Abstract
Fruiting bodies of Amanita muscaria and topsoil beneath from six background areas in northern regions of Poland were investigated for the concentration levels of Ag, Al, Ba, Ca, Cd, Co, Cu, Fe, Hg, K, Mg, Mn, Na, Rb, Sr, and Zn. In addition, the bioconcentration factors (BCF values) were studied for each of these metallic elements. Similar to studies from other basidiomycetes, A. muscaria showed species-specific affinities to some elements, resulting in their bioconcentration in mycelium and fruiting bodies. This mushroom growing in soils with different levels of the geogenic metallic elements (Ag, Al, Ba, Ca, Co, Cu, Fe, Hg, K, Mg, Mn, Na, Rb, Sr, and Zn) showed signs of homeostatic accumulation in fruiting bodies of several of these elements, while Cd appeared to be accumulated at a rate dependent of the concentration level in the soil substrate. This species is an efficient bio-concentrator of K, Mg, Cd, Cu, Hg, Rb, and Zn and hence also contributes to the natural cycling of these metallic elements in forest ecosystems.
Keywords: Amanita muscaria, Environment, Metallic contaminants, Bioconcentration, Forest, Fungi, Geochemical cycle
Introduction
Amanita muscaria (fly agaric) is a spectacular and well recognizable ectomycorrhizal mushroom that is native and common in the conifer and deciduous forests of the temperate and boreal zones of the northern hemisphere. In recent decades, it was introduced by forestry into the southern hemisphere and hence became a cosmopolitan species (Reid and Eicker 1991).
Fly agaric is widely known as psychoactive due to the hallucinogenic effects of some of its compounds. This mycorrhizal species is considered as specifically efficient for the bio-concentration of vanadium and cadmium in fruiting bodies (Drewnowska et al. 2013; Falandysz et al. 2007a, b; Lepp et al. 1987; Vetter 2005). Amanita muscaria is considered as a toadstool, i.e., an inedible or poisonous mushroom. A case of intoxication and coma of a young man after ingestion of A. muscaria was reported in one study from Poland (Mikaszewska-Sokolewicz et al. 2016). Due to its reputation, A. muscaria is usually avoided and rarely foraged; therefore, its fruiting bodies are abundant and available for research. Traditionally, it has also been used for catching flies (Lumpert and Kreft 2016). Little is known on the bio-concentration potential of heavy metals in A. muscaria across different soil types and geochemical variations and from areas with various amounts of pollution.
Ibotenic acid and muscimol are the two important psychoactive compounds in the fruiting bodies of Amanita muscaria. Muscimol is considered the principal psychoactive component and it is much more potent than ibotenic acid. After ingestion or drying of A. muscaria fruiting bodies, decarboxylases convert ibotenic acid ((S)-2-amino-2-(3-hydroxyisoxazol-5-yl) acetic acid) to muscimol. Both muscimol and ibotenic acid activate the neurotransmitter gamma-aminobutyric acid (GABA) receptors and A. muscaria can affect neuronal activity in the central regions of the brain (Michelot and Melendez-Howell 2003; Ogawa et al. 2015). While there is no doubt that the consumption of A. muscaria can lead to poisonings (Mikaszewska-Sokolewicz et al. 2016), the consumption of A. muscaria or its products for hallucinatory effects by the native Siberian shamans and possibly also in other locations in Eurasia was frequently not accompanied by symptoms of poisonings (Wasson 1968; http 2016). Therefore, the effect of treatment of the fruitbodies before consumption may be important (Feeney 2010). Other aspects of A. muscaria toxicology are discussed by Michelot and Melendez-Howell (2003), Tsujikawa et al. (2006), and by Vendramin and Brvar (2014).
Muscimol and ibotenic acid are well soluble in water—with a solubility of up to 100 mM for muscimol and of up to 10 mM for ibotenic acid (ChemSpider 2017). Blanching or parboiling of mushrooms is a common treatment before cooking: they are boiled in excess water for 10–15 min and the waste water is subsequently discarded (Falandysz and Drewnowska 2017). As reported by some authors, Amanita muscaria fruiting bodies (de-peeled caps and young stipes) sliced into small pieces and blanched (parboiled) for 15 min or blanched twice (for 5 and 5 min) with a large excess of water will lose their muscimol and ibotenic acid after the waste water is discarded. This treatment detoxifies the mushrooms from the hallucinogens and renders them well edible (Rubel and Arora 2008; Marley 2010).
It is worth to mention that blanching of mushroom’s fruiting bodies can lead also to partial but significant loss of some other organic constituents and numerous minerals during such preparation (Biekman et al. 1996; Drewnowska et al. 2017a, b; Falandysz and Drewnowska 2015 and 2017).
In the region of the Nagano prefecture in Japan, it is a tradition to preserve caps of the fruiting bodies of A. muscaria in salt after blanching (Phipps et al. 2000). Apart from Japan, there are also reports of culinary use of A. muscaria as pickles from Lithuania, Finland, Russia, and very recently from the North America (Rubel and Arora 2008).
The aim of this work was to assess the bioconcentration and bio-indication potential of Amanita muscaria fruiting bodies for the metallic elements Ag, Al, Ba, Ca, Cd, Co, Cu, Fe, Hg, K, Mg, Mn, Na, Rb, Sr, and Zn. Samples of fruiting bodies were collected together with the top layer of soil beneath. Six locations of forested or woodland regions from northern Poland were selected. These areas are not subject to direct impacts from local or regional heavy metals emitters. Forest soils in the northern part of Poland are slightly acidic and differ regionally and locally according to the differences in soil parent material; podzolic, brown, and rusty soils were formed where conifers dominate. In the region of Pomerania (Kaszuby and Kociewie region), poor sands with or without clay background (remains of a recent glacial epoch) are frequently dominant (DRRiP 2000). Planted scots pines (Pinus silvestris) and spruce (Picea abies) are predominant, with small enclaves of birch (Betula spp., often self-introduced) occasionally forming mixed stands. Locally, European larch (Larix decidua), European beech (Fagus sylvatica), or oak trees (Quercus petraea or Quercus robur) are planted. Older forests may include maples (Acer platanoides) and European hornbeam (Carpinus betulus), while Alnus glutinosa, the common alder, is frequent in wetlands.
Materials and methods
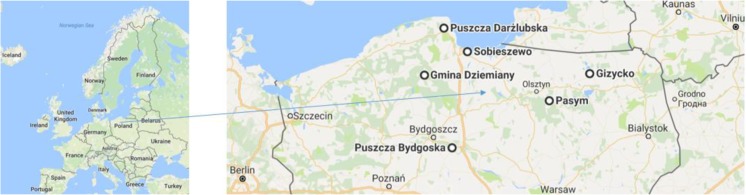
Fruiting bodies of A. muscaria and the surface soil layer (0–10 cm) underneath the fruiting bodies were collected from six sites: Sobieszewo Island forest (N 53° 48′ 22″ E 18° 18′ 50″), Puszcza Darżlubska (Darżlubska Wilderness) in the vicinity of Wejherowo, forests in the County of Dziemiany, Bydgoska Wilderness in the Pomerania region, forests near the town of Pasym, and in the vicinity of Giżycko town in the Warmia and Masuria region of Poland (Fig. 1; Table 1). At each site 8–15 composite fruiting body samples were taken. Each sample consisted of 24–45 well-developed fruiting bodies. Caps and stipes were separated. Likewise, 8–15 composite topsoil samples were taken from underneath the fruiting bodies.
Fig. 1.
Sampling sites of A. muscaria: Sobiszewo (Sobieszewo Island), Puszcza Darżlubska (Darżlubska Wilderness), Gmina Dziemiany (Dziemiany), Puszcza Bydgoska (Bydgoska forests), Pasym, and Giżycko
Table 1.
Elements content of the caps (C) and stipes (S) of Amanita muscaria and beneath forest soil, and elements’ BCF values (mean ± SD, median value, and range)
| Element | Matrix | Site | |||||
|---|---|---|---|---|---|---|---|
| Sobieszewo Island n = 8 (24)* |
Darżlubska Wilderness, Wejherowo n = 15 (44) |
Kaszuby, Dziemiany n = 15 (23) |
Bydgoska forests n = 15 (45) |
Pasym, Warmia n = 15 (30) |
Giżycko, Warmia n = 15 (28) |
||
| mg kg−1 dm | |||||||
| K | Cap | 39,000 ± 2000 | 38,000 ± 6000 | 50,000 ± 9000 | 43,000 ± 5000 | 34,000 ± 6000 | 40,000 ± 3000 |
| 40,000 | 37,000 | 53,000 | 45,000 | 36,000 | 40,000 | ||
| 36,000–41,000 | 29,000–49,000 | 31,000–61,000 | 35,000–51,000 | 25,000–45,000 | 34,000–46,000 | ||
| Stipe | 32,000 ± 4000 | 24,000 ± 8000 | 38,000 ± 10,000 | 38,000 ± 4000 | 20,000 ± 5000 | 25,000 ± 6000 | |
| 31,000 | 25,000 | 38,000 | 38,000 | 19,000 | 26,000 | ||
| 29,000–40,000 | 800–34,000 | 19,000–56,000 | 32,000–44,000 | 15,000–34,000 | 17,000–33,000 | ||
| Soil PT | 540 ± 150 | 460 ± 110 | 800 ± 460 | 320 ± 170 | 580 ± 170 | 330 ± 20 | |
| 480 | 430 | 740 | 380 | 600 | 330 | ||
| 440–810 | 310–630 | 290–1400 | 300–780 | 360–760 | 300–350 | ||
| Soil E | 31 ± 2 | 85 ± 37 | 110 ± 60 | 43 ± 16 | 98 ± 49 | 38 ± 15 | |
| 31 | 86 | 110 | 43 | 91 | 45 | ||
| 28–34 | 42–14 | 33–180 | 26–61 | 55–160 | 15–46 | ||
| aBCFC-pt | 76 ± 15 | 120 ± 16 | 120 ± 120 | 140 ± 17 | 77 ± 47 | 120 ± 7 | |
| 82 | 120 | 95 | 150 | 83 | 120 | ||
| 49–88 | 110–130 | 38–210 | 130–160 | 43–110 | 110–120 | ||
| aBCFS-pt | 64 ± 18 | 77 ± 3 | 66 ± 55 | 110 ± 12 | 37 ± 16 | 70 ± 15 | |
| 69 | 77 | 69 | 110 | 34 | 69 | ||
| 39–83 | 75–80 | 27–100 | 100–120 | 26–48 | 59–80 | ||
| bBCFC-e | 1200 ± 59 | 740 ± 340 | 1100 ± 1100 | 800 ± 95 | 570 ± 220 | 1800 ± 1200 | |
| 1200 | 720 | 1400 | 820 | 590 | 1300 | ||
| 1200–1300 | 500–980 | 290–1800 | 740–870 | 410–720 | 900–2600 | ||
| bBCFS-e | 1100 ± 140 | 470 ± 180 | 560 ± 500 | 610 ± 10 | 280 ± 48 | 1100 ± 980 | |
| 980 | 480 | 350 | 610 | 280 | 1200 | ||
| 920–1200 | 340–590 | 210–920 | 600–620 | 250–320 | 450–1800 | ||
| Mg | Cap | 920 ± 60 | 870 ± 10 | 850 ± 150 | 1100 ± 100 | 980 ± 100 | 1100 ± 100 |
| 920 | 850 | 880 | 1100 | 1000 | 1100 | ||
| 850–1000 | 750–1100 | 580–1200 | 910–1300 | 780–1100 | 1000–1300 | ||
| Stipe | 560 ± 480 | 490 ± 70 | 460 ± 90 | 610 ± 40 | 530 ± 110 | 490 ± 90 | |
| 560 | 490 | 440 | 600 | 530 | 500 | ||
| 500–620 | 370–580 | 300–610 | 570–690 | 390–690 | 340–670 | ||
| Soil PT | 590 ± 0.340 | 720 ± 270 | 1000 ± 100 | 390 ± 10 | 1200 ± 400 | 540 ± 20 | |
| 450 | 730 | 1100 | 400 | 1200 | 540 | ||
| 410–1200 | 410–1100 | 850–1200 | 370–410 | 780–1900 | 500–560 | ||
| Soil E | 110 ± 10 | 300 ± 140 | 350 ± 120 | 160 ± 40 | 370 ± 190 | 90 ± 14 | |
| 100 | 320 | 330 | 160 | 350 | 92 | ||
| 90–120 | 130–500 | 220–510 | 120–200 | 170–620 | 71–110 | ||
| BCFC-pt | 1.9 ± 0.6 | 1.7 ± 0.5 | 0.98 ± 0.73 | 2.7 ± 0.2 | 0.97 ± 0.39 | 2.1 ± 0.1 | |
| 2.0 | 1.4 | 0.65 | 2.7 | 0.82 | 2.1 | ||
| 0.76–2.3 | 1.4–2.1 | 0.46–1.5 | 2.5–2.7 | 0.69–1.2 | 2.0–2.3 | ||
| BCFS-pt | 1.1 ± 0.4 | 0.95 ± 0.31 | 0.41 ± 0.28 | 1.5 ± 0.1 | 0.55 ± 0.07 | 1.1 ± 0.2 | |
| 1.3 | 0.88 | 0.47 | 1.5 | 0.55 | 1.0 | ||
| 0.45–1.5 | 0.73–1.2 | 0.21–0.61 | 1.5–1.6 | 0.50–0.60 | 0.95–1.2 | ||
| BCFC-e | 8.8 ± 1.2 | 4.6 ± 1.8 | 4.2 ± 3.9 | 5.7 ± 0.6 | 3.5 ± 0.1 | 13 ± 0 | |
| 8.5 | 5.0 | 2.6 | 5.7 | 3.5 | 13 | ||
| 7.6–10 | 3.3–5.9 | 1.4–6.9 | 5.2–6.1 | 3.5–3.5 | 13–13 | ||
| BCFS-e | 5.3 ± 0.6 | 2.5 ± 1.1 | 1.7 ± 1.6 | 3.2 ± 0.4 | 2.2 ± 1.1 | 6.4 ± 1.1 | |
| 5.0 | 2.4 | 1.2 | 3.3 | 2.3 | 6.8 | ||
| 4.8–6.0 | 1.8–3.3 | 0.62–2.8 | 3.0–3.5 | 1.4–3.0 | 5.6–7.2 | ||
| mg kg −1 dm | |||||||
| Ag | Cap | 0.70 ± 0.09 | 0.50 ± 0.10 | 3.9 ± 1.4 | 0.81 ± 0.16 | 0.72 ± 0.13 | 0.72 ± 0.18 |
| 0.72 | 0.48 | 3.3 | 0.81 | 0.70 | 0.70 | ||
| 0.55–0.79 | 0.39–0.74 | 2.3–6.4 | 0.68–1.1 | 0.54–0.89 | 0.43–1.0 | ||
| Stipe | 0.78 ± 0.33 | 0.63 ± 0.21 | 1.4 ± 0.6 | 0.86 ± 0.11 | 0.44 ± 0.10 | 0.53 ± 0.30 | |
| 0.72 | 0.57 | 1.2 | 0.86 | 0.48 | 0.55 | ||
| 0.45–1.3 | 0.34–1.2 | 0.77–2.5 | 0.71–1.1 | 0.27–0.57 | 0.16–1.0 | ||
| Soil PT | WD | WD | 5.4 ± 3.0 | 1.8 ± 0.3 | 2.5 ± 1.5 | 1.5 ± 0.3 | |
| 5.0 | 1.8 | 3.0 | 1.4 | ||||
| 2.2–9.3 | 1.7–2.2 | 0.44–3.6 | 1.2–1.9 | ||||
| BCFC-pt | WD | WD | 1.6 ± 1.9 | 0.49 ± 0.03 | 0.85 ± 0.90 | 0.53 ± 0.09 | |
| 1.8 | 0.49 | 0.87 | 0.53 | ||||
| 0.27–2.9 | 0.46–0.50 | 0.21–1.5 | 0.46–0.59 | ||||
| BCFS-pt | WD | WD | 0.33 ± 0.34 | 0.52 ± 0.03 | 0.47 ± 0.45 | 0.39 ± 0.24 | |
| 0.38 | 0.52 | 0.50 | 0.45 | ||||
| 0.09–0.57 | 0.49–0.54 | 0.15–0.79 | 0.22–0.57 | ||||
| Al | Cap | 490 ± 390 | 270 ± 160 | 190 ± 140 | 420 ± 200 | 170 ± 82 | 110 ± 140 |
| 300 | 290 | 140 | 380 | 180 | 68 | ||
| 210–1300 | 48–590 | 74–540 | 110–780 | 69–320 | 43–510 | ||
| Stipe | 48 ± 47 | 210 ± 100 | 200 ± 110 | 200 ± 68 | 110 ± 35 | 58 ± 33 | |
| 34 | 220 | 160 | 200 | 110 | 61 | ||
| 7.0–140 | 88–400 | 64–410 | 83–350 | 61–170 | 18–100 | ||
| Soil PT | 5900 ± 4200 | 16,000 ± 2700 | 26,000 ± 3800 | 8600 ± 7200 | 18,000 ± 4500 | 14,000 ± 960 | |
| 4000 | 15,000 | 27,000 | 8500 | 17,000 | 14,000 | ||
| 3800–13,000 | 12,000–20,000 | 20,000–30,000 | 7800–9600 | 14,000–25,000 | 13,000–15,000 | ||
| Soil E | 1100 ± 42 | 6600 ± 2600 | 6700 ± 1400 | 2600 ± 810 | 5200 ± 1900 | 3400 ± 210 | |
| 1100 | 6700 | 6200 | 2400 | 5300 | 3400 | ||
| 1000–1100 | 3400–10,000 | 5800–8700 | 1800–3700 | 3200–6900 | 3200–3600 | ||
| BCFC-pt | 0.082 ± 0.051 | 0.015 ± 0.010 | 0.004 ± 0.001 | 0.039 ± 0.004 | 0.008 ± 0.006 | 0.006 ± 0.001 | |
| 0.069 | 0.014 | 0.004 | 0.039 | 0.007 | 0.006 | ||
| 0.023–0.15 | 0.008–0.022 | 0.004–0.005 | 0.036–0.042 | 0.003–0.012 | 0.006–0.007 | ||
| BCFS-pt | 0.009 ± 0.007 | 0.009 ± 0.004 | 0.013 ± 0.002 | 0.030 ± 0.014 | 0.006 ± 0.001 | 0.005 ± 0.001 | |
| 0.008 | 0.009 | 0.013 | 0.028 | 0.006 | 0.005 | ||
| 0.001–0.019 | 0.006–0.012 | 0.011–0.014 | 0.020–0.041 | 0.005–0.007 | 0.004–0.006 | ||
| BCFC-e | 0.35 ± 0.16 | 0.027 ± 0.019 | 0.036 ± 0.023 | 0.14 ± 0.03 | 0.021 ± 0.013 | 0.027 ± 0.006 | |
| 0.26 | 0.022 | 0.042 | 0.14 | 0.023 | 0.028 | ||
| 0.22–0.59 | 0.014–0.040 | 0.020–0.052 | 0.12–0.16 | 0.012–0.031 | 0.023–0.031 | ||
| BCFS-e | 0.034 ± 0.023 | 0.016 ± 0.007 | 0.033 ± 0.017 | 0.11 ± 0.06 | 0.018 ± 0.001 | 0.023 ± 0.007 | |
| 0.030 | 0.014 | 0.035 | 0.09 | 0.018 | 0.022 | ||
| 0.009–0.067 | 0.011–0.021 | 0.021–0.045 | 0.07–0.16 | 0.017–0.018 | 0.018–0.028 | ||
| Ba | Cap | 2.0 ± 1.0 | 1.4 ± 0.4 | 1.2 ± 0.6 | 1.8 ± 0.9 | 0.72 ± 0.27 | 0.88 ± 0.77 |
| 1.6 | 1.2 | 1.2 | 1.6 | 0.69 | 0.51 | ||
| 1.2–4.2 | 0.99–2.0 | 0.37–2.7 | 0.61–3.6 | 0.36–1.3 | 0.41–2.9 | ||
| Stipe | 0.50 ± 0.40 | 1.5 ± 0.2 | 1.4 ± 0.7 | 1.1 ± 0.4 | 0.69 ± 0.27 | 0.73 ± 0.38 | |
| 0.40 | 1.6 | 1.3 | 1.3 | 0..66 | 0.75 | ||
| 0.10–1.3 | 1.2–2.0 | 0.63–3.0 | 0.45–1.6 | 0.35–1.2 | 0.23–1.3 | ||
| Soil PT | 73 ± 54 | 160 ± 45 | 130 ± 15 | 87 ± 14 | 120 ± 28 | 67 ± 5 | |
| 49 | 150 | 130 | 90 | 130 | 67 | ||
| 45–170 | 94–220 | 120–150 | 69–99 | 89–160 | 60–72 | ||
| Soil E | 41 ± 4 | 120 ± 28 | 86 ± 4 | 76 ± 17 | 110 ± 21 | 35 ± 2 | |
| 41 | 120 | 86 | 78 | 110 | 34 | ||
| 37–47 | 78–160 | 82–91 | 56–92 | 80–130 | 33–37 | ||
| BCFC-pt | 0.031 ± 0.014 | 0.013 ± 0.002 | 0.014 ± 0.013 | 0.013 ± 0.002 | 0.006 ± 0.001 | 0.019 ± 0.001 | |
| 0.035 | 0.013 | 0.018 | 0.013 | 0.006 | 0.019 | ||
| 0.009–0.043 | 0.012–0.015 | 0.005–0.023 | 0.012–0.014 | 0.005–0.007 | 0.018–0.020 | ||
| BCFS-pt | 0.008 ± 0.005 | 0.014 ± 0.001 | 0.017 ± 0.012 | 0.015 ± 0.003 | 0.006 ± 0.001 | 0.014 ± 0.004 | |
| 0.008 | 0.015 | 0.013 | 0.015 | 0.006 | 0.016 | ||
| 0.002–0.014 | 0.014–0.015 | 0.009–0.026 | 0.013–0.017 | 0.005–0.006 | 0.011–0.016 | ||
| BCFC-e | 0.042 ± 0.010 | 0.015 ± 0.001 | 0.021 ± 0.017 | 0.014 ± 0.002 | 0.006 ± 0.001 | 0.039 ± 0.001 | |
| 0.037 | 0.015 | 0.036 | 0.014 | 0.006 | 0.039 | ||
| 0.032–0.057 | 0.014–0.16 | 0.010–0.033 | 0.013–0.015 | 0.005–0.007 | 0.038–0.039 | ||
| BCFS-e | 0.010 ± 0.005 | 0.017 ± 0.001 | 0.026 ± 0.015 | 0.016 ± 0.003 | 0.006 ± 0.001 | 0.027 ± 0.007 | |
| 0.010 | 0.017 | 0.024 | 0.016 | 0.006 | 0.026 | ||
| 0.004–0.016 | 0.016–0.017 | 0.016–0.037 | 0.014–0.018 | 0.006–0.007 | 0.022–0.032 | ||
| Ca | Cap | 99 ± 49 | 150 ± 50 | 120 ± 61 | 160 ± 60 | 110 ± 38 | 98 ± 52 |
| 99 | 160 | 84 | 130 | 110 | 87 | ||
| 41–180 | 75–220 | 48–240 | 92–250 | 54–170 | 39–240 | ||
| Stipe | 76 ± 48 | 240 ± 71 | 120 ± 67 | 200 ± 79 | 120 ± 71 | 99 ± 55 | |
| 77 | 260 | 120 | 220 | 100 | 95 | ||
| 34–170 | 110–330 | 41–230 | 79–330 | 48–290 | 39–220 | ||
| Soil PT | 640 ± 340 | 1800 ± 1400 | 510 ± 110 | 380 ± 130 | 810 ± 740 | 200 ± 27 | |
| 490 | 1400 | 520 | 390 | 720 | 190 | ||
| 380–1200 | 600–4400 | 360–640 | 240–490 | 180–1600 | 170–230 | ||
| Soil E | 530 ± 65 | 1700 ± 1000 | 250 ± 170 | 310 ± 220 | 740 ± 500 | 44 ± 10 | |
| 500 | 1500 | 230 | 320 | 690 | 41 | ||
| 460–610 | 620–3400 | 81–460 | 54–530 | 270–1300 | 37–59 | ||
| BCFC-pt | 0.18 ± 0.09 | 0.25 ± 0.13 | 0.27 ± 0.19 | 0.25 ± 0.01 | 0.51 ± 0.15 | 0.46 ± 0.10 | |
| 0.20 | 0.26 | 0.28 | 0.25 | 0.55 | 0.46 | ||
| 0.04–0.29 | 0.15–0.34 | 0.13–0.40 | 0.24–0.26 | 0.40–0.62 | 0.38–0.53 | ||
| BCFS-pt | 0.12 ± 0.07 | 0.33 ± 0.12 | 0.29 ± 0.21 | 0.44 ± 0.01 | 0.63 ± 0.18 | 0.50 ± 0.13 | |
| 0.11 | 0.32 | 0.24 | 0.44 | 0.61 | 0.51 | ||
| 0.03–0.19 | 0.25–0.42 | 0.14–0.44 | 0.43–0.44 | 0.50–0.80 | 0.41–0.59 | ||
| BCFC-e | 0.18 ± 0.07 | 0.27 ± 0.12 | 1.4 ± 1.7 | 0.25 ± 0.01 | 0.89 ± 0.06 | 2.1 ± 0.8 | |
| 0.17 | 0.28 | 1.3 | 0.25 | 0.89 | 2.2 | ||
| 0.09–0.27 | 0.18–0.35 | 0.18–2.6 | 0.24–0.26 | 0.84–0.93 | 1.5–2.7 | ||
| BCFS-e | 0.13 ± 0.05 | 0.37 ± 0.09 | 1.5 ± 1.8 | 0.44 ± 0.05 | 1.2 ± 0.6 | 2.2 ± 0.2 | |
| 0.16 | 0.36 | 1.4 | 0.44 | 1.2 | 2.3 | ||
| 0.06–0.18 | 0.30–0.43 | 0.20–2.8 | 0.41–0.47 | 0.76–1.6 | 2.0–2.4 | ||
| Cd | Cap | 19 ± 10 | 10 ± 3 | 15 ± 6 | 21 ± 5 | 14 ± 5 | 13 ± 7 |
| 13 | 10 | 15 | 21 | 14 | 11 | ||
| 9.0–36 | 5.7–14 | 4.0–25 | 9.0–25 | 7.0–26 | 6.0–32 | ||
| Stipe | 10 ± 6 | 4.2 ± 1.7 | 8.7 ± 5.5 | 10 ± 4 | 5.6 ± 2.2 | 7.5 ± 7.4 | |
| 6.0 | 4.2 | 7.4 | 11 | 4.9 | 3.6 | ||
| 5.0–22 | 2.0–7.1 | 1.4–20 | 4.0–17 | 3.4–10 | 2.1–27 | ||
| Soil PT | 0.036 ± 0.027 | 0.098 ± 0.062 | 0.049 ± 0.013 | 0.052 ± 0.017 | 0.050 ± 0.017 | 0.014 ± 0.003 | |
| 0.026 | 0.086 | 0.046 | 0.055 | 0.049 | 0.013 | ||
| 0.017–0.083 | 0.035–0.21 | 0.036–0.066 | 0.029–0.068 | 0.034–0.068 | 0.012–0.018 | ||
| Soil E | 0.025 ± 0.001 | 0.097 ± 0.036 | 0.047 ± 0.016 | 0.034 ± 0.011 | 0.036 ± 0.009 | 0.005 ± 0.003 | |
| 0.025 | 0.10 | 0.049 | 0.019 | 0.037 | 0.006 | ||
| 0.024–0.026 | 0.049–0.14 | 0.028–0.061 | 0.029–0.050 | 0.025–0.048 | 0.002–0.008 | ||
| BCFC-pt | 840 ± 580 | 200 ± 17 | 330 ± 6 | 440 ± 19 | 290 ± 140 | 1100 ± 440 | |
| 280 | 200 | 330 | 440 | 240 | 1300 | ||
| 120–900 | 180–210 | 320–330 | 430–450 | 190–390 | 830–1400 | ||
| BCFS-pt | 440 ± 340 | 94 ± 12 | 130 ± 16 | 240 ± 11 | 110 ± 18 | 530 ± 260 | |
| 280 | 91 | 130 | 240 | 110 | 510 | ||
| 120–900 | 86–100 | 120–150 | 230–250 | 99–120 | 340–710 | ||
| BCFC-e | 890 ± 420 | 270 ± 30 | 350 ± 200 | 840 ± 78 | 360 ± 240 | 2900 ± 870 | |
| 990 | 270 | 400 | 810 | 380 | 2700 | ||
| 450–1400 | 250–290 | 210–500 | 770–490 | 190–530 | 2300–3500 | ||
| BCFS-e | 460 ± 270 | 130 ± 13 | 150 ± 90 | 460 ± 27 | 130 ± 8 | 1300 ± 220 | |
| 410 | 130 | 180 | 460 | 130 | 1200 | ||
| 210–850 | 120–140 | 83–210 | 420–490 | 120–140 | 1100–1400 | ||
| Co | Cap | 0.32 ± 0.06 | 0.16 ± 0.04 | 0.20 ± 0.15 | 0.34 ± 0.10 | 0.25 ± 0.03 | 0.51 ± 0.23 |
| 0.31 | 0.15 | 0.19 | 0.30 | 0.25 | 0.44 | ||
| 0.25–0.40 | 0.10–0.24 | 0.04–0.54 | 0.24–0.61 | 0.22–0.31 | 0.29–1.1 | ||
| Stipe | 0.36 ± 0.05 | 0.15 ± 0.02 | 0.16 ± 0.12 | 0.28 ± 0.08 | 0.24 ± 0.03 | 0.40 ± 0.15 | |
| 0.35 | 0.14 | 0.13 | 0.26 | 0.24 | 0.37 | ||
| 0.29–0.45 | 0.12–0.19 | 0.03–0.42 | 0.22–0.53 | 0.19–0.29 | 0.23–0.78 | ||
| Soil PT | 0.56 ± 0.36 | 0.91 ± 0.25 | 1.4 ± 0.5 | 0.39 ± 0.04 | 0.86 ± 0.10 | 0.45 ± 0.02 | |
| 0.40 | 0.86 | 1.5 | 0.39 | 0.84 | 0.45 | ||
| 0.37–1.2 | 0.59–1.3 | 0.78–2.1 | 0.35–0.44 | 0.77–0.99 | 0.42–0.47 | ||
| Soil E | 0.22 ± 0.01 | 0.53 ± 0.24 | 1.1 ± 0.5 | 0.28 ± 0.10 | 0.60 ± 0.05 | 0.16 ± 0.01 | |
| 0.23 | 0.47 | 1.2 | 0.24 | 0.58 | 0.45 | ||
| 0.21–0.23 | 0.30–0.90 | 0.49–1.7 | 0.22–0.43 | 0.57–0.67 | 0.42–0.47 | ||
| BCFC-pt | 0.62 ± 0.17 | 0.20 ± 0.04 | 0.31 ± 0.26 | 0.76 ± 0.07 | 0.26 ± 0.05 | 1.6 ± 1.1 | |
| 0.69 | 0.20 | 0.32 | 0.76 | 0.26 | 1.7 | ||
| 0.32–0.74 | 0.18–0.23 | 0.13–0.49 | 0.72–0.81 | 0.23–0.30 | 0.83–2.4 | ||
| BCFS-pt | 0.76 ± 0.28 | 0.20 ± 0.04 | 0.30 ± 0.27 | 0.70 ± 0.12 | 0.24 ± 0.01 | 1.1 ± 0.9 | |
| 0.80 | 0.20 | 0.27 | 0.71 | 0.24 | 1.0 | ||
| 0.31–1.0 | 0.17–0.22 | 0.11–0.49 | 0.62–0.79 | 0.23–0.25 | 0.50–1.8 | ||
| BCFC-e | 1.3 ± 0.2 | 0.33 ± 0.05 | 0.47 ± 0.44 | 1.2 ± 0.1 | 0.39 ± 0.01 | 4.6 ± 2.8 | |
| 1.2 | 0.33 | 0.58 | 1.2 | 0.39 | 4.8 | ||
| 1.2–1.6 | 0.29–0.37 | 0.15–0.78 | 1.2–1.3 | 0.38–0.40 | 2.6–6.6 | ||
| BCFS-e | 1.6 ± 0.2 | 0.32 ± 0.05 | 0.45 ± 0.46 | 1.1 ± 0.0 | 0.37 ± 0.04 | 3.2 ± 2.3 | |
| 1.5 | 0.32 | 0.51 | 1.1 | 0.37 | 3.0 | ||
| 1.4–1.9 | 0.28–0.35 | 0.12–0.78 | 1.1–1.1 | 0.34–0.39 | 1.5–4.9 | ||
| Cu | Cap | 50 ± 19 | 35 ± 15 | 39 ± 11 | 40 ± 4 | 41 ± 8 | 33 ± 11 |
| 48 | 30 | 37 | 39 | 43 | 30 | ||
| 25–85 | 19–69 | 22–62 | 36–50 | 28–53 | 18–52 | ||
| Stipe | 27 ± 12 | 10 ± 2 | 16 ± 5 | 17 ± 4 | 15 ± 4 | 14 ± 4 | |
| 24 | 10 | 17 | 15 | 14 | 14 | ||
| 13–50 | 6.6–14 | 8.0–24 | 13–23 | 10–20 | 7.9–20 | ||
| Soil PT | 1.0 ± 1.1 | 1.9 ± 0.9 | 4.0 ± 2.3 | 1.0 ± 0.5 | 1.6 ± 0.5 | 0.86 ± 0.11 | |
| 0.52 | 1.8 | 4.4 | 1.1 | 1.5 | 0.89 | ||
| 0.33–3.0 | 0.76–3.1 | 0.86–6.2 | 0.35–1.6 | 1.1–2.3 | 0.69–0.95 | ||
| Soil E | 0.32 ± 0.03 | 1.0 ± 0.4 | 2.3 ± 1.6 | 0.78 ± 0.38 | 1.4 ± 1.4 | 0.36 ± .0.03 | |
| 0.31 | 0.98 | 2.3 | 0.84 | 0.82 | 0.35 | ||
| 0.29–0.37 | 0.46–1.5 | 0.37–4.3 | 0.30–1.1 | 0.67–3.5 | 0.33–0.41 | ||
| BCFC-pt | 110 ± 73 | 46 ± 10 | 39 ± 47 | 34 ± 1 | 27 ± 6 | 43 ± 2 | |
| 130 | 41 | 18 | 34 | 27 | 43 | ||
| 15–190 | 39–53 | 6.0–72 | 34–35 | 23–32 | 41–45 | ||
| BCFS-pt | 56 ± 38 | 11 ± 0 | 11 ± 12 | 13 ± 1 | 12 ± 1 | 16 ± 1 | |
| 61 | 11 | 7.0 | 13 | 12 | 16 | ||
| 7.0–98 | 11–11 | 2.0–20 | 13–14 | 11–13 | 15–16 | ||
| BCFC-e | 180 ± 38 | 76 ± 18 | 90 ± 11 | 67 ± 62 | 53 ± 1 | 110 ± 3 | |
| 160 | 72 | 76 | 84 | 53 | 110 | ||
| 130–230 | 63–89 | 11–170 | 23–110 | 53–54 | 110–120 | ||
| BCFS-e | 90 ± 27 | 18 ± 1 | 25 ± 30 | 26 ± 24 | 24 ± 8 | 42 ± 1 | |
| 79 | 18 | 28 | 38 | 28 | 42 | ||
| 66–140 | 17–18 | 4.0–46 | 9.0–43 | 18–30 | 42–43 | ||
| Fe | Cap | 310 ± 400 | 140 ± 68 | 130 ± 79 | 240 ± 97 | 74 ± 31 | 180 ± 160 |
| 170 | 120 | 100 | 230 | 65 | 110 | ||
| 49–800 | 33–250 | 60–320 | 85–430 | 37–140 | 77–610 | ||
| Stipe | 83 ± 93 | 110 ± 56 | 130 ± 62 | 110 ± 29 | 51 ± 13 | 79 ± 53 | |
| 60 | 97 | 110 | 110 | 54 | 74 | ||
| 13–280 | 48–220 | 54–260 | 56–150 | 35–66 | 26–220 | ||
| Soil PT | 2500 ± 600 | 2800 ± 560 | 3500 ± 850 | 1000 ± 680 | 2600 ± 620 | 2100 ± 94 | |
| 2200 | 3000 | 3400 | 1200 | 2600 | 2000 | ||
| 2000–3500 | 2000–3400 | 2600–4600 | 1000–1500 | 1900–3400 | 2000–2200 | ||
| Soil E | 850 ± 32 | 1400 ± 550 | 1900 ± 310 | 620 ± 150 | 1400 ± 430 | 1100 ± 120 | |
| 860 | 1300 | 1900 | 620 | 1400 | 1100 | ||
| 800–890 | 540–2200 | 1700–2400 | 450–790 | 920–1800 | 990–1300 | ||
| BCFC-pt | 0.080 ± 0.057 | 0.044 ± 0.025 | 0.062 ± 0.063 | 0.32 ± 0.07 | 0.020 ± 0.012 | 0.084 ± 0.018 | |
| 0.078 | 0.042 | 0.019 | 0.34 | 0.023 | 0.084 | ||
| 0.027–0.17 | 0.026–0.062 | 0.017–0.11 | 0.28–0.37 | 0.012–0.029 | 0.072–0.097 | ||
| BCFS-pt | 0.026 ± 0.020 | 0.028 ± 0.010 | 0.060 ± 0.056 | 0.21 ± 0.06 | 0.016 ± 0.006 | 0.074 ± 0.034 | |
| 0.028 | 0.029 | 0.037 | 0.23 | 0.016 | 0.075 | ||
| 0.007–0.057 | 0.021–0.035 | 0.021–0.10 | 0.17–0.26 | 0.012–0.021 | 0.050–0.099 | ||
| BCFC-e | 0.22 ± 0.13 | 0.065 ± 0.034 | 0.086 ± 0.073 | WD | 0.034 ± 0.015 | 0.17 ± 0.01 | |
| 0.21 | 0.054 | 0.061 | 0.039 | 0.17 | |||
| 0.08–0.43 | 0.041–0.090 | 0.034–0.14 | 0.023–0.045 | 0.16–0.18 | |||
| BCFS-e | 0.069 ± 0.045 | 0.041 ± 0.012 | 0.085 ± 0.063 | WD | 0.028 ± 0.007 | 0.16 ± 0.09 | |
| 0.074 | 0.041 | 0.052 | 0.026 | 0.18 | |||
| 0.020–0.14 | 0.033–0.050 | 0.041–0.13 | 0.023–0.033 | 0.09–0.22 | |||
| Hg | Cap | 0.76 | 0.40 | 0.22 | 0.45 | 0.75 | 0.68 |
| Stipe | 0.45 | 0.24 | 0.21 | 0.23 | 0.45 | 0.35 | |
| Soil | 0.015 | 0.054 | 0.010 | 0.013 | 0.031 | 0.031 | |
| BCFC | 49 | 17 | 19 | 51 | 29 | 22 | |
| BCFS | 28 | 10 | 18 | 23 | 15 | 19 | |
| Mn | Cap | 8.3 ± 2.5 | 8.6 ± 3.7 | 14 ± 6 | 17 ± 4 | 11 ± 3 | 11 ± 6 |
| 7.6 | 8.6 | 14 | 18 | 10 | 9.4 | ||
| 6.1–12 | 5.1–19 | 6.0–24 | 10–23 | 8.9–18 | 6.2–29 | ||
| Stipe | 6.0 ± 3 | 7.3 ± 2.5 | 14 ± 7 | 14 ± 6 | 10 ± 5 | 13 ± 8 | |
| 4.5 | 6.3 | 12 | 12 | 8.0 | 11 | ||
| 4.1–12 | 3.9–12 | 6.0–29 | 7.0–25 | 4.7–22 | 4.8–29 | ||
| Soil PT | 37 ± 29 | 100 ± 60 | 86 ± 11 | 82 ± 44 | 74 ± 9 | 23 ± 2 | |
| 23 | 76 | 84 | 84 | 76 | 23 | ||
| 22–89 | 60–200 | 74–100 | 37–120 | 63–83 | 20–26 | ||
| Soil E | 22 ± 1 | 100 ± 46 | 88 ± 15 | 45 ± 38 | 71 ± 11 | 13 ± 1 | |
| 22 | 97 | 84 | 44 | 72 | 13 | ||
| 21–23 | 53–180 | 75–110 | 10–94 | 56–82 | 12–15 | ||
| BCFC-pt | 0.29 ± 0.12 | 0.18 ± 0.11 | 0.18 ± 0.07 | 0.21 ± 0.07 | 0.13 ± 0.00 | 0.54 ± 0.08 | |
| 0.31 | 0.13 | 0.17 | 0.23 | 0.13 | 0.56 | ||
| 0.08–0.41 | 0.10–0.26 | 0.14–0.23 | 0.15–0.26 | 0.13–0.13 | 0.48–0.59 | ||
| BCFS-pt | 0.22 ± 0.14 | 0.11 ± 0.02 | 0.18 ± 0.10 | 0.27 ± 0.12 | 0.17 ± 0.14 | 0.51 ± 0.01 | |
| 0.20 | 0.11 | 0.14 | 0.33 | 0.12 | 0.51 | ||
| 0.05–0.44 | 0.09–0.13 | 0.11–0.25 | 0.19–0.36 | 0.07–0.27 | 0.50–0.52 | ||
| BCFC-e | 0.36 ± 0.08 | 0.20 ± 0.12 | 0.18 ± 0.06 | WD | 0.13 ± 0.00 | 1.0 ± 0.0 | |
| 0.34 | 0.24 | 0.18 | 0.13 | 1.0 | |||
| 0.27–0.49 | 0.11–0.29 | 0.14–0.23 | 0.13–0.13 | 1.0–1.0 | |||
| BCFS-e | 0.27 ± 0.14 | 0.12 ± 0.02 | 0.18 ± 0.09 | WD | 0.17 ± 0.14 | 0.99 ± 0.18 | |
| 0.21 | 0.12 | 0.14 | 0.12 | 0.91 | |||
| 0.18–0.52 | 0.11–0.14 | 0.12–0.25 | 0.07–0.27 | 0.86–1.1 | |||
| Na | Cap | 59 ± 12 | 230 ± 500 | 53 ± 43 | 30 ± 7 | 390 ± 490 | 120 ± 200 |
| 63 | 47 | 31 | 32 | 110 | 37 | ||
| 39–76 | 23–1700 | 10–150 | 19–41 | 53–1500 | 16–670 | ||
| Stipe | 100 ± 36 | 730 ± 1400 | 130 ± 210 | 92 ± 39 | 710 ± 730 | 750 ± 940 | |
| 110 | 270 | 41 | 100 | 350 | 370 | ||
| 54–150 | 49–5000 | 21–640 | 23–150 | 130–2100 | 23–2600 | ||
| Soil PT | 16 ± 11 | 34 ± 12 | 24 ± 4 | 12 ± 1 | 20 ± 4 | 15 ± 1 | |
| 12 | 31 | 23 | 12 | 22 | 15 | ||
| 10–35 | 20–53 | 20–30 | 11–14 | 15–24 | 14–16 | ||
| Soil E | 6.2 ± 2.8 | 23 ± 7 | 13 ± 7 | 8.6 ± 2.3 | 14 ± 3 | 16 ± 13 | |
| 5.8 | 24 | 11 | 8.7 | 13 | 11 | ||
| 3.0–10 | 12–30 | 7.7–23 | 5.8–11 | 12–18 | 8.0–36 | ||
| BCFC-pt | 4.6 ± 1.9 | 1.6 ± 0.4 | 3.2 ± 1.6 | 1.8 ± 0.4 | 18 ± 18 | 6.4 ± 1.8 | |
| 5.0 | 1.6 | 3.2 | 1.8 | 9.0 | 6.0 | ||
| 1.4–6.3 | 1.3–1.9 | 2.1–4.4 | 1.5–2.1 | 5.0–31 | 5.2–7.7 | ||
| BCFS-pt | 8.2 ± 3.8 | 4.2 ± 3.6 | 0.68 ± 0.52 | 8.0 ± 0.1 | 42 ± 34 | 40 ± 3 | |
| 9.2 | 2.5 | 0.98 | 8.0 | 27 | 40 | ||
| 2.3–11 | 1.7–6.8 | 0.32–1.5 | 8.0–8.1 | 18–67 | 39–42 | ||
| BCFC-e | 11 ± 6 | 1.7 ± 0.4 | 8.9 ± 3.9 | 2.1 ± 0.3 | 34 ± 40 | 7.5 ± 5.4 | |
| 9.1 | 1.7 | 8.4 | 2.2 | 48 | 7.9 | ||
| 4.8–21 | 1.4–2.0 | 6.1–12 | 1.9–2.3 | 6.0–62 | 3.7–11 | ||
| BCFS-e | 21 ± 12 | 4.4 ± 3.8 | 1.8 ± 1.3 | 9.5 ± 0.8 | 77 ± 80 | 56 ± 53 | |
| 19 | 4.1 | 1.5 | 9.4 | 69 | 58 | ||
| 8.0–37 | 1.8–7.1 | 0.9–2.8 | 8.9–10 | 20–130 | 19–93 | ||
| Rb | Cap | 220 ± 56 | 340 ± 270 | 320 ± 88 | 140 ± 50 | 500 ± 170 | 620 ± 390 |
| 210 | 260 | 310 | 110 | 470 | 470 | ||
| 120–300 | 170–1100 | 240–420 | 80–210 | 320–820 | 270–1500 | ||
| Stipe | 93 ± 36 | 88 ± 21 | 160 ± 89 | 66 ± 14 | 130 ± 71 | 280 ± 110 | |
| 86 | 88 | 110 | 66 | 110 | 310 | ||
| 58–160 | 53–110 | 45–290 | 47–100 | 57–320 | 140–480 | ||
| Soil PT | 2.3 ± 1.3 | 4.1 ± 1.0 | 1.1 ± 0.2 | 1.6 ± 1.0 | 3.9 ± 0.9 | 2.2 ± 0.1 | |
| 1.7 | 4.4 | 1.2 | 2.0 | 4.0 | 2.3 | ||
| 1.5–4.7 | 2.7–5.2 | 0.88–1.4 | 0.02–2.3 | 2.7–5.0 | 2.0–2.4 | ||
| Soil E | 0.26 ± 0.02 | 1.1 ± 0.7 | 0.86 ± 0.35 | 0.69 ± 0.19 | 0.90 ± 0.22 | 0.26 ± 0.15 | |
| 0.27 | 1.0 | 0.94 | 0.66 | 0.90 | 0.27 | ||
| 0.23–0.28 | 0.21–2.0 | 0.31–1.2 | 0.49–0.94 | 0.66–1.1 | 0.22–0.30 | ||
| BCFC-pt | 130 ± 52 | 180 ± 110 | 190 ± 45 | 230 ± 65 | 150 ± 74 | 320 ± 280 | |
| 130 | 130 | 180 | 220 | 130 | 390 | ||
| 45–180 | 100–260 | 160–220 | 180–270 | 100–210 | 130–540 | ||
| BCFS-pt | 57 ± 28 | 34 ± 11 | 97 ± 21 | 92 ± 4 | 35 ± 5 | 120 ± 60 | |
| 51 | 39 | 87 | 92 | 35 | 120 | ||
| 18–94 | 26–42 | 72–110 | 89–95 | 32–39 | 80–160 | ||
| BCFC-e | 930 ± 120 | 610 ± 340 | 620 ± 110 | 4400 ± 6200 | 670 ± 72 | 3000 ± 2200 | |
| 930 | 670 | 590 | 2500 | 680 | 2600 | ||
| 760–1100 | 370–850 | 490–700 | 57–8800 | 620–720 | 1500–4600 | ||
| BCFS-e | 400 ± 100 | 120 ± 50 | 130 ± 32 | 1500 ± 2100 | 160 ± 45 | 1100 ± 380 | |
| 370 | 110 | 120 | 990 | 150 | 1200 | ||
| 310–580 | 87–160 | 190 ± 45 | 28–3100 | 130–190 | 880–1400 | ||
| Sr | Cap | 0.68 ± 0.36 | 0.45 ± 0.15 | 0.41 ± 019 | 0.70 ± 0.27 | 0.30 ± 0.12 | 0.24 ± 0.20 |
| 0.56 | 0.40 | 0.36 | 0.69 | 0.28 | 0.19 | ||
| 0.35–1.4 | 0.26–0.75 | 0.15–0.98 | 0.33–1.2 | 0.14–0.58 | 0.12–0.82 | ||
| Stipe | 0.22 ± .0.18 | 0.48 ± 0.09 | 0.41 ± 0.19 | 0.65 ± 0.23 | 0.27 ± 0.11 | 0.27 ± 0.16 | |
| 0.20 | 0.47 | 0.37 | 0.68 | 0.26 | 0.26 | ||
| 0.03–0.55 | 0.35–0.63 | 0.21–0.88 | 0.30–1.0 | 0.12–0.46 | 0.08–0.59 | ||
| Soil PT | 19 ± 8 | 35 ± 10 | 34 ± 10 | 14 ± 10 | 26 ± 6 | 18 ± 1 | |
| 15 | 34 | 34 | 17 | 28 | 18 | ||
| 15–33 | 23–51 | 23–44 | 1.0–22 | 17–31 | 17–19 | ||
| Soil E | 7.6 ± 0.5 | 17 ± 6 | 8.2 ± 5.3 | 7.4 ± 5.5 | 11 ± 8 | 16 ± 1 | |
| 7.4 | 17 | 8.8 | 8.1 | 11 | 16 | ||
| 7.1–8.2 | 9.0–25 | 2.3–13 | 0.97–12 | 3.9–19 | 15–18 | ||
| BCFC-pt | 0.035 ± 0.012 | 0.017 ± 0.008 | 0.024 ± 0.025 | 0.047 ± 0.001 | 0.013 ± 0.004 | 0.012 ± 0.002 | |
| 0.037 | 0.019 | 0.036 | 0.047 | 0.013 | 0.012 | ||
| 0.016–0.048 | 0.011–0.023 | 0.007–0.042 | 0.046–0.047 | 0.010–0.016 | 0.011–0.013 | ||
| BCFS-pt | 0.011 ± 0.007 | 0.017 ± 0.001 | 0.023 ± 0.022 | 0.065 ± 0.001 | 0.013 ± 0.005 | 0.017 ± 0.005 | |
| 0.012 | 0.017 | 0.025 | 0.065 | 0.013 | 0.017 | ||
| 0.004–0.021 | 0.017–0.018 | 0.007–0.038 | 0.065–0.066 | 0.009–0.017 | 0.013–0.021 | ||
| BCFC-e | 0.079 ± 0.022 | 0.035 ± 0.008 | 0.23 ± 0.29 | WD | 0.071 ± 0.055 | 0.14 ± 0.03 | |
| 0.079 | 0.035 | 0.27 | 0.071 | 0.14 | |||
| 0.052–0.11 | 0.030–0.041 | 0.02–0.43 | 0.069–0.072 | 0.11–0.16 | |||
| BCFS-e | 0.026 ± 0.014 | 0.038 ± 0.010 | 0.21 ± 0.26 | WD | 0.079 ± 0.055 | 0.19 ± 0.03 | |
| 0.027 | 0.033 | 0.26 | 0.088 | 0.19 | |||
| 0.009–0.042 | 0.031–0.045 | 0.02–0.39 | 0.040–0.12 | 0.17–0.22 | |||
| Zn | Cap | 140 ± 31 | 96 ± 25 | 140 ± 37 | 180 ± 27 | 120 ± 30 | 150 ± 35 |
| 140 | 89 | 140 | 180 | 130 | 130 | ||
| 120–190 | 65–150 | 86–200 | 140–240 | 71–170 | 99–200 | ||
| Stipe | 91 ± 21 | 56 ± 10 | 82 ± 25 | 130 ± 27 | 73 ± 13 | 83 ± 23 | |
| 95 | 52 | 81 | 130 | 73 | 80 | ||
| 66–120 | 45–73 | 46–140 | 95–193 | 57–93 | 49–130 | ||
| Soil PT | 14 ± 11 | 16 ± 6 | 14 ± 2 | 7.2 ± 4.1 | 15 ± 3 | 6.2 ± 0.5 | |
| 8.0 | 15 | 14 | 7.4 | 15 | 6.2 | ||
| 6.0–31 | 9.0–25 | 12–15 | 2.6–12 | 12–19 | 5.5–6.7 | ||
| Soil E | 7.3 ± 0.6 | 10 ± 3 | 8.2 ± 1.8 | 5.6 ± 4.2 | 15 ± 5 | 2.6 ± 0.2 | |
| 7.2 | 12 | 8.3 | 5.3 | 16 | 2.6 | ||
| 6.5–8.3 | 6.0–13 | 6.0–10 | 0.80–11 | 8.0–19 | 2.4–2.9 | ||
| BCFC-pt | 16 ± 8 | 10 ± 2 | 13 ± 0 | 18 ± 4 | 8.8 ± 5.4 | 21 ± 5 | |
| 20 | 10 | 13 | 18 | 8.3 | 23 | ||
| 4.0–24 | 9.0–12 | 13–13 | 16–21 | 5.0–13 | 17–25 | ||
| BCFS-pt | 9.7 ± 5.6 | 5.3 ± 0.1 | 6.2 ± 0.2 | 12 ± 3 | 5.1 ± 1.4 | 13 ± 2 | |
| 11 | 5.3 | 6.2 | 12 | 5.8 | 13 | ||
| 2.2–15 | 5.2–5.4 | 6.1–6.3 | 11–14 | 4.1–6.1 | 12–15 | ||
| BCFC-e | 22 ± 5 | 15 ± 12 | 23 ± 2 | 120 ± 150 | 11 ± 10 | 54 ± 6 | |
| 19 | 12 | 23 | 49 | 13 | 53 | ||
| 17–30 | 7.0–24 | 22–25 | 19–230 | 4.0–18 | 50–58 | ||
| BCFS-e | 13 ± 1 | 7.3 ± 4.6 | 11 ± 1 | 85 ± 100 | 6.0 ± 3.8 | 34 ± 0 | |
| 11 | 7.4 | 11 | 29 | 6.4 | 34 | ||
| 9.0–17 | 4.1–11 | 11–12 | 13–160 | 3.3–8.7 | 34–34 | ||
* Pooled samples (nubmer of individuals is given in parentheses). PT pseudo total, E extractable or leachable, WD without data. aBCFC-pt and aBCFS-pt (values of BCF respectively for caps and stipes calculated using data for the “pseudo-total” fraction of element in soil), bBCFC-e and bBCFS-e (values of BCF respectively for caps and stipes calculated using data for an extractable-leachable fraction of element in soil)
After collecting, mushrooms were cleaned up from debris of the litter and soil substrate. For each sample, caps and stipes (bottom part cut off) were separated from the fruiting bodies, sliced with a plastic knife, and dried at 65 °C for 24 h to constant mass using a commercial vegetable dehydrator with plastic trays (Falandysz et al. 2015). Dried caps and stipes were ground into a fine powder using a porcelain mortar, kept in air tight polyethylene bags, and stored under dry conditions.
Subsamples of 0.2–0.3 g of the fungal material were weighed into polytetrafluoroethylene (PTFE) pressure vessels and digested with 5 mL of concentrated nitric acid (HNO3) (Suprapure, Merck). After initial pre-digestion at room temperature and normal pressure for 24 h in semi-closed vessels, further digestion of the fungal material was achieved under pressure in an automatic digestion system (MLS 1200) in a microwave oven, and elements were determined by inductively coupled plasma-optical emission spectrometry (ICP-OES; Optima 2000 DV, Perkin-Elmer) with external standards using yttrium (20 mg L−1) (Brzostowski et al., 2011a, b). Mercury was determined by a validated method of cold vapor atomic absorption spectroscopy (CV-AAS) (Falandysz 1990).
Soil substratum samples (0–10 cm layer) underneath fruiting bodies were collected using plastic tools (knives and spoons) and were packed into sealed polyethylene bags. To obtain air-dried samples, opened polyethylene bags were covered with sheets of paper towels, kept in vertical position, and air-dried at room temperature for 16–18 weeks in the laboratory. Further, the soil samples were sieved through a 2-mm pore size plastic sieve and then stored until analysis for 4 to 8 weeks in brand new sealed polyethylene bags in a closed plastic box under dry conditions.
Before chemical analyses, topsoil samples were dried in an electric oven at 40 °C for 48 h. The extractable (labile) metallic elements from the soil samples (1.5 g) were extracted using 10 mL of 20% nitric acid solution (Suprapure, Merck) in open PTFE vessels that were gently heated up to 105 °C for 2 h (Kučak and Blanuša 1998). The pseudo-total metallic elements from the soil samples (1.0 g) were initially cold digested with 15 mL of concentrated nitric acid solution (65%, analytical grade; Suprapure, Merck) in Pyrex glass round bottom flasks for 16 h and further were hot digested for 2.5 h (Sastre et al. 2002). Analytical methods were validated and controlled as described in detail in previous articles (Brzostowski et al. 2009, 2011a, b). The statistical analyses were performed with the Statistica® program package (Statsoft, Inc.), using a nonparametric Mann-Whitney U test.
Results and discussion
Bioconcentration potential
The bioconcentration potential of a fungal species for the accumulation of elements is assessed using the bioconcentration factor (BCF) which is calculated as the quotient of concentration levels in the mushroom divided by the concentration in the topsoil (or other substrate). Depending on fungal species, its mycelium can penetrate into the surficial organic layer and also the deeper mineral soil, and determined BCF is always an estimated indication of elements uptake. In other words, the BCF relates to and assesses the potential of mycelia for the uptake of chemical element(s) from soil and soil solution and its accumulation in a fruiting body and its morphological parts (Brzostowski et al. 2011a). This idea is similar to the “dose-effect” concept by Paracelsus and relates to the total concentration of an element in a fruiting body (its chemical forms can be different depending on substrate, e.g., soil fractions and soil solution). Trace elements that are well absorbed by mycelium, both from geogenic and anthropogenic sources, include Ag, Cd, Cu, Hg, Rb, and Zn, and they can be very well bio-concentrated and reach high values of BCF (BCF > 1) (Falandysz and Danisiewicz 1995; Falandysz 2017; Li et al. 2016; Tyler 1982). Heavy metals (Cd, Hg, Pb, Ni) that originate from anthropogenic sources in polluted areas, may be well bio-concentrated or accumulated by some species while other elements are accumulated due to geogenic anomalies (Árvay et al. 2014; Barkan et al. 1998; Cejpková et al. 2016; Collin-Hansen et al. 2005; Kojta et al. 2015; Petkovšek and Pokorny, 2013). Other macro metallic elements that are in excess in soil substrata (e.g., Al, Ca, Fe) are excluded by the mycelia, and quantities accumulated in fruiting bodies are relatively low although they exceed the load of typical trace metallic elements (Ba, Co, Li, Ni, Sr). Pb, a widespread pollutant, also produces low BCF values in mushrooms (usually < 1) (Proskura et al. 2017).
The BCF value strongly depends on the determination method for the respective chemical element and its various chemical forms in soil and substrate. There are numerous methods, using different reagents, to determine the total, pseudo-total, or leachable content of a particular element (Cejpková et al. 2016; Davidson 2013; Lipka and Falandysz 2017; Kučak and Blanuša 1998; Proskura et al. 2017; Sastre et al. 2002). With the exception of Mn, the BCF values of the elements in A. muscaria were usually significantly higher for the labile than the “pseudo-total” fraction (Table 1). Mushrooms, both mycorrhizal and saprobic, are active participants in soil ecosystems; and through their filamentous structure, the hyphae are able to secrete organic acids and enzymes (Falandysz and Borovička 2013). They are able to modulate the pH in the vicinity of the hyphal filaments in order to better absorb inorganic compounds. The chelation and absorption features of a mushroom are species-specific and it is impossible to mimic them with chemical means.
Scarcity of some mineral nutrients or the requirement for another one may cause and accelerate co-absorption of other elements with similar chemical and physical characteristics although they are useless or toxic. Some elements can be co-associated for particular species or mushrooms from particular sites (e.g., Hg and Se) (Vogel-Mikus et al. 2016). Extensive studies from the northern hemisphere, where topsoil is usually slightly acidic, revealed that soil pH did not affect the bioconcentration and levels of Cd, Cu, Pb, and Zn (Gast et al. 1988) or Ag, Al, Ba, Ca, Cd, Co, Cr, Cu, Fe, Hg, K, Mg, Mn, Na, Ni, Sr, Pb, Rb, and Zn (Brzostowski et al. 2011a) in mushrooms. On the other hand, mushrooms may be able to adapt to small or geogenic concentrations of toxic elements, e.g., Ag, Cd, Hg, or Se via the formation of hardly soluble inorganic compounds (AgS, HgSe, HgS) and metalorganic complexes of Ag, Cd, Hg, and Se with firm bonds (Osobová et al. 2011; Schmitt and Meisch 1985; Vogel-Mikus et al. 2016).
Barium and strontium are similar chemically and physically to the essential element Ca, and all of them are weakly bioconcentrated (BCF around 1 or < 1) in mushrooms, e.g., (Brzostowski et al. 2011a); this is confirmed for our data on A. muscaria (Table 1). Barium and strontium in soil are in a form that is hardly soluble, and this can be one of the reasons for their weak bioconcentration by mushrooms. The same is the case for Ca which as a leachable fraction is relatively weakly bio-concentrated in caps and stipes of fruiting bodies. Its uptake can be in part dependent on co-occurrence of elements being in competition with Ca, such as Ba, Sr, and rare earth elements, or also due to possible chemical differences in Ca compounds in soil (Falandysz et al. 2017c). Ba and Sr from the highly weathered laterite read earths, red earths, and yellow earths of Yunnan in Asia, which are low in Ca, can be better bio-concentrated by mushrooms than from the European soils which are usually richer in Ca. Possibly, some of these species may have a preference for Ba and Sr from the laterite read earths, red earths, and yellow earths (Falandysz et al. 2017a, b; Saniewski et al. 2016). A. muscaria from the northern regions of Poland often bio-excluded Ba, Ca, and Sr by but also the metallic elements Ag, Al, Fe, Mn, and usually also Co (Table 1).
Fe and Al are both abundant in soils but commonly only minor in mushrooms (an exception for Fe is Suillus variegatus) (Drbal et al. 1975; Falandysz et al. 2001), and therefore, under typical environmental and soil conditions, these elements are bio-excluded by mushrooms. However, some doubts were raised about the accuracy of data reported earlier on Fe and Al in mushrooms (Borovička and Řanda, 2007).
Amanita muscaria, like all terrestrial mushrooms studied so far, was characterized by high BCF values for potassium (K). Fruiting bodies of mushrooms, e.g., A. muscaria, are physiologically rich in K, which is a major metallic cation (in this study median values of K in the caps were in the range of 36,000–53,000 mg kg−1 dm among the sites; Table 1). Also, parasitic mushrooms that develop rhizomorphs (mycelial cords), connecting soil, and infected plant, e.g., as is the case for some Armillaria species, are rich in K (48,000 ± 5700 mg kg−1 dm in the caps and 59,000 ± 40,000 mg kg−1 dm in the stipes) (Falandysz et al. 1992).
The median BCF values for leachable K by A. muscaria were in the range of 590–1400 for caps and 280–1200 for stipes, but substantially lower (in the ranges 82–150 and 34–110) for the “pseudo-total” fraction of K in soil. As expected, the values for hot extraction with concentrated (65%) nitric acid were much higher (medians in range 380–740 mg kg−1 dry matter, dm) than the values for cold-extraction with 20% nitric acid (medians in range 31–100 mg kg−1 dm) (Table 1).
The element Rb was even better bio-concentrated by A. muscaria than K, with an efficiency (median values of BCF) in the range of 670–2600 for caps and 110–1200 for stipes (leachable fraction), while the “pseudo-total” fraction was in the range of 130–220 for caps and 35–120 for stipes (Table 1). It is well known that Rb can be an abundant element in fruiting bodies of A. muscaria and in other mushrooms. For example, the median values of Rb were at 110 mg kg−1 dm in caps and 65 mg kg−1 dm in stipes of A. muscaria fruiting bodies (Drewnowska et al. 2013), and at 500 (21–1600) mg kg−1 dm in whole fruiting bodies of Amanita rubescens Pers. (Tyler 1982).
The fraction of magnesium in soils that was leachable with 20% nitric acid was easily taken up by A. muscaria and further bio-concentrated by its fruiting bodies in all the sites studied. The median BCF values calculated for leachable Mg in caps were in the range of 2.6–13 and for stipes in the range of 1.2–6.8. As expected, most of the Mg in the examined soils was in the pool of non-leachable, “pseudo-total” Mg (Table 1). “Pseudo-total” (and possible total) Mg, which is a geogenic metallic element in a top layer of forest soils, dominates and is less accessible for the mycelia. The median BCF values calculated for the “pseudo-total” Mg in A. muscaria from northern Poland were in the range of 0.65–2.7 for caps and in the range of 0.47–1.5 for stipes.
Among of the essential elements, the micro-constituents such as Cu and Zn were well bio-concentrated by A. muscaria in fruiting bodies, while less so for Na but still with a BCF > 1. The median BCF values for leachable Cu, Zn, and Na in caps were in the range, respectively, 53–160, 12–53, and 1.7–48, and for stipes were 18–79, 6.4–34, and 1.5–69 (Table 1). Also, the BCF values of Cu, Zn, and Na for the “pseudo-total” fraction were relatively high (usually > 1), i.e., the medians for caps and stipes respectively were in the ranges 18–130, 8.3–23, and 1.6–9.0 and 7.0–61, 5.3–13, and 0.98–40. This is discussed in detail below.
Macro elements in mushrooms
Potassium
The median concentrations of K in A. muscaria caps ranged from 36,000–53,000 mg kg−1 dry matter (dm) and from 19,000 to 38,000 mg kg−1 dm in stipes (Table 1). The caps were substantially richer in K than the stipes—the median values of the QC/S index (which was calculated in the fruiting bodies as the quotient of metallic element concentration in caps divided by the concentration in the stipes) were in the range 1.7–1.9 for five sites and at 2.3 in the Giżycko site. Potassium in whole fruiting bodies of A. muscaria was at 33000 ± 6000 mg kg−1 dm in a study by Vetter (2005). Mushrooms from two sites (Dziemiany and Bydgoska forest) showed potassium in a substantially greater concentration than for the Pasym site (p < 0.05; Man-Whitney U test). The potassium concentration in fruiting bodies of A. muscaria correlated positively with cadmium (r = 0.61; p < 0.05) and cobalt (r = 0.65; p < 0.05).
The Dziemiany site was also characterized by greater K content in topsoil—both the “pseudo-total” and labile fractions, i.e., the median values were respectively at 74,000 and 11,000 mg kg−1 dm. Elsewhere in this study, the range of median values for the “pseudo-total” fraction was at 33–60, and for the labile fraction it was at 31–91 mg kg−1 dm.
As was mentioned, K was efficiently bio-concentrated by A. muscaria in fruiting bodies. The median values of BCF for the caps differed significantly for the sites and for the labile fraction were in the range 1200–1400 at the sites of Sobieszewo Island, Giżycko, and Dziemiany, and much lower for the three other sites which ranged from 590 to 820.
Magnesium
In all six sites, A. muscaria fruiting bodies contained Mg in similar (p > 0.05) concentrations (Table 1). Medians for caps were at 850–1100 mg kg−1 dm, with a total range of 580–1300 mg kg−1 dm, and for stipes the medians were at 440–600 mg kg−1 dm, with a total range of 300–680 mg kg−1 dm. The median values of the index QC/S for magnesium were in the range of 1.7–1.9 in five sites and 2.3 in the Giżycko site.
The sites differed in concentration levels of Mg in topsoil. The “pseudo-total” fraction of magnesium was at 400 to 1200 mg g−1 dm (medians) and the labile fraction was in the range of 92–310 mg g−1 dm. Magnesium was bio-included by A. muscaria, and the median values of BCF for this element in caps were in the range of 0.65–2.7 for the “pseudo-total” fraction and in the range of 2.6–13 for the labile fraction, and in stipes respectively at 0.47–1.5 and 1.2–6.8.
Rubidium
Amanita muscaria contained Rb in the caps in the range (medians) from 110 to 470 mg kg−1 dm, while for the stipes those values were substantially smaller and ranged from 66 to 310 mg kg−1 dm (QC/S index at 1.8–2.7 in five sites and 4.0 in the Pasym site).
Topsoil contained Rb in the “pseudo-total” fraction in the range (medians) from 1.2 to 4.4 mg kg−1 dm and in a much smaller portion in the labile fraction (range 0.27–1.0 mg kg−1 dm). The concentration levels of the labile fraction of Rb in forest topsoil were significantly lower at two sites: the Sobieszewo Island site, which is under impact by aerosol from the Baltic Sea and the most eastern location of this study in the Giżycko site (medians respectively at 0.27 and 0.27 mg kg−1 dm and in other sites at 0.66–1.0 mg kg−1 dm; p < 0.05). Rubidium was highly bio-concentrated in fruiting bodies of A. muscaria, and the median BCF values for the labile fraction were in the range of 670–2600. Due to the large difference between the contents of the labile and “pseudo-total” fraction of Rb in topsoil substrata, the BCF values for the “pseudo-total” fraction were much lower than for the labile fraction (Table 1).
Calcium
The median values of Ca concentration in the caps were in the range of 84–160 mg kg−1 dm and were at similar or greater level in the stipes in particular sites (range 77–260 mg kg−1 dm; QC/S index at 0.61–1.4). The woodland areas investigated differed significantly in content both of the “pseudo-total” and the labile fraction of calcium in topsoil. The Darżlubska Wilderness site was richer both in the “pseudo-total” and the labile fraction of Ca (medians respectively at 1400 and 1500 mg kg−1 dm) than were other locations. The “pseudo-total” fraction of Ca was in the range of 190–720 mg kg−1 dm in other sites. The difference in the content of the labile fraction of Ca in topsoil substrata between the sites was even greater than for the “pseudo-total” fraction, e.g., as mentioned, the median value was at 1500 mg kg−1 dm in the Darżlubska site, and for the other four sites it was in the range 230–690 mg kg−1 dm, while the lowest value of 41 mg kg−1 dm was found in the Giżycko site.
The median values of BCF for leachable Ca from caps and stipes of A. muscaria were respectively in a wide range: 0.17–2.2 and 0.16–2.3, and for “pseudo-total” Ca were 0.20–0.55 and 0.11–0.61. The BCF values calculated for the leachable fraction of Ca in caps and stipes of A. muscaria showed on inverse association with the content of the element in soil. Such an association indicates a tendency to maintain Ca homeostasis in the fruiting bodies.
Soils from the sites examined also highly differed in Ca content (p < 0.01; Mann-Whitney U test). The median concentration values for the leachable fraction of Ca was as low as 41 mg kg−1 dm for the Giżycko site and as high as 1500 mg kg−1 dm for the Darżlubska Wilderness site in the region nearby to the town of Wejherowo.
The median Ca values in caps were in the range from 84 mg kg−1 dm (Dziemiany site in the region of Kaszuby) to 160 mg kg−1 dm (Darżlubska Wilderness). Stipes tend to provide a better matrix for Ca than caps in mature fruiting bodies as Ca concentrations in stipes are typically greater than in caps (Kułdo et al. 2014; Kojta et al. 2016). In this study, the median values of the QC/S index for Ca were in the range of 0.61–1.0 at four sites (Darżlubska Wilderness, Bydgoskie forests, Dziemiany, and Pasym) and in the range of 1.1–1.4 at two sites (Giżycko and Sobieszewo Island).
Sodium
A half or more of Na determined in topsoil was in the leachable fraction for the study sites: the medians ranged from 5.8 to 24 mg kg−1 dm, while the “pseudo-total” fraction was in the range of 12–31 mg kg−1 dm. The Na concentrations in the caps of A. muscaria were half or lower than the levels in stipes, and the medians for the sites were respectively in the range of 31–110 mg kg−1 dm and 41–370 mg kg−1 dm. The BCF of Na in A. muscaria exceeded 1 for all locations, with median values in the range of 1.7–48 for caps and 1.5–69 for stipes (Table 1).
Edible mushrooms collected from the wild or cultivated are considered as low sodium food items (Vetter 2003).
Trace elements
Manganese
The leachable fraction of Mn was relatively low in topsoil from the seaside region of Sobieszewo Island and also from the outskirts of the Giżycko site in the Great Lakes region of NE Poland, i.e., they were at 22 and 13 mg kg−1 dm (medians). Topsoil in other sites was richer in leachable Mn, with 44 mg kg−1 dm in the Bydgoska forests and in a range of 77–97 mg kg−1 dm for the other sites. Leachable Mn was at the same level as the “pseudo-total” fraction at four sites, while it was around 40% lower than the “pseudo-total” Mn in the Bydgoska forests and the Giżycko sites (Table 1).
Amanita muscaria bio-concentrated Mn with similar efficiency in caps and stipes of fruiting bodies. The median values of BCF for leachable Mn in caps were in the range of 0.13–1.0 and in stipes at 0.12–0.91, while for the “pseudo-total” fraction they were respectively in the ranges 0.13–0.56 and 0.11–0.51.
Aluminum and Iron
Forest soils showed the leachable fraction of Al in the range of 1100–6700 mg kg−1 dm (medians) and of Fe at 620–1900 mg kg−1 dm, while the “pseudo-total” fractions were respectively in the range of 4000–27,000 mg Al kg−1 dm and 1200–3400 mg Fe kg−1 dm. With a high content of Al and Fe in soil substrata and under the typically slightly acidic pH conditions of forest soils from the northern regions of Poland, mycelia usually highly restrict their uptake of Al and Fe (Brzostowski et al. 2011a). The median values of BCF both for Al and Fe in A. muscaria were much lower than 1 (Table 1). Nevertheless, Al and Fe, with median contents in the caps in the range of 68–300 mg Al kg−1 dm and 65–230 mg Fe kg−1 dm, and in the stipes with 34–220 mg Al kg−1 dm and 54–110 mg Fe kg−1 dm, were at the levels of Na, Rb, or Zn and higher than those of Cu (Table 1).
Copper and zinc
Leachable copper values, when based on chemical extraction in vitro, were the highest (around 80%) in topsoil from the Bydgoska forest site and at the lowest level (around 30%) at the Giżycko site. In four study sites, leachable and “pseudo-total” Cu concentrations were approximately equal (Table 1). Soils from the background areas of northern Poland in this study reflect the typical composition of the parent rock and are without known geochemical anomalies in the upper lithosphere, while soil types are diverse and display a mosaic-like pattern (www. 2017a). The situation is different in the region of the Świętokrzyskie Mountains in southcentral Poland and towards the south, where some minerals, the copper, zinc, lead, and iron ores are or were major resources (Brzezicha-Cirocka et al. 2016), as well as in southwestern Poland with a large Cu ore deposit in the Legnica region (in the European part of the Circum Pacific Mercuriferous Belt anomaly, and these deposits are enriched with associated elements (Gustin et al. 1999; www. 2017b).
The efficiency of Amanita muscaria Cu bioconcentration varied among the sites. The median BCF values for the leachable and “pseudo-total” fractions were respectively in the range of: 53–160 and 18–130 for caps, and 18–79 and 7.0–61 for stipes (Table 1). Leachable Cu median levels were in the range of 0.31–2.3 mg kg−1 dm and the “pseudo-total” at 0.52–4.4 mg kg−1 dm, while quantities sequestered in the fruiting bodies were in the range of 30–48 mg kg−1 dm for caps and 10–24 mg kg−1 dm for stipes.
Clearly, A. muscaria itself was a better extractor and regulator of Cu uptake and sequestration when compared to predictions based on the concept of the “leachable” fraction of Cu in soil that is extracted by diluted (20%) nitric acid (Table 1).
The requirement of A. muscaria for Zn is greater than for Cu. Median quantities of Zn determined in mushrooms were in the ranges of 89–180 mg kg−1 dm for caps and 52–130 mg kg−1 dm for stipes. In the three sites of Sobieszewo Island, Darżlubska Wilderness, and Pasym, almost 90% of Zn was in the leachable fraction (median concentration levels between 7.2–16 mg kg−1 dm for the leachable fraction and 8.0–15 mg kg−1 dm for the “pseudo-total”) while in smaller proportion for the other sites (median concentration levels between 2.6–8.3 mg kg−1 for leachable Zn and 6.2–14 mg kg−1 for the “pseudo-total”; Table 1). In this aspect, certain sites were statistically different (0.01 < p < 0.05). Hence, zinc was easier available for mushroom at the Sobieszewo Island, Darżlubska Wilderness, and Pasym sites—with median BCF in the range of 12–19 for caps and 6.4–11 for stipes, while less available at the Dziemiany, Bydgoska forests, and Giżycko sites—with median BCF in the range of 23–53 for caps and 11–34 for stipes (Table 1).
Cobalt
Topsoil Co concentrations were in the range of 0.23–1.2 mg kg−1 dm for the leachable fraction and slightly higher, i.e., in the range of 0.39–1.5 mg kg−1 dm for the “pseudo-total” fraction.
Cobalt in the lithosphere is associated with Fe and Mn and under oxidizing acidic conditions is considered as a relatively mobile metallic element, but its migration can be inhibited when adsorbed onto hydroxides of Fe and Mn (Kabata-Pendias and Pendias 1999). A high ratio of the labile to the “pseudo-total” fraction of Co in forest topsoils in this study suggested a high mobility. On the other hand, the concentration levels of Co in this study seem typical for sandy soils in Poland and were in the range of 0.1–1.2 mg kg−1 dm, while at 0.2–34 mg kg−1 dm in organic soils (mean 3 mg kg−1 dm), at 5.5–19 mg kg−1 dm (mean 10.8 mg kg−1 dm) in alluvial soils and at 4–29 mg kg−1 dm (mean 3 mg kg−1 dm) in clayey soils (Kabata-Pendias and Pendias 1999).
The median values of Co in caps were in the range of 0.15–0.44 mg kg−1 dm and in stipes were at 0.13–0.37 mg kg−1 dm, i.e., the distribution between the caps and stipes of the fruiting bodies was largely equal, while the concentration levels were relatively low. Vetter (2005) reported, as determined by inductively coupled plasma-mass spectrometry, on a greater content of Co in whole fruiting bodies of A. muscaria at 1.4 ± 1.3 mg kg−1 dm, and for six other Amanita species, the values ranged from a non-detectable level (< 0.05 mg kg−1 dm) to 1.8 ± 4.0 mg kg−1 dm in A. phalloides (Fr.) Link. A. fulva Fr., a species which was not studied by Vetter (2005), was collected from a forest of different soil parent material in Poland and contained Co in the caps in the range of 0.028 ± 0.001–0.098 ± 0.061 mg kg−1 dm and in the stipes at 0.050 ± 0.001–0.11 ± 0.08 mg kg−1 dm, as determined by inductively coupled plasma-mass spectroscopy with dynamic reactive cells (ICP-DRC-MS) (Falandysz et al. 2017a). Cantharellus cibarius Fr., sampled from several sites in Poland and examined by ICP-DRC-MS, contained the element in fruiting bodies at the median level of 0.56 mg kg−1 dm but Co was much lower, i.e., from 0.046 to 0.076 mg kg−1 dm in Cantharellus tubaeformis Fr. (Falandysz et al. 2017b). Hence, species-specific Co accumulation of fruiting bodies is likely to account for the differences found in mushroom species that grow under the same forest soil conditions.
Amanita muscaria bio-excluded Co at three sites (Darżlubska Wilderness, Dziemiany, and Giżycko) where the median values of BCF for the leachable fraction were < 1, while they were in the range of 1.2–4.8 for caps and 1.1–3.0 for stipes at the three other sites (Island of Sobieszewo, Bydgoska forests, and Pasym) (Table 1). In another study, Amanita fulva also bio-excluded Co (BCF around 0.05) (Falandysz et al. 2017a).
Barium and strontium
Barium and strontium were closely correlated in fruiting bodies of A. muscaria in this study (r = 0.82; p < 0.01). Both Ba and Sr positively correlated with Ca (p < 0.05; Student’s t test) in the examined A. muscaria. The median values of Ba contents were in the ranges of 0.51–1.6 mg kg−1 dm in caps and 0.40–1.6 mg kg−1 dm in stipes, and of Sr were in the range of 0.19–0.69 and 0.20–0.68 mg kg−1 dm (Table 1).
As mentioned, both Ba and Sr were characterized by very low BCF values for the caps and stipes (BCF much lower than 1). The median values of Ba content in soils were in the range of 34–120 and of Sr at 7.4–17 mg kg−1 dm in the leachable fraction while at 49–150 and 15–34 for the “pseudo-total” fraction.
Toxic elements
Cadmium, mercury, and silver
The elements Cd, Hg, and Ag were minor constituents in forest topsoils from the background areas in this study, i.e., the median values for leachable Cd were in the range of 0.006–0.10 mg kg−1 dm and for the “pseudo-total” Cd at 0.013–0.086 mg kg−1 dm; total Hg was at 0.010–0.051 mg kg−1 dm, and the “pseudo-total” Ag was at 1.4–5.0 mg kg−1 dm (four sites) (Table 1).
As was mentioned, Cd and Hg were well bio-concentrated by A. muscaria, but Ag only weakly. The median values of BCF for leachable Cd ranged from 270 to 2700 (caps) and from 130 to 1200 (stipes). For total Hg, they were in the range of 17–51 (caps) and 10–28 (stipes). Hence, both for Cd and Hg, A. muscaria plays a role in their cycling between deeper strata and the surface soil in a forest ecosystem. As assessed from the data for the “pseudo-total” fraction, silver was rather weakly bio-concentrated by A. muscaria at some sites and was bio-excluded in others. The median value of BCF for Ag in caps was 1.8 at the Dziemiany site and was in the range of 0.49–0.87 elsewhere, while for stipes, it was in the range of 0.38–0.52 at all the sites with data (Table 1).
The elements Cd, Hg, and Ag are closely correlated and are strong chalcophiles. They form compounds in mushrooms via bonds with thiols in glutathione and metallothionein-like polypeptides (Cd, Ag, Hg), bonds with phosphoglycoprotein (Cd) where sulfur is absent, while some can occur as organometallic compounds such as methylmercury (MeHg), in thiols in larger organic molecule such as glutathione, cysteine, and thiol (-SH) rich proteins or in inorganic compounds, e.g., HgS, SeHg in the cytosol and its structures and in the cell walls in general (Fischer et al. 1995; Vogel-Mikus et al. 2016; Kavčič et al. 2018; Kruse and Lommel 1979; Osobová et al. 2011; Schmitt and Meisch 1985).
Some Amanita species from the section Lepidella bio-concentrate (hyperaccumulate) Ag with high efficiency, e.g., Amanita strobiliformis (Paulet ex Vittad.) Bertill. (Borovička et al. 2007). Mercury, as can be concluded from the studies performed so far on several Amanita species that grew under typical environmental conditions, was bio-concentrated by mycelium in fruiting bodies with similar efficiency in A. muscaria, A. rubescens, A. fulva, and A. vaginata (Bull.) Lam. (Drewnowska et al. 2014; Drewnowska et al. 2012a, b; Falandysz et al. 2007b; Falandysz and Drewnowska 2015; Lipka and Falandysz 2017; Nasr and Arp 2011; Nasr et al. 2011). Studies of Amanita echinocephala (Vittad.) Quél., A. manginiana Hariot et Patouillard, and A. vaginata from Yunnan soils with elevated geogenic Hg revealed a mercury accumulation at concentration levels in the range of 2.9–7.3 mg kg−1 dm (caps) and at 1.8–4.2 mg kg−1 dm (stipes) (Falandysz et al. 2016).
Both Cd and Pb form weaker bonds with biomolecules when compared to Ag, which is a highly reactive cation in contact with peptides or proteins. The Ag ion is highly toxic for unicellular organisms and lower trophic web aquatic biota but also in general for the proteins in cells. Some mushroom species accumulate Ag with high efficiency and have elevated Ag levels, but nothing is known of the associated risk for consumers of these mushrooms (Byrne and Tušek-Žnidarič 1990; Borovicka et al. 2010; Falandysz et al. 1994a, b).
Conclusions
Amanita muscaria growing in soils with different levels of the geogenic metallic elements (Ag, Al, Ba, Ca, Co, Cu, Fe, Hg, K, Mg, Mn, Na, Rb, Sr, and Zn) showed signs of homeostatic accumulation in fruiting bodies of several of these elements, while Cd appeared to be accumulated at a rate dependent of the concentration level in the soil substrate. This species is an efficient bio-concentrator of K, Mg, Cd, Cu, Hg, Rb, and Zn and hence also contributes to the natural cycling of these metallic elements in forest ecosystems.
Acknowledgements
Technical support by Michał Bajerowicz, Ilona Bochentin, Izabela Domin, Celina Gołacka, Krzysztof Lipka, Alina Pękacka and Kamila Strupińska is acknowledged.
References
- Árvay J, Tomáša J, Hauptvogl M, Kopernická M, Kováčik A, Bajčan D, Massányi P. Contamination of wild-grown edible mushrooms by heavy metals in a former mercury-mining area. J Environ Sci Health B. 2014;49:815–827. doi: 10.1080/03601234.2014.938550. [DOI] [PubMed] [Google Scholar]
- Barcan VS, Kovnatsky EF, Smetannikova MS. Absorption of heavy metals in wild berries and edible mushrooms in an area affected by smelter emissions. Water Air Soil Pollut. 1998;103:173–195. doi: 10.1023/A:1004972632578. [DOI] [Google Scholar]
- Biekman ESA, Kroese-Hoedeman HI, Schijven HPHM. Loss of solutes during blanching of mushrooms (Agaricus bisporus) as a result of shrinkage and extraction. J Food Eng. 1996;28:139–152. doi: 10.1016/0260-8774(95)00030-5. [DOI] [Google Scholar]
- Borovička J, Řanda Z. Distribution of iron, cobalt, zinc and selenium in macrofungi. Mycol Prog. 2007;6:249–259. doi: 10.1007/s11557-007-0544-y. [DOI] [Google Scholar]
- Borovička J, Řanda Z, Jelínek E, Kotrba P, Dunn CE. Hyperaccumulation of silver by Amanita strobiliformis and related species of the section Lepidella. Mycol Res. 2007;111:1339–1344. doi: 10.1016/j.mycres.2007.08.015. [DOI] [PubMed] [Google Scholar]
- Borovička J, Dunn CE, Gryndler M, Mihaljevič M, Jelínek E, Rohovec J, Rohoškoná M, Řanda Z. Bioaccumulation of silver in macrofungi and ectomycorrhizae from the vicinity of the Morsko gold deposit, Czech Republic. Soil Biol Biochem. 2010;42:83–91. doi: 10.1016/j.soilbio.2009.10.003. [DOI] [Google Scholar]
- Byrne AR, Tušek-Žnidarič M. Studies on the uptake and binding of trace metals in fungi. Part I. Accumulation and characterization of mercury and silver in the cultivated mushroom, Agaricus bisporus. Appl Organomet Chem. 1990;4:43–48. doi: 10.1002/aoc.590040108. [DOI] [Google Scholar]
- Brzezicha-Cirocka J, Mędyk M, Falandysz J, Szefer P. Bio- and toxic elements in edible wild mushrooms from two regions of potentially different environmental conditions in eastern Poland. Environ Sci Pollut Res. 2016;23:21517–21522. doi: 10.1007/s11356-016-7371-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brzostowski A, Bielawski L, Orlikowska A, Plichta S, Falandysz J. Instrumental analysis of metals profile in Poison Pax (Paxillus involutus) collected at two sites in Bory Tucholskie. Chem Anal (Warsaw) 2009;54:907–919. [Google Scholar]
- Brzostowski A, Falandysz J, Jarzyńska G, Zhang D. Bioconcentration potential of metallic elements by oison Pax (Paxillus involutus) mushroom. J Environ Sci Health A. 2011;46:378–393. doi: 10.1080/10934529.2011.542387. [DOI] [PubMed] [Google Scholar]
- Brzostowski A, Jarzyńska G, Kojta AK, Wydmańska D, Falandysz J. Variations in metal levels accumulated in Poison Pax (Paxillus involutus) mushroom collected at one site over four years. J Environ Sci Health A. 2011;46:581–588. doi: 10.1080/10934529.2011.562827. [DOI] [PubMed] [Google Scholar]
- Cejpková J, Gryndler M, Hršelová H, Kotrba P, Řanda Z, Borovička J. Bioaccumulation of heavy metals, metalloids, and chlorine in ectomycorrhizae from smelter-polluted area. Environ Pollut. 2016;218:176–185. doi: 10.1016/j.envpol.2016.08.009. [DOI] [PubMed] [Google Scholar]
- ChemSpider (2017) ChemSpider Search and share bbfcgchemistry. Royal Society of Chemistry. http://www.chemspider.com/Chemical-Structure.4116.html# (retrived on March 11, 2017)
- Collin-Hansen C, Andersen RA, Steinnes E. Damage to DNA and lipids in Boletus edulis exposed to heavy metals. Mycol Res. 2005;109:1386–1396. doi: 10.1017/S0953756205004016. [DOI] [PubMed] [Google Scholar]
- Davidson ChM (2013) Methods for the determination of heavy metals and metalloids in soils. pp 97–140. In Heavy metals in soils: Trace metals and metalloids in soils and their bioavailability. Environ Pollut 22, Springer Science + Business Media, Dordrecht
- Drbal K, Kalač P, Šeflová A, Šefl J. Iron and manganese content in some edible macrofungi (In Czech) Czech Mycol. 1975;29:110–114. [Google Scholar]
- Drewnowska M, Jarzyńska G, Kojta AK. Mercury in European blusher, Amanita rubescens, mushroom and soil. Bioconcentration potential and intake assessment. J Environ Sci Health B. 2012;47:466–474. doi: 10.1080/03601234.2012.663609. [DOI] [PubMed] [Google Scholar]
- Drewnowska M, Jarzyńska G, Sąpór A, Nnorom IC, Sajwan KS, Falandysz J. Mercury in Russula mushrooms: bioconcentration by yellow-ocher brittle gills Russula ochroleuca. J Environ Sci Health A. 2012;47:1577–1591. doi: 10.1080/10934529.2012.680420. [DOI] [PubMed] [Google Scholar]
- Drewnowska M, Lipka K, Jarzyńska G, Danisiewicz-Czupryńska D, Falandysz J. Investigation on metallic elements of fly agaric, Amanita muscaria, fungus and the forest soils from the Mazurian Lakes District of Poland. Fresenius Environ Bull. 2013;22:455–460. [Google Scholar]
- Drewnowska M, Nnorom IC, Falandysz J. Mercury in the Tawny Grisette, Amanita vaginata Fr. and soil below the fruiting bodies. J Environ Sci Health B. 2014;49:521–526. doi: 10.1080/03601234.2014.896677. [DOI] [PubMed] [Google Scholar]
- Drewnowska M, Falandysz J, Chudzińska M, Hanć A, Saba M, Barałkiewicz D. Leaching of arsenic and sixteen metallic elements from Amanita fulva mushrooms after food processing. LWT Food Sci Technol. 2017;84:861–866. doi: 10.1016/j.lwt.2017.04.066. [DOI] [Google Scholar]
- Drewnowska M, Hanc A, Barałkiewicz D, Falandysz J. Pickling of chanterelle Cantharellus cibarius mushrooms highly reduce cadmium contamination. Environ Sci Pollut Res. 2017;24:21733–21738. doi: 10.1007/s11356-017-9819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DRRiP – Departament Rozwoju Regionalnego i Przestrzennego (2000) http://pomorskie.eu/-/gleby-w-wojewodztwie-pomorskim. (retrieved 14 March 2017)
- Falandysz J. Mercury content of squid Loligo opalescens. Food Chem. 1990;38(3):171–177. doi: 10.1016/0308-8146(90)90191-6. [DOI] [Google Scholar]
- Falandysz J. Mercury accumulation of three Lactarius mushroom species. Food Chem. 2017;214:96–101. doi: 10.1016/j.foodchem.2016.07.062. [DOI] [PubMed] [Google Scholar]
- Falandysz J, Borovička J. Macro and trace mineral constituents and radionuclides in mushrooms: health benefits and risks. Appl Microbiol Biotechnol. 2013;97:477–501. doi: 10.1007/s00253-012-4552-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falandysz J, Danisiewicz D. Bioconcentration factors (BCF) of silver in wild Agaricus campestris. Bull Environ Contam Toxicol. 1995;55:122–129. doi: 10.1007/BF00212398. [DOI] [PubMed] [Google Scholar]
- Falandysz J, Drewnowska M. Distribution of mercury in Amanita fulva (Schaeff.) Secr. mushrooms: accumulation, loss in cooking and dietary intake. Ecotoxicol Environ Saf. 2015;115:49–54. doi: 10.1016/j.ecoenv.2015.02.004. [DOI] [PubMed] [Google Scholar]
- Falandysz J, Drewnowska M. Cooking can decrease mercury contamination of a mushroom meal: Cantharellus cibarius and Amanita fulva. Environ Sci Pollut Res. 2017;24:13352–13357. doi: 10.1007/s11356-017-8933-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falandysz J, Sicińska B, Bona H, Kohnke D. Metale w opieńce miodowej Armillariella mellea. Bromatol Chem Toksykol. 1992;25:71–176. [Google Scholar]
- Falandysz J, Bona H, Danisiewicz D. Silver content of wild-grown mushrooms from northern Poland. Z Lebensm Unters Forsch. 1994;199:222–224. doi: 10.1007/BF01193449. [DOI] [PubMed] [Google Scholar]
- Falandysz J, Bona H, Danisiewicz D. Silver uptake by champignons Agaricus bisporus from artificially enriched substrate. Z Lebensm Unters Forsch. 1994;199:225–228. doi: 10.1007/BF01193450. [DOI] [PubMed] [Google Scholar]
- Falandysz J, Szymczyk K, Ichihashi H, Bielawski L, Gucia M, Frankowska A, Yamasaki S. ICP/MS and ICP/AES elemental analysis (38 elements) of edible wild mushrooms growing in Poland. Food Addit Contam. 2001;18:503–513. doi: 10.1080/02652030119625. [DOI] [PubMed] [Google Scholar]
- Falandysz J, Lipka K, Kawano M, Brzostowski A, Dadej M, Jędrusiak A, Puzyn T. Mercury content and its bioconcentration factors in wild mushrooms at Łukta and Morąg, northeastern Poland. J Agric Food Chem. 2003;51:2832–2836. doi: 10.1021/jf026016l. [DOI] [PubMed] [Google Scholar]
- Falandysz J, Kunito T, Kubota R, Lipka K, Mazur A, Falandysz JJ, Tanabe S. Selected elements in fly agaric Amanita muscaria. J Environ Sci Health A. 2007;42:1615–1623. doi: 10.1080/10934520701517853. [DOI] [PubMed] [Google Scholar]
- Falandysz J, Lipka K, Mazur A. Mercury and its bioconcentration factors in fly agaric (Amanita muscaria) from spatially distant sites in Poland. J Environ Sci Health A. 2007;42:1625–1630. doi: 10.1080/10934520701517879. [DOI] [PubMed] [Google Scholar]
- Falandysz J, Zhang J, Wang Y, Krasińska G, Kojta A, Saba M, Shen T, Li T, Liu H. Evaluation of the mercury contamination in mushrooms of genus Leccinum from two different regions of the world: accumulation, distribution and probable dietary intake. Sci Total Environ. 2015;537:470–478. doi: 10.1016/j.scitotenv.2015.07.159. [DOI] [PubMed] [Google Scholar]
- Falandysz J, Saba M, Liu H-G, Li T, Wang J-P, Wiejak A, Zhang J, Wang Y-Z, Zhang D (2016) Mercury in forest mushrooms and topsoil from the Yunnan highlands and the subalpine region of the Minya Konka summit in the Eastern Tibetan Plateau. Environ Sci Pollut Res 23(23):23730–23741 [DOI] [PMC free article] [PubMed]
- Falandysz J, Drewnowska M, Chudzińska M, Barałkiewicz D. Accumulation and distribution of metallic elements and metalloids in edible Amanita fulva mushrooms. Ecotoxicol Environ Saf. 2017;137:265–271. doi: 10.1016/j.ecoenv.2016.12.014. [DOI] [PubMed] [Google Scholar]
- Falandysz J, Chudzińska M, Barałkiewicz D, Drewnowska M, Hanć A. Toxic elements and bio-metals in Cantharellus mushrooms from Poland and China. Environ Sci Pollut Res. 2017;24:11472–11482. doi: 10.1007/s11356-017-8554-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falandysz J, Sapkota A, Mędyk M, Feng X. Rare earth elements in parasol mushroom Macrolepiota procera. Food Chem. 2017;221:24–28. doi: 10.1016/j.foodchem.2016.10.047. [DOI] [PubMed] [Google Scholar]
- Feeney K. Revisiting Wasson’s Soma: exploring the effects of preparation on the chemistry of Amanita muscaria. J Psychoactive Drugs. 2010;42:499–506. doi: 10.1080/02791072.2010.10400712. [DOI] [PubMed] [Google Scholar]
- Fischer RG, Rapsomanikis S, Andreae MO, Baldi F. Bioaccumulation of methylmercury and transformation of inorganic mercury by macrofungi. Environ Sci Technol. 1995;29:993–999. doi: 10.1021/es00004a020. [DOI] [PubMed] [Google Scholar]
- Gast CH, Jansen E, Bierling J, Haanstra L. Heavy metals in mushrooms and their relationship with soil characteristics. Chemosphere. 1988;17:789–799. doi: 10.1016/0045-6535(88)90258-5. [DOI] [Google Scholar]
- Gustin MS, Lindberg S, Marsik F, Casimir A, Ebinghaus R, Edwards G, Hubble-Fitzgerald C, Kemp R, Kock H, Leonard T, London J, Majewski M, Montecinos C, Owens J, Pilote M, Poissant L, Rasmussen P, Schaedlich F, Schneeberger D, Schroeder W, Sommar J, Turner R, Vette A, Wallschlaeger D, Xiao Z, Zhang H. Nevada STORMS project: measurement of mercury emissions from naturally enriched surfaces. J Geophys Res. 1999;104:21831–21844. doi: 10.1029/1999JD900351. [DOI] [Google Scholar]
- http (2016) https://en.wikipedia.org/wiki/Amanita_muscaria#Siberia (retrieved on January 18, 2018)
- Kabata-Pendias A, Pendias H. Biogeochemia pierwiastków śladowych. Warszawa: Wydawnictwo Naukowe PWN; 1999. [Google Scholar]
- Kavčič A, Petric M, Vogel-Mikuš K. Chemical speciation using high energy resolution PIXE spectroscopy in the tender X-ray range. Nucl Instrum Methods Phys Res Sect B. 2018;417:65–69. doi: 10.1016/j.nimb.2017.06.009. [DOI] [Google Scholar]
- Kojta AK, Wang Y, Zhang J, Li T, Saba M, Falandysz J. Mercury contamination of Fungi genus Xerocomus in the Yunnan Province in China and the region of Europe. J Environ Sci Health Part A. 2015;50:1342–1350. doi: 10.1080/10934529.2015.1059108. [DOI] [PubMed] [Google Scholar]
- Kojta AK, Gucia M, Krasińska G, Saba M, Nnorom IC, Falandysz J. Mineral constituents of edible field parasol (Macrolepiota procera) mushrooms and the underlying substrate from upland regions of Poland: bioconcentration potential, intake benefits, and toxicological risk. Pol J Environ Stud. 2016;25:2445–2460. doi: 10.15244/pjoes/62997. [DOI] [Google Scholar]
- Kruse H, Lommel A. Untersuchungen über cadmiumbindende Proteine im Schaf-Champignon (Agaricus arvensis Schff. ex Fr.) Z Lebensm Untrers Forsch. 1979;168:444–447. doi: 10.1007/BF01479258. [DOI] [PubMed] [Google Scholar]
- Kučak A, Blanuša M. Comparison of two extraction procedures for determination of trace metals in soil by atomic absorption spectrometry. Arh Hig Rada Toksikol. 1998;49:327–334. [PubMed] [Google Scholar]
- Kułdo E, Jarzyńska G, Gucia M, Falandysz J. Mineral constituents of edible parasol mushroom Macrolepiota procera (Scop. ex Fr.) Sing and soils beneath its fruiting bodies collected from a rural forest area. Chem Pap. 2014;68:484–492. [Google Scholar]
- Lepp NW, Harrison SCS, Morrell BG. A role for Amanita muscaria L. in the circulation of cadmium and vanadium in non-polluted woodland. Environ Geochem Health. 1987;9:61–64. doi: 10.1007/BF02057276. [DOI] [PubMed] [Google Scholar]
- Li Q, Li S, Huang W, Liu C, Xiong C, Zheng L. Mineral constituents of a prized edible mushroom (Tricholoma matsutake) and soils beneath the fruiting bodies from the production areas across China. J Mt Sci. 2016;13:2046–2052. doi: 10.1007/s11629-015-3568-9. [DOI] [Google Scholar]
- Lipka K, Falandysz J. Accumulation of metallic elements by Amanita muscaria from rural lowland and industrial upland regions. J Environ Sci Health B. 2017;52:184–190. doi: 10.1080/03601234.2017.1261547. [DOI] [PubMed] [Google Scholar]
- Lipka K, Saba M, Falandysz J (2018) Preferential accumulation of inorganic elements in Amanita muscaria from North-eastern Poland. J Environ Sci Health Part A 53; in press, 10.1080/10934529.2018.1470805, 2018 [DOI] [PubMed]
- Lumpert M, Kreft S. Catching flies with Amanita muscaria: traditional recipes from Slovenia and their efficacy in the extraction of ibotenic acid. J Ethnopharmacol. 2016;187:1–8. doi: 10.1016/j.jep.2016.04.009. [DOI] [PubMed] [Google Scholar]
- Marley G (2010) Chanterelle Dreams, Amanita Nightmares: The Love, Lore, and Mystique of Mushrooms. Chelsea Green Publishing. White River Junction, Vermont, USA
- Michelot D, Melendez-Howell M. Amanita muscaria: chemistry, biology, toxicology and ethnomycology. Mycol Res. 2003;107:131–146. doi: 10.1017/S0953756203007305. [DOI] [PubMed] [Google Scholar]
- Mikaszewska-Sokolewicz MA, Pankowska S, Janiak M, Pruszczyk P, Lazowski T, Jankowski K. Coma in the course of severe poisoning after consumption of red fly agaric (Amanita muscaria) Acta Biochim Pol. 2016;63:181–182. doi: 10.18388/abp.2015_1170. [DOI] [PubMed] [Google Scholar]
- Nasr M, Arp PA. Hg concentrations and accumulations in fungal fruiting bodies, as influenced by forest soil substrates and moss carpets. Appl Geochem. 2011;26:1905–1917. doi: 10.1016/j.apgeochem.2011.06.014. [DOI] [Google Scholar]
- Nasr M, Malloch DW, Arp PA. Quantifying Hg within ectomycorrhizal fruiting bodies from emergence to senescence. Fungal Biol. 2011;116:1163–1177. doi: 10.1016/j.funbio.2012.09.002. [DOI] [PubMed] [Google Scholar]
- Ogawa Y, Sato H, Yamamoto M, Tada H, Hashimoto T. Burst suppression electroencephalogram with mushroom poisoning, Amanita pantherina. Epilepsy Behav Case Rep. 2015;4:82–83. doi: 10.1016/j.ebcr.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osobová M, Urban V, Jedelský PL, Borovička J, Gryndler M, Ruml T, Kotrba P. Three metallothionein isoforms and sequestration of intracellular silver in the hyperaccumulator Amanita strobiliformis. New Phytol. 2011;90:916–926. doi: 10.1111/j.1469-8137.2010.03634.x. [DOI] [PubMed] [Google Scholar]
- Petkovšek SAS, Pokorny B. Lead and cadmium in mushrooms from the vicinity of two large emission sources in Slovenia. Sci Total Environ. 2013;443:944–954. doi: 10.1016/j.scitotenv.2012.11.007. [DOI] [PubMed] [Google Scholar]
- Proskura N, Podlasińska J, Skopicz-Radkiewicz L. Chemical composition and bioaccumulation ability of Boletus badius (Fr.) Fr. collected in western Poland. Chemosphere. 2017;168:106–111. doi: 10.1016/j.chemosphere.2016.10.003. [DOI] [PubMed] [Google Scholar]
- Phipps AG, Bennett BC, Downum KR. Japanese use of Beni-tengu-dake (Amanita muscaria) and the efficacy of traditional detoxification methods. Miami, Florida: Florida International University; 2000. [Google Scholar]
- Reid DA, Eicker A. South African fungi: the genus Amanita. Mycol Res. 1991;95:80–95. doi: 10.1016/S0953-7562(09)81364-6. [DOI] [Google Scholar]
- Rubel W, Arora D. A study of cultural bias in field guide determinations of mushroom edibility using the iconic mushroom, Amanita muscaria, as an example. Econ Bot. 2008;62:223–243. doi: 10.1007/s12231-008-9040-9. [DOI] [Google Scholar]
- Saniewski M, Zalewska T, Krasińska G, Szylke N, Wang Y, Falandysz J. 90Sr in King Bolete Boletus edulis and certain other mushrooms consumed in Europe and China. Sci Total Environ. 2016;543:287–294. doi: 10.1016/j.scitotenv.2015.11.042. [DOI] [PubMed] [Google Scholar]
- Sastre J, Sahuquillo A, Vidal M, Rauret G. Determination of Cd, Cu, Pb and Zn in environmental samples: microwave-assisted total digestion versus aqua regia and nitric acid digestion. Anal Chim Acta. 2002;462:59–72. doi: 10.1016/S0003-2670(02)00307-0. [DOI] [Google Scholar]
- Schmitt JA, Meisch HU. Cadmium in mushrooms—distribution, growth effects, and binding. Trace Elem Med. 1985;2:163–166. [Google Scholar]
- Stefanović V, Trifković J, Mutić J, Tešić Ž. Metal accumulation capacity of parasol mushroom (Macrolepiota procera) from Rasina region (Serbia) Environ Sci Pollut Res. 2016;23:13178–13190. doi: 10.1007/s11356-016-6486-7. [DOI] [PubMed] [Google Scholar]
- Širić I, Kasap A, Bedeković D, Falandysz J. Lead, cadmium and mercury contents and bioaccumulation potential of wild edible saprophytic and ectomycorrhizal mushrooms, Croatia. J Environ Sci Health B. 2017;52:156–165. doi: 10.1080/03601234.2017.1261538. [DOI] [PubMed] [Google Scholar]
- Tsujikawa K, Mohri H, Kuwayama K, Miyaguchi H, Iwata Y, Gohda A, Fukushima S, Inoue H, Kishi T. Analysis of hallucinogenic constituents in Amanita mushrooms circulated in Japan. Forensic Sci Int. 2006;164:172–178. doi: 10.1016/j.forsciint.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Tyler G. Accumulation and exclusion of metals in Collybia peronata and Amanita rubescens. Trans Br Mycol Soc. 1982;79:239–245. doi: 10.1016/S0007-1536(82)80109-5. [DOI] [Google Scholar]
- Vendramin A, Brvar M. Amanita muscaria and Amanita pantherina poisoning: two syndromes. Toxicon. 2014;90:269–272. doi: 10.1016/j.toxicon.2014.08.067. [DOI] [PubMed] [Google Scholar]
- Vetter J. Data on sodium content of common edible mushrooms. Food Chem. 2003;81:589–593. doi: 10.1016/S0308-8146(02)00501-0. [DOI] [Google Scholar]
- Vetter J. Mineral composition of basidiomes of Amanita species. Mycol Res. 2005;109:746–750. doi: 10.1017/S0953756205002455. [DOI] [PubMed] [Google Scholar]
- Vogel-Mikuš K, Debeljak M, Kavčič A, Murn T, Arčon I, Kodre A, van Elteren JT, Budič B, Kump P, Mikuš K, Migliori A, Czyzycki M, Karydas A (2016) Localization and bioavailability of mercury and selenium in edible mushrooms Boletus edulis and Scutiger pes caprae. Abstract book of the 18th international conference on heavy metals in the environment, 12 to 15 September 2016, Ghent, Belgium, pp 611–612
- Wasson RG. Soma: divine mushroom of immortality. New York: Harcourt Brace Jovanovich; 1968. [Google Scholar]
- www (2017a) http://geografia_liceum.republika.pl/pol_gleby.htm (retrieved on March 25, 2017
- www (2017b) http://www.infomine.com/library/publications/docs/InternationalMining/ Chadwick2009aa.pdf (retrieved on March 23, 2017)