of antiviral and virucidal activity of a high molecular weight hyaluronic acid. Virol J. 2011;8:141. doi: 10.1186/1743-422X-8-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iannitti T, Elhensheri M, Bingol AO, Palmieri B. Preliminary histopathological study of intra-articular injection of a novel highly cross-linked hyaluronic acid in a rabbit model of knee osteoarthritis. J Mol Histol. 2013;44(2):191–201. doi: 10.1007/s10735-012-9457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iannitti T, Rottigni V, Palmieri B. A pilot study to compare two different hyaluronic acid compounds for treatment of knee osteoarthritis. Int J Immunopathol Pharmacol. 2012;25(4):1093–1098. doi: 10.1177/039463201202500426. [DOI] [PubMed] [Google Scholar]
- 21.Palmieri B, Rottigni V, Iannitti T. Preliminary study of highly cross-linked hyaluronic acid-based combination therapy for management of knee osteoarthritis-related pain. Drug Des Devel Ther. 2013;7:7–12. doi: 10.2147/DDDT.S37330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iannitti T, Rottigni V, Torricelli F, Palmieri B. Combination therapy of hyaluronic acid mesotherapic injections and sclerotherapy for treatment of lower leg telangiectasia without major venous insufficiency: a preliminary clinical study. Clin Appl Thromb Hemost. 2014;20(3):326–30. [DOI] [PubMed]
- 23.Littara A, Palmieri B, Rottigni V, Iannitti T. A clinical study to assess the effectiveness of a hyaluronic acid-based procedure for treatment of premature ejaculation. Int J Impot Res. 2013;25(3):117–120. doi: 10.1038/ijir.2013.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iannitti T, Bingol AO, Rottigni V, Palmieri B. Biocompatibility and rheological properties of a new highly viscoelastic hyaluronic acid in a rat model for biomaterial implants and first-in-man clinical investigation in esthetic and restorative medicine. Int J Pharm. 2013;456:583–592. doi: 10.1016/j.ijpharm.2013.06.066. [DOI] [PubMed] [Google Scholar]
- 25.Iannitti T, Capone S, Palmieri B. Short review on face rejuvenation procedures: focus on preoperative antiseptic and anesthetic delivery by JetPeel-3 (a high pressure oxygen delivery device) Minerva Chir. 2011;66(3 Suppl 1):1–8. [PubMed] [Google Scholar]
- 26.Marusza W, Mlynarczyk G, Olszanski R, Netsvyetayeva I, Obrowski M, Iannitti T, Palmieri B. Probable biofilm formation in the cheek as a complication of soft tissue filler resulting from improper endodontic treatment of tooth 16. Int J Nanomedicine. 2012;7:1441–1447. doi: 10.2147/IJN.S27994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Di Cerbo A, Palmieri B. The economic impact of second opinion in pathology. Saudi Med J. 2012;33(10):1051–1052. [PubMed] [Google Scholar]
- 28.Palmieri B, Iannitti T. The Web Babel syndrome. Patient Educ Couns. 2011;85(2):331–333. doi: 10.1016/j.pec.2011.02.019. [DOI] [PubMed] [Google Scholar]
- 29.Palmieri B, Iannitti T, Capone S, Fistetto G, Arisi E. Second opinion clinic: is the Web Babel syndrome treatable? Clin Ter. 2011;162(6):575–583. [PubMed] [Google Scholar]
- 30.Palmieri B, Laurino C, Vadalà M. The “second opinion medical network”. Int J Pathol Clin Res. 2017;3(1):1–7. doi: 10.1002/cjp2.63. [DOI] [Google Scholar]
- 31.Wunsch A, B P. The role of second opinion in oncology: an update. Eur J Oncol. 2013;18(3):3–10. [Google Scholar]
- 32.Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney system. International workshop on the histopathology of gastritis, Houston 1994. Am J Surg Pathol. 1996;20(10):1161–1181. doi: 10.1097/00000478-199610000-00001. [DOI] [PubMed] [Google Scholar]
- 33.Batchelor HK. Novel bioadhesive formulations in drug delivery. Drug Delivery Companies Report Autumn/Winter; 2004. p. 16–19.
- 34.Di Simone MP, Baldi F, Vasina V, Scorrano F, Bacci ML, Ferrieri A, Poggioli G. Barrier effect of Esoxx((R)) on esophageal mucosal damage: experimental study on ex-vivo swine model. Clin Exp Gastroenterol. 2012;5:103–107. doi: 10.2147/CEG.S31404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crandall LA, Roberts GM. Observations on administration of chondroitin in peptic ulcers. Proc Soc Exp Biol Med. 1933;30:704–708. doi: 10.3181/00379727-30-6638. [DOI] [Google Scholar]
- 36.Fialkova MA, Smirnova T, Ivanova GI, Aboiants RK, Golubeva VF. The effect of chondroitin sulfate preparations on wound healing and the strength of the surgical scar. Biull Eksp Biol Med. 1989;108(9):350–351. [PubMed] [Google Scholar]
- 37.Harrison SE, Soll DB, Shayegan M, Clinch T. A new and effective protective agent for intraocular lens insertion. Ophthalmology. 1982;89(11):1254–1260. doi: 10.1016/S0161-6420(82)34653-9. [DOI] [PubMed] [Google Scholar]
- 38.Barthe L, Woodley J, Lavit M, Przybylski C, Philibert C, Houin G. In vitro intestinal degradation and absorption of chondroitin sulfate, a glycosaminoglycan drug. Arzneimittelforschung. 2004;54(5):286–292. doi: 10.1055/s-0031-1296972. [DOI] [PubMed] [Google Scholar]
- 39.Sekino T, Murata K. Age-dependent constitutional change in acidic glycosaminoglycans in human esophagus. Digestion. 1978;18(5–6):319–328. doi: 10.1159/000198219. [DOI] [PubMed] [Google Scholar]
- 40.Sekino T, Murata K, Saito Y. Acidic glycosaminoglycans in human esophagus tissue. Tohoku J Exp Med. 1979;127(3):273–280. doi: 10.1620/tjem.127.273. [DOI] [PubMed] [Google Scholar]
- 41.Volpi N. Oral bioavailability of chondroitin sulfate (Condrosulf) and its constituents in healthy male volunteers. Osteoarthr Cartil. 2002;10(10):768–777. doi: 10.1053/joca.2002.0824. [DOI] [PubMed] [Google Scholar]
- 42.Steinhoff G, Ittah B, Rowan S. The efficacy of intravesicular sterile sodium chondroitin sulfate 0.2% in potassium tested positive patients with interstitial cystitis. Adv Exp Med Biol. 2003;539(Pt B):731–739. doi: 10.1007/978-1-4419-8889-8_45. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 2745 kb)