Abstract
Aim
Planar myocardial 123I-meta-iodobenzylguanidine (123I-mIBG) scintigraphy is a highly reproducible technique. However, differences in collimator use are one of the most important factors that cause variation among institutions and studies in heart-to-mediastinum (H/M) ratio. Therefore, standardization among various gamma camera-collimator combinations is needed. Previously, a phantom has been developed to cross-calibrate different acquisition conditions in Japan. For further cross-calibration of European myocardial 123I-mIBG imaging, the aim of this study was to collect 123I-mIBG data for H/M ratios from common European gamma camera vendors.
Methods
210 experiments were performed in 27 European institutions. Based on these experiments, conversion coefficients for each gamma camera-collimator combination were calculated. An averaged conversion coefficient of 0.88 was used to calculate a standardized H/M ratio.
Results
On average, LE-collimator-derived H/M ratios were significantly lower compared to ME-collimator-derived H/M ratios. The mean conversion coefficients ranged from 0.553 to 0.605 for the LE-collimator group and from 0.824 to 0.895 for the ME-collimator group.
Conclusion
Clinically established H/M ratios can be converted into standardized H/M ratios using cross-calibrated conversion coefficients. This standardization is important for identifying appropriate thresholds for adequate risk stratification. In addition, this cross-calibration enables comparison between different national and international data.
Keywords: 123I-mIBG scintigraphy, standardization, heart-to-mediastinum ratio, calibration phantom, collimator
Introduction
Cardiac 123I-mIBG scintigraphy, a non-invasive imaging technique to assess cardiac sympathetic activity, has been shown to be of clinical value, especially for the assessment of prognosis, in many cardiac diseases.1 – 5 The quantification method is essential to differentiate normal and abnormal cardiac sympathetic activity and to distinguish high- and low-risk groups. The heart-to-mediastinum (H/M) ratio is a simple method to correct for background and is highly reproducible with small inter- and intra-observer variation.6 However, standardization of acquisition and analysis is needed. The lack of standardization between different institutions is one of the factors that have hampered wide scale clinical implementation of cardiac 123I-mIBG scintigraphy. International efforts have been made to harmonize and standardize cardiac 123I-mIBG scintigraphy.7 These recommendations include proposals for patient preparation, administered dose of 123I-mIBG activity (MBq), scanning parameters, and analysis of the acquired data to obtain the most used semi-quantitative parameters [i.e., early and late H/M ratio and 123I-mIBG washout (WO)].
Collimator choice is one of the most important factors causing variation among institutions and studies.8,9 In addition to 159 keV photons, 123I emits high-energy photons of 529 keV which penetrate the relatively thin septa of low-energy (LE) collimators. This penetration leads to degradation of image quality and ultimately introduces variation in H/M ratios.10 Medium-energy (ME) collimators have thicker septa and lower photon penetration compared to LE collimators and therefore have improved image quality and accuracy in myocardial 123I-mIBG imaging, however, at the expense of spatial resolution.11 – 13 Consequently, the use of ME collimators is recommended for estimation the H/M ratios.7 However, LE collimators are still commonly applied for cardiac 123I-mIBG scintigraphy because of their wide availability.7 In addition, although the nomenclature of collimators is classified into 2 major groups of LE and ME collimators, various types of collimators have been developed depending on the clinical purpose. The variety in the collimator types used has hampered multicentre comparison of cardiac 123I-mIBG scintigraphy-derived parameters and single-centre results could not easily be extrapolated to other institutions.14
In Japan, a phantom for planar cardiac 123I-mIBG imaging has been developed to cross-calibrate different acquisition conditions.15 This phantom has been used to calculate conversion coefficients for different gamma camera-collimator combinations in Japan.16 With these conversion coefficients, various conditions can be converted to standard H/M ratios. As an extension of this phantom study, the purpose of this study was to accumulate H/M ratios from common gamma camera vendors in Europe and compare these data with the data from Japan.
Methods
Phantom Design and Experiment
A light-weight calibration phantom was used as previously described.17 111 MBq 123I was mixed with 4450 ml water to fill the phantom. Since all organ parts were connected as one compartment, no radionuclide concentration adjustment was required for each organ separately. A 3 cm acrylic plate was placed over the phantom to simulate human body attenuation, when imaging was performed. The 256 × 256 matrix images were acquired from the anterior and posterior views for 5 minutes, comparable to clinical planar cardiac 123I-mIBG imaging (Figure 1). The energy window was centered at 159 keV with a 15% window. The phantom was placed centrally under the gamma camera head with a 5 cm distance between the phantom and collimator surface. The experiments were performed using 210 conditions in 27 institutions in Europe (see ‘‘Appendix’’ for list of all participating institutions).
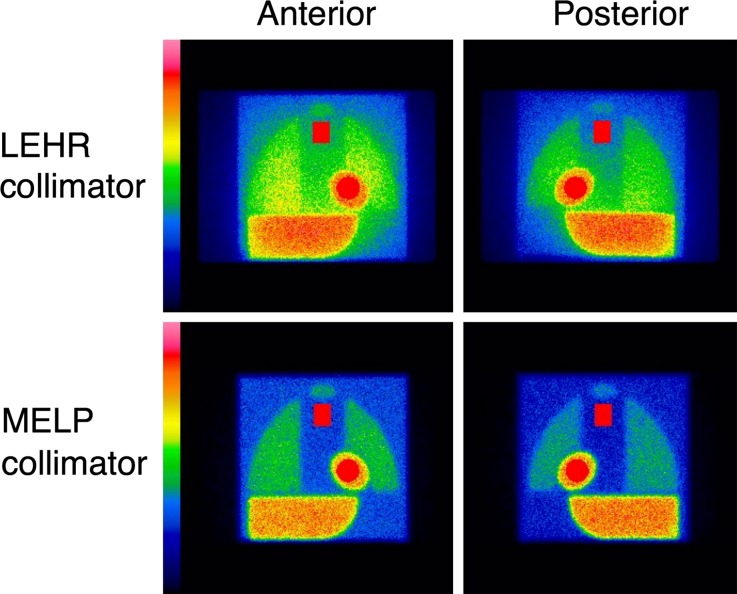
Figure 1.
Example of planar 123I-mIBG images of the phantom in anterior (left panels) and posterior (right panels) view with Symbia system (Siemens, Erlangen, Germany). Note the difference in image quality due to septal penetration or scatter between low-energy and medium-energy collimators. LEHR low energy high resolution; MELP medium energy low penetration
Mathematical Reference Value of H/M Ratio
All 123I-mIBG phantom images were acquired in each participating institution. Data were anonymized and were sent to the Kanazawa University in Japan for central analysis. H/M ratios were mathematically calculated, assuming a linear attenuation coefficient (μ) of 123I for water as 0.147 cm−1. The standard equation for attenuation was used (i.e., exponential of −μx, where x stands for the thickness of the attenuating material). The mathematical calculated reference H/M ratio was corrected for attenuation, while Compton scatter and septal penetration of gamma rays were not included. The reference H/M ratios determined by the structure of the phantom were 2.60 and 3.50 (respectively, anterior and posterior acquisition). Instead of the original phantom used in the Japanese studies, a new light-weight phantom was used in the European study. In the latter phantom, although the dimensions of the phantom were identical, some acrylic parts were made hollow to fill with non-radioactive water. To obtain identical results compared to the original phantom, minor differences in reference values derived from the light-weight phantom were adjusted. The phantom dimensions of the original light-weighted phantom type were measured by CT scan, and the attenuation in the water and acrylics was recalculated. The adjustment of minor difference of phantom design using linear regression line resulted in agreement of conversion coefficients using low energy high resolution (LEHR), low-medium-energy general purpose (LMEGP), and medium energy general purpose (MEGP) collimators.
Cross Calibration
In this study, two H/M ratios (anterior and posterior acquisitions) from each institution were plotted against the reference values (Figure 2). A linear regression equation was calculated using the formula:
(* denotes multiplication), in which the line always passes on the coordinate (1, 1). The coefficient K i (i.e., slope of the regression line for each institution) was used to convert the institutional H/M ratios to the reference values (H/M ratioref). In the second step, the H/M ratioref was converted to a standardized H/M ratio using the K std. This process can be summarized as:
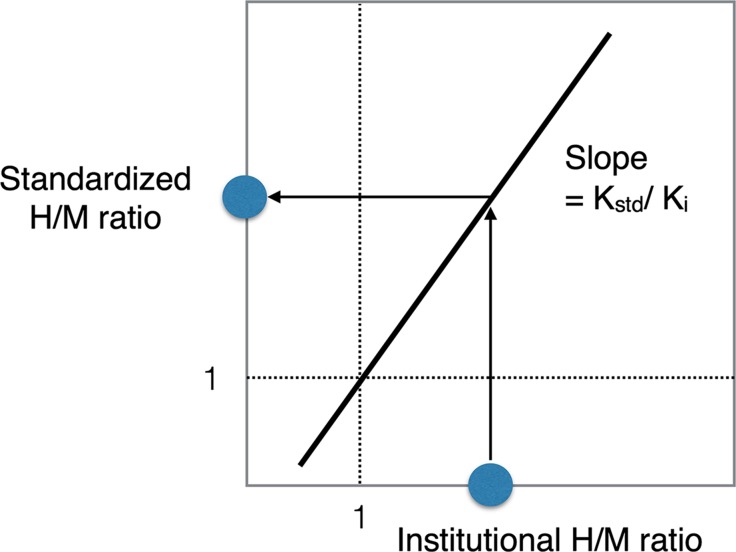
Figure 2.
Conversion of H/M ratio from an institutional condition A (H/M ratioA) to the standard value (H/M ratiostandard). The slope of the regression line of the institutional condition, coefficient K i, and the averaged coefficient of common ME collimators, coefficient K std = 0.88, allows for the calculation of a conversion coefficient corrected to a common ME-collimator type
The K std was 0.88, defined as the average K values for typical ME collimators. The rationale for this conversion to the common ME-collimator type is based on the recommendation to use ME collimators.
Statistics
The data are shown as mean ± standard deviation. Differences among groups were examined by one-way analysis of variance and Student’s t test. The linear regression equation of the H/M ratios between two conditions was calculated by the least square method. The statistics software JMP (version 11, SAS Institute Inc., Cary, NC, USA) was used and mathematical calculation was based on Mathematica 10 (Wolfram Research Inc., Champaign, IL, USA).
Results
210 123I-mIBG phantom studies were performed in 27 institutions in Austria, Belgium, the Netherlands, and the United Kingdom including camera vendors of Siemens (n = 148), GE (n = 44) and Philips (n = 18). Collimator types were divided into 2 groups: LE and ME. The LE group included LEHR, general purpose (LEGP), and all-purpose (LEAP) collimators. The ME group included LMEGP, MEGP, and low-penetration (MELP) collimators.
H/M Ratio Measured in Two Phantom Conditions
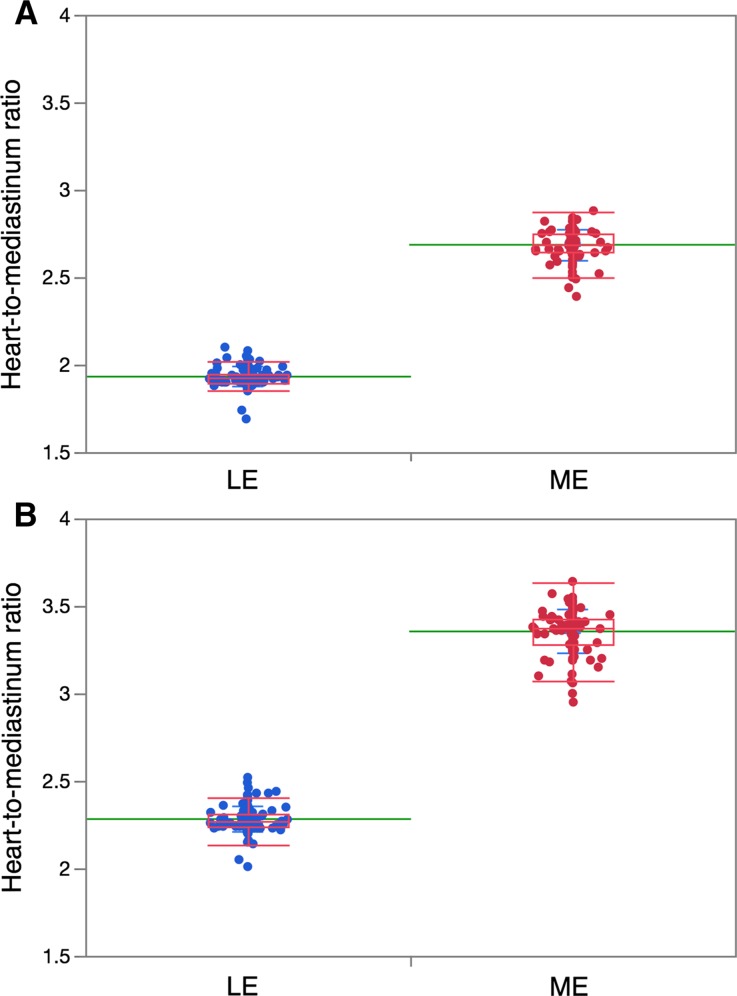
Overall, the LE-collimator group showed lower H/M ratios compared with the ME-collimator group. For the phantom H/M ratio of 2.60, the LE-collimator (n = 113)- and ME-collimator (n = 97)-derived H/M ratios were 1.932 ± 0.056 and 2.685 ± 0.088, respectively, (p = <0.0001). Similarly, for the phantom H/M ratio of 3.50, the LE-collimator- and ME-collimator- derived H/M ratios were 2.281 ± 0.074 and 3.354 ± 0.124, respectively, (p < 0.0001) (Figure 3).
Figure 3.
Individual data points and box-whisker plots of H/M ratios using phantoms with the reference H/M ratio of 2.60 (panel A) and 3.50 (panel B). Green lines denote mean values. The box plot shows median and the 1st and 3rd quartile, and the ends of the whiskers are ± 1.5 *(interquartile range)
Conversion Coefficients Determined by 2 Data Points
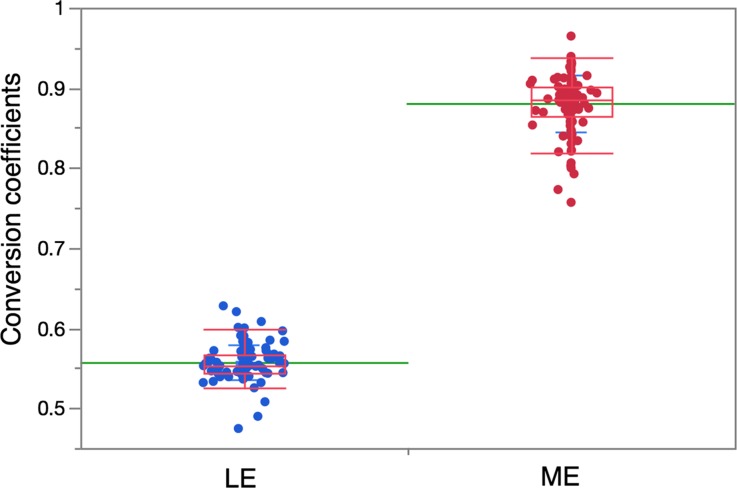
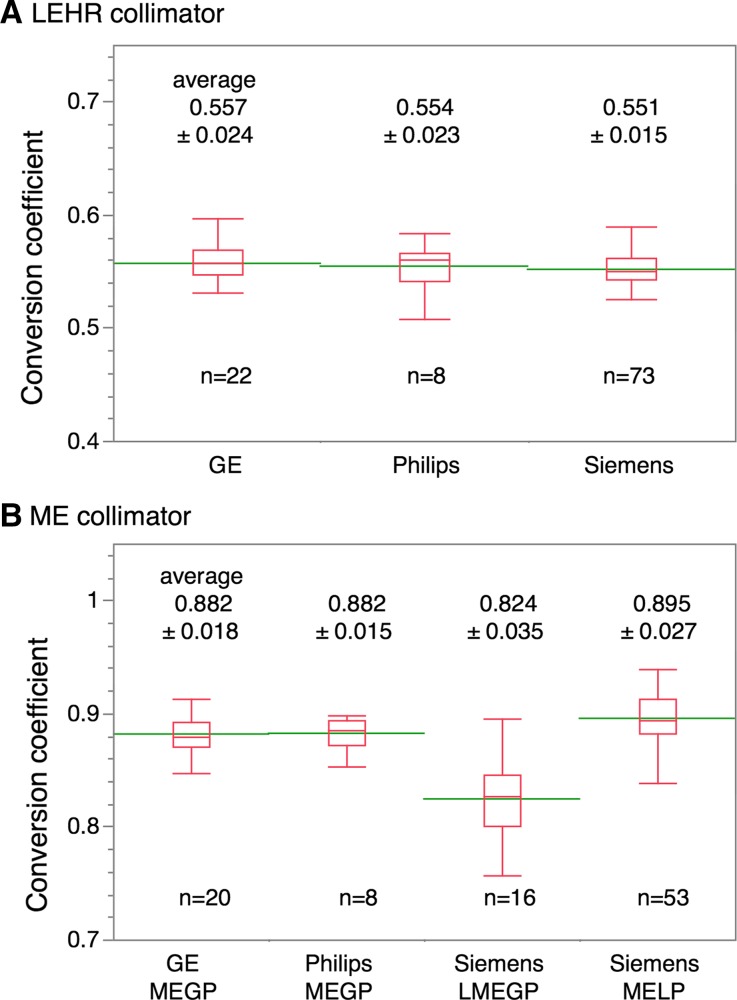
The conversion coefficients to the reference value are summarized according to the main collimator names: 3 LE sub-groups and 3 ME sub-groups. (Table 1) The average conversion coefficients were 0.553 for LEHR, 0.605 for LEAP, 0.570 for LEGP, 0.824 for LMEGP, and 0.882 for MEGP, and the highest was 0.895 for MELP types. When the conversion coefficients were divided into a LE and a ME group, the average values were, respectively, 0.556 ± 0.021 and 0.880 ± 0.036 (p < 0.0001) (Figure 4). Figure 5 shows conversion coefficients of the most common used LE- and ME-collimator types per vendor.
Table 1.
Conversion coefficient of collimators: European vs. Japanese studies
| Europe | Japan* | P values between Europe and Japan | |||||
|---|---|---|---|---|---|---|---|
| N | Mean | SD | N | Mean | SD | ||
| LEHR | 103 | 0.553 | 0.018 | 73 | 0.552 | 0.048 | 0.85 |
| LEGP+LEAP group** | 10 | 0.591 | 0.024 | 25 | 0.648 | 0.036 | <0.0001 |
| LEGP | 4 | 0.570 | 0.011 | 17 | 0.654 | 0.037 | 0.0003 |
| LEAP | 6 | 0.605 | 0.020 | 2 | 0.624 | 0.014 | 0.15 |
| LMEGP | 16 | 0.824 | 0.035 | 46 | 0.829 | 0.055 | 0.74 |
| MEGP+MELP group | 81 | 0.891 | 0.025 | 53 | 0.895 | 0.061 | 0.60 |
| MEGP*** | 28 | 0.882 | 0.017 | 40 | 0.878 | 0.054 | 0.71 |
| MELP | 53 | 0.895 | 0.027 | 13 | 0.950 | 0.051 | <0.0001 |
*Data from J Nucl Cardiol 2014; 21: 970–978
**LE general-all-purpose collimator is included in Japanese study
*** MEGP, ME general-all purpose, and ME collimators are included in Japanese study
Figure 4.
Conversion coefficients to the reference values for the LE- and ME-collimator groups. Data points and box-whisker plots are also shown. Green lines denote mean values. The box plot shows median and the 1st and 3rd quartile, and the ends of the whiskers are ± 1.5 *(interquartile range)
Figure 5.
Conversion coefficients of the most common used LE- and ME-collimator types per vendor. Green lines denote mean values. The box plot shows median and the 1st and 3rd quartile, and the ends of the whiskers are ± 1.5 *(interquartile range)
Comparison Between European and Japanese Conversion Coefficients
Overall, there were no significant differences when the European conversion coefficients of LEHR, LEAP, LMEGP, and MEGP collimators were compared with the Japanese conversion coefficients (Table 1). Only the conversion coefficients for LEGP and MELP collimators differed significantly (p < 0.0001). However, when the conversion coefficients for MEGP and MELP were combined, the difference was no longer statistically significant. In contrast when the conversion coefficients for LEGP and LEAP were combined, the statistical significant difference persisted between the European and Japanese data.
Discussion
These are the results of the first European myocardial 123I-mIBG cross-calibration phantom study to calculate conversion coefficients for specific individual gamma camera-collimator combinations. The cross-calibration allowed for a conversion of institutional H/M ratios to standardized H/M ratios. These conversion coefficients will facilitate multicentre comparison of myocardial 123I-mIBG results and enable the extrapolation of the outcome of single- and multicentre studies to other institutions.
The design of collimator septa and apertures has influence on the septal penetration. ME collimators, as recommend by the EANM Cardiovascular Committee,7 have thicker septa and lower penetration compared to LE collimators. The difference in collimator types is therefore one of the most important factors that affect variation in H/M ratios. It has been shown that H/M ratios derived from LE collimators are significantly lower compared to those from ME collimators.8,17 This has been confirmed by the previous cross-calibration phantom study in Japan showing significant underestimation of H/M ratios derived from LE collimators.16 As H/M ratios help differentiating high-risk and low-risk groups, this could have clinical implications. However, after correction to standardized H/M ratios, LE and ME collimators showed comparable values.16
The present study shows that the conversion coefficients of the LE collimators are lower compared to the ME collimators which is in line with previous phantom studies in Japan (Table 1). The conversion coefficients for most LE- and ME-collimator sub-groups did not show any statistical differences between Europe and Japan. However, there was a significant difference in the LEGP and MELP sub-group.
There are several factors that could explain variation in the LEGP and MELP sub-group between the European and Japanese institutions. Most likely, the small number of LEGP and MELP collimators may have resulted in a limited statistical power. In addition, these differences may be explained by small differences in the phantom used. In contrast to the original designed phantom used in Japan, a light-weight phantom was used for the current European study. After careful examination of both cross-calibration phantoms by CT scanner (Symbia T6/16, Siemens. Erlangen, Germany), under the same conditions (120 mAs and 130 kV), there was a small difference of <1 mm of the 123I-mIBG compartment between the conventional and light-weight types. Furthermore, one could expect minor differences between Japanese and European camera combinations due to differences in the design of the collimator septa and apertures and gamma camera crystals. However, we have confirmed with the manufacturers that both collimators and gamma cameras used are manufactured identical for Europe and Japan. The differences between LEAP and MELP may also be explained by small variations in acquisition. In Europe and Japan, both energy windows of 15% and 20% have been used according to local protocol. The acquisition time ranged from 3 to 10 minutes in Japan and was 5 minutes in Europe. The distance from collimator to phantom was the same in Europe and Japan. Moreover, although 123I was manufactured by different companies in Japan (FUJIFILM RI Pharma, Tokyo, Japan) and Europe (GE Healthcare, Eindhoven, The Netherlands), both products showed no contamination of other isotopes. Finally, there might be a difference between used acrylics of the original phantom and water in some compartments of the light-weight phantom. Although both water and the used acrylics have an almost identical decay coefficient with a very similar scatter pattern, this minor difference in phantom design may explain the found differences in coefficient values. In summary although there is no variation between Europe and Japan for most LE and ME groups, there is a minimal difference in the LEAP and MELP collimator group. As shown, there is a variety of possible explanations for this small difference. However, except for the relative small number of experiments, the above mentioned factors are true for all comparisons between the European and Japanese data. Hence, if valid, these factors would have caused also differences between the other collimators groups. Therefore, this difference is most likely driven for the largest part by the relative small number of experiments. Of course variation in phantom design, variation in energy windows, and variation in acquisition time may have also contributed.
Our study has some limitations. Compared to the Japanese cross-calibration study, we only used 2 (2.60 and 3.50) instead of 4 (1.35, 1.80, 2.60, and 3.50) references H/M ratios. However, conversion coefficients from 2 data points were nearly identical to those 4 data points.16 In addition, this cross-calibration method only corrects for high-energy photons coming from liver and lungs, which are the most important contributors of counts overestimation in the mediastinum and heart.8 However, this method does not correct for high-energy photons coming other organs like kidney and bladder. In addition, it is important to realize that phantom data are only an approximation of the clinical setting. Therefore, the validity of the conversion coefficients is not guaranteed.
In conclusion, differences in gamma camera-collimator combinations can be corrected to standardize ME-collimator values with the use of a cross-calibration phantom. This method can readily be applied, reducing variation in outcome measures and thereby further the clinical role of myocardial 123I-mIBG scintigraphy.
New Knowledge Gained
Standardization of H/M ratios has impact on patient management. Most importantly, standardization of H/M ratio allows for the development of a universal prognostic threshold. This could be established by reanalyzing databases from a number of 123I-mIBG studies previously published. In addition, future multicentre studies should aim for the use of a standardized H/M ratio, overcoming the impact of gamma camera and collimator differences. Finally, to further stress the importance of standardized H/M ratios, standardized values are essential in risk models for cardiac mortality.18
Acknowledgments
Funding
This study was funded by a research Grant from GE healthcare B.V. (Grant number ADR-14-03).
Disclosure
K. Nakajima, MD, PhD, has a collaboration to develop software with FUJIFILM RI Pharma, Co. Ltd, Tokyo, Japan. All other authors (D.O. Verschure, E. Poel, K. Okuda, B.L.F. van Eck-Smit, G.A.Somsen, H.J. Verberne) declare that he/she has no conflict of interest.
Abbreviations
- 123I-mIBG
123I-meta-iodobenzylguanidine
- H/M ratio
Heart-to-mediastinum ratio
- LE
Low energy
- LEAP
Low energy all-purpose
- LEHR
Low energy high resolution
- LMEGP
Low-medium-energy general purpose
- ME
Medium energy
- MEGP
Medium energy general purpose
- MELP
Medium energy low penetration
- WO
Washout
Appendix
Participating institutions included (alphabetical order):
Academic Medical Center (Amsterdam, the Netherlands),
Amphia ziekenhuis (Breda, the Netherlands),
AZ Groeninge (Kortijk, Belgium),
AZ Maria Middelares (Gent, Belgium),
AZ Sint Jan (Brugge, Belgium),
Diakonessenhuis (Utrecht, the Netherlands),
Erasmus Medical Center (Rotterdam, the Netherlands),
Gelderse Vallei (Ede, the Netherlands),
Groene Hart ziekenhuis (Gouda, the Netherlands),
Jeroen Bosch Ziekenhuis (Den Bosch, the Netherlands),
Leids University Medical Center (Leiden, the Netherlands),
Medical Center Leeuwarden (Leeuwarden, the Netherlands),
Onze Lieve Vrouw (Aalst, Belgium),
OLVG oost (Amsterdam, the Netherlands),
OLVG west (Amsterdam, the Netherlands),
Noordwest ziekenhuisgroep (Alkmaar, the Netherlands),
Sint Augustinus (Wilrijk, Belgium),
Spaarne Gasthuis (Hoofddorp, the Netherlands),
Spaarne Gasthuis (Haarlem, the Netherlands),
University Hospital of North Durham (Durham, United Kingdom),
University Medical Center Groningen (Groningen, the Netherlands),
University Medical Center Utrecht (Utrecht, the Netherlands),
UZ Antwerpen (Edegem, Belgium),
UZ Brussel (Brussel, Belgium),
UZ Leuven (Leuven, Belgium)
Wilhelminenspital (Vienna, Austria),
Zuyderland Medical Center (Heerlen, the Netherlands)
References
- 1.Schofer J, Spielmann R, Schuchert A, Weber K, Schluter M. Iodine-123 meta-iodobenzylguanidine scintigraphy: a noninvasive method to demonstrate myocardial adrenergic nervous system disintegrity in patients with idiopathic dilated cardiomyopathy. J Am Coll Cardiol. 1988;12:1252–1258. doi: 10.1016/0735-1097(88)92608-3. [DOI] [PubMed] [Google Scholar]
- 2.Wichter T, Hindricks G, Lerch H, Bartenstein P, Borggrefe M, Schober O, et al. Regional myocardial sympathetic dysinnervation in arrhythmogenic right ventricular cardiomyopathy. an analysis using 123I-meta-iodobenzylguanidine scintigraphy. Circulation. 1994;89:667–683. doi: 10.1161/01.CIR.89.2.667. [DOI] [PubMed] [Google Scholar]
- 3.Shimizu M, Ino H, Yamaguchi M, Terai H, Hayashi K, Nakajima K, et al. Heterogeneity of cardiac sympathetic nerve activity and systolic dysfunction in patients with hypertrophic cardiomyopathy. J Nucl Med. 2002;43:15–20. [PubMed] [Google Scholar]
- 4.Jacobson AF, Senior R, Cerqueira MD, Wong ND, Thomas GS, Lopez VA, et al. Myocardial iodine-123 meta-iodobenzylguanidine imaging and cardiac events in heart failure. results of the prospective ADMIRE-HF (AdreView Myocardial Imaging for Risk Evaluation in Heart Failure) study. J Am Coll Cardiol. 2010;55:2212–2221. doi: 10.1016/j.jacc.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 5.Verschure DO, Veltman CE, Manrique A, Somsen GA, Koutelou M, Katsikis A, et al. For what endpoint does myocardial 123I-MIBG scintigraphy have the greatest prognostic value in patients with chronic heart failure? results of a pooled individual patient data meta-analysis. Eur Heart J Cardiovasc Imaging. 2014;15:996–1003. doi: 10.1093/ehjci/jeu044. [DOI] [PubMed] [Google Scholar]
- 6.Verschure DO, Bongers V, Hagen P, Somsen GA, van Eck-Smit BF, Verberne HJ. Impact of a predefined mediastinal ROI on inter-observer variability of planar 123I-MIBG heart-to-mediastinum ratio. J Nucl Cardiol. 2014;21:605–613. doi: 10.1007/s12350-014-9854-z. [DOI] [PubMed] [Google Scholar]
- 7.Flotats A, Carrió I, Agostini D, Le Guludec D, Marcassa C, Schaffers M, et al. Proposal for standardization of 123I-metaiodobenzylguanidine (MIBG) cardiac sympathetic imaging by the EANM Cardiovascular Committee and the European Council of Nuclear Cardiology. Eur J Nucl Med Mol Imaging. 2010;37:1802–1812. doi: 10.1007/s00259-010-1491-4. [DOI] [PubMed] [Google Scholar]
- 8.Verschure DO, de Wit TC, Bongers V, Hagen PJ, Sonneck-Koenne C, D’Aron J, et al. 123I-MIBG heart-to-mediastinum ratio is influenced by high-energy photon penetration of collimator septa from liver and lung activity. Nucl Med Commun. 2015;36:279–285. doi: 10.1097/MNM.0000000000000238. [DOI] [PubMed] [Google Scholar]
- 9.Verberne HJ, Feenstra C, de Jong WM, Somsen GA, van Eck-Smit BL, Busemann Sokole E. Influence of collimator choice and simulated clinical conditions on 123I-MIBG heart/mediastinum ratios: a phantom study. Eur J Nucl Med Mol Imaging. 2005;32:1100–1107. doi: 10.1007/s00259-005-1810-3. [DOI] [PubMed] [Google Scholar]
- 10.Inoue Y, Suzuki A, Shirouzu I, Machida T, Yoshizawa Y, Akita F, et al. Effect of collimator choice on quantitative assessment of cardiac iodine 123 MIBG uptake. J Nucl Cardiol. 2003;10:623–632. doi: 10.1016/S1071-3581(03)00652-4. [DOI] [PubMed] [Google Scholar]
- 11.Inoue Y, Shirouzu I, Machida T, Yoshizawa Y, Akita F, Doi I, et al. Physical characteristics of low and medium energy collimators for 123I imaging and simultaneous dual-isotope imaging. Nucl Med Commun. 2003;24:1195–1202. doi: 10.1097/00006231-200311000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Macey DJ, DeNardo GL, DeNardo SJ, Hines HH. Comparison of low- and medium-energy collimators for SPECT imaging with iodine-123-labeled antibodies. J Nucl Med. 1986;27:1467–1474. [PubMed] [Google Scholar]
- 13.Geeter FD, Franken PR, Defrise M, Andries H, Saelens E, Bossuyt A. Optimal collimator choice for sequential iodine-123 and technetium-99m imaging. Eur J Nucl Med. 1996;23:768–774. doi: 10.1007/BF00843705. [DOI] [PubMed] [Google Scholar]
- 14.Verberne HJ, Habraken JB, van Eck-Smit BL, Agostini D, Jacobson AF. Variations in 123I-metaiodobenzylguanidine (MIBG) late heart mediastinal ratios in chronic heart failure: a need for standardisation and validation. Eur J Nucl Med Mol Imaging. 2008;35:547–553. doi: 10.1007/s00259-007-0611-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakajima K, Okuda K, Matsuo S, Yoshita M, Taki J, Yamada M, et al. Standardization of metaiodobenzylguanidine heart to mediastinum ratio using a calibration phantom: effects of correction on normal databases and a multicentre study. Eur J Nucl Med Mol Imaging. 2012;39:113–119. doi: 10.1007/s00259-011-1963-1. [DOI] [PubMed] [Google Scholar]
- 16.Nakajima K, Okuda K, Yoshimura M, Matsuo S, Wakabayashi H, Imanishi Y, et al. Multicenter cross-calibration of I-123 metaiodobenzylguanidine heart-to-mediastinum ratios to overcome camera-collimator variations. J Nucl Cardiol. 2014;21:970–978. doi: 10.1007/s12350-014-9916-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakajima K, Matsubara K, Ishikawa T, Motomura N, Maeda R, Akhter N, et al. Correction of iodine-123-labeled meta-iodobenzylguanidine uptake with multi-window methods for standardization of the heart-to-mediastinum ratio. J Nucl Cardiol. 2007;14:843–851. doi: 10.1016/j.nuclcard.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 18.Nakajima K, Nakata T, Matsuo S, Jacobson AF (2015) Creation of mortality risk charts using 123I meta-iodobenzylguanidine heart-to-mediastinum ratio in patients with heart failure: 2- and 5-year risk models. Eur Heart J Cardiovasc Imaging. Epub ahead of print. [DOI] [PMC free article] [PubMed]