Abstract
Introduction
To describe the 12-month efficacy and safety of goniotomy performed using the Kahook Dual Blade (KDB) in combination with cataract surgery in eyes with medically treated open-angle glaucoma (OAG).
Methods
This was a prospective, interventional case series conducted at seven centers in North America. Consecutive patients with medically treated OAG and visually significant cataract underwent phacoemulsification combined with goniotomy (PE + goniotomy) using KDB. Indications for glaucoma surgery included reduction of intraocular pressure (IOP) and reduction of IOP-lowering medications. De-identified data were collected and included pre-, intra-, and postoperative data on IOP, the use of IOP-lowering medications, and adverse events through 12 months of follow-up.
Results
Among 52 eyes undergoing surgery, mean IOP was reduced from 16.8 ± 0.6 mmHg at baseline to 12.4 ± 0.3 mmHg at month 12 (P < 0.001), a 26.2% reduction. Mean IOP across time points ranged from 12.4–13.3 mmHg during follow-up. The mean number of topical IOP-lowering medications was reduced from 1.6 ± 0.2 at baseline to 0.8 ± 0.1 at month 12 (P < 0.05), a 50.0% reduction. At month 12, 57.7% of eyes had IOP reduction ≥ 20% from baseline, and 63.5% were on ≥ 1 fewer IOP-lowering medications. In subgroup analysis, 84.6% of eyes with lower mean baseline IOP were using ≥ 1 fewer medications at month 12, and 100% of eyes with higher mean baseline IOP had IOP reductions ≥ 20%. The most common postoperative adverse events were pain/irritation (n = 4, 7.7%), opacification of the posterior lens capsule (n = 2, 3.8%), and IOP spike > 10 mmHg (n = 2, 3.8%).
Conclusion
PE + goniotomy using the KDB significantly lowers both IOP and dependence on IOP-lowering medications in eyes with OAG. Adverse events were not sight-threatening and typically resolved spontaneously.
Funding
New World Medical, Inc.
Keywords: Cataract surgery, Glaucoma, Goniotomy, Intraocular pressure, Micro-incisional glaucoma surgery, Micro-invasive glaucoma surgery, Ophthalmology, Phacoemulsification, Trabecular meshwork
Introduction
The goal of therapy for open-angle glaucoma (OAG) is reduction of intraocular pressure (IOP) to prevent damage to the optic nerve and preserve visual function. Reduction of IOP can be achieved with medical therapy, laser procedures, and incisional surgery. In general, surgery is the most effective method of IOP reduction, but is also the most prone to adverse events, some of them sight-threatening. Traditional glaucoma surgical procedures, trabeculectomy and tube-shunt implantation, can provide greater IOP reductions and attain lower target IOP than medications or laser procedures [1–4]. These procedures, however, require the formation of a filtering bleb, which over time can develop leaks that can lead to hypotony and infections [5]. In recent years, substantial innovation in surgical technique has sought to develop a procedure that provides IOP reduction comparable to that achieved with traditional surgery, but with a more favorable safety profile. Generally, these procedures avoid the formation of a bleb by shunting fluid across the obstructed trabecular meshwork (TM) into Schlemm’s canal or into the suprachoroidal space. Overall, these procedures, termed micro-incisional glaucoma surgeries (MIGS), are safer than traditional glaucoma surgery, but have not consistently delivered comparable IOP reductions [6, 7]. More recently, in recognition of the bleb’s key role in achieving significant IOP reductions, bleb-based MIGS procedures have been developed [8, 9]. Long-term safety of these procedures has yet to be established.
The Kahook Dual Blade (KDB; New World Medical, Rancho Cucamonga, CA, USA) is a single-use ophthalmic knife designed to perform a goniotomy procedure. The traditional goniotomy knife, usually a microvitreoretinal blade, is used to incise the trabecular meshwork behind Schwalbe’s line and produces a single incision in the TM. Unfortunately, this technique does not remove TM tissue and may result in injuries to the adjacent sclera [10]. Potential reasons for short- and long-term failure of incisional goniotomy include fusion of residual TM leaflets as well as scleral damage with resulting inflammation and localized scarring response [11, 12]. The KDB is a new ophthalmic blade, designed to remove the TM in a more complete fashion with minimal residual TM leaflets and less collateral damage [10].
Herein, we present 12-month efficacy and safety outcomes of a series of cases in which patients with both medically treated OAG and visually significant cataract underwent phacoemulsification combined with goniotomy (PE + goniotomy) using the KDB.
Methods
This was a prospective, interventional case series of patients with both visually significant cataract and glaucoma undergoing PE + goniotomy using the KDB. De-identified data were collected from the practices at seven centers in North America using a standardized set of data collection forms.
This study was initiated after Institutional Review Board approval at each site. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study. Patients enrolled in this study were aged 18 or older with concurrent cataract and mild to severe glaucoma and scheduled for PE + goniotomy with KDB. In this study, indications for goniotomy were to reduce IOP and/or to reduce reliance on IOP-lowering medications at the time of cataract surgery in eyes with medically treated OAG while avoiding the formation of a filtering bleb and its associated risk profile. Patients in this study were selected for goniotomy if they had glaucoma that, in the judgment of the treating physician, would benefit from TM excision to remove diseased tissue and allow for unimpeded egress of aqueous into the distal outflow system.
This study had no formal protocol and was intended to descriptively characterize the impact of goniotomy with KDB on IOP and IOP medication use when combined with cataract surgery. Surgeons were invited to submit de-identified data collected prospectively on consecutive patients undergoing combined cataract and goniotomy in whom the indication for surgery was determined at each surgeon’s discretion. The study was not registered as the ICMJE guidelines did not require it. Baseline and operative data were collected on the day of surgery, and the clinical course was assessed at 1 day, 1 week, and 1, 3, 6, and 12 months, postoperatively. Individual IOP goals were tailored to each individual patient at the discretion of each investigator; postoperatively, IOP-lowering medications were withdrawn or added as deemed necessary by each investigator.
Each investigator performed goniotomy using the KDB according to the manufacturer’s directions for use. Goniotomy via TM excision performed with the KDB has been described elsewhere [13]. The KDB has a distal tip that pierces the TM and enters Schlemm’s canal (Fig. 1). As the instrument is advanced along Schlemm’s canal, the TM is elevated on the KDB ramp and guided toward two parallel blades. Unlike a standard goniotomy knife that simply incises the TM, leaving contiguous anterior and posterior flaps, the KDB excises a strip of TM, leaving a direct opening for aqueous to pass from the anterior chamber into Schlemm’s canal. Thus, goniotomy with KDB removes diseased tissue at the site of aqueous outflow obstruction, restoring the natural aqueous outflow pathway without the formation of a filtering bleb.
Fig. 1.
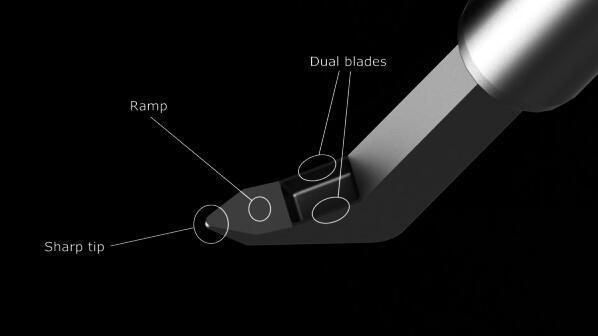
Schematic of the single-use Kahook Dual Blade
The primary outcome for this study was IOP reduction from preoperative baseline. Mixed model analysis with a diagonal covariance matrix was utilized to evaluate longitudinal IOP changes over time and was adjusted for multiplicity using Bonferroni’s method. Within-eye changes from baseline were compared using a paired t test. Two threshold measures of success were also defined: (1) an IOP reduction ≥ 20% from baseline and (2) IOP-lowering medical regimen reduced by ≥ 1 medication compared with preoperative therapy. The proportion of eyes attaining each of these threshold success criteria was reported at each time point. To best characterize success in meeting individual subject goals, a subgroup analysis was conducted to characterize IOP changes and medication changes over time in two subgroups: those with IOP greater than or equal to the median IOP and those with IOP less than the median IOP. Because the indication for surgery was not uniformly recorded in this study, it is assumed that the IOP reduction in the higher IOP subgroup approximates the IOP reductions expected in eyes undergoing surgery primarily for IOP reduction, while the medication reduction in the lower IOP subgroup approximates the medication reductions expected in eyes undergoing surgery for medication reduction. Safety was evaluated by tabulating both solicited and unsolicited adverse events from the intraoperative period through last follow-up. As this study was descriptive in nature and not designed to test a pre-specified hypothesis, no formal power/sample size analysis was conducted. Statistical analysis was conducted using SAS (PC Version 9.4, SAS Institute, Cary, NC, USA).
Results
Overall, data from 52 patients were included in this analysis, of whom 44 (84.6%) had primary OAG, 4 (7.7%) had pigmentary glaucoma, and 2 each (3.9% each) had normal tension glaucoma or exfoliation glaucoma. The majority of eyes (43/52, 82.7%) were using IOP-lowering medications at baseline, most commonly a prostaglandin analog (35/44, 79.6%).
Intraocular pressure data at baseline and each follow-up time point are given in Table 1 and Fig. 2. From a mean preoperative IOP of 16.8 ± 0.6 mmHg while using a mean of 1.6 ± 0.2 topical IOP-lowering medications, statistically significant IOP reduction was observed in the full sample as soon as postoperative day 1 and remained significant at every postoperative time point through 12 months of follow-up, ranging from 3.5 to 4.4 mmHg (P < 0.001 at each time point), a 20.8–26.2% reduction. Similarly, the mean number of IOP-lowering medications was significantly lower at almost every postoperative visit compared to baseline, ranging from 0.5 to 1.0 (P < 0.05 at each time point except month 3), a 37.5–68.8% reduction (Table 2). At the month 12 assessment, mean IOP was 12.4 ± 0.3 mmHg (a mean of − 4.4 mmHg from baseline; P < 0.001), a − 26.2% reduction. Eyes required 0.8 ± 0.1 medications on average (a mean reduction of 0.8 medications from baseline; P < 0.05), a 50.0% reduction.
Table 1.
Mean IOP over time: all eyes and low and high subgroups
| All eyes (N = 52) | Baseline IOP ≤ 16.5 mmHg (n = 26) | Baseline IOP > 16.5 mmHg (n = 26) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean IOP (mmHg) ± SE* | Mean difference (mmHg) | IOP change (%) | Mean IOP (mmHg) ± SE | Mean difference (mmHg) | IOP change (%) | Mean IOP (mmHg) ± SE | Mean difference (mmHg) | IOP change (%) | |
| Baseline | 16.8 (0.6) | – | 13.1 (0.4) | – | 20.5 (0.6) | – | |||
| Day 1 | 12.7 (0.5) | − 4.1 | − 24.4 | 12.3 (0.6) | − 0.8 | − 6.1 | 13.2 (0.8) | − 7.3 | − 35.6 |
| Week 1 | 12.7 (0.6) | − 4.1 | − 24.4 | 12.4 (1.0) | − 0.7 | − 5.3 | 13.1 (0.7) | − 7.4 | − 36.1 |
| Month 1 | 13.3 (0.5) | − 3.5 | − 20.8 | 13.4 (0.9) | 0.3 | 2.3 | 13.2 (0.5) | − 7.3 | − 35.6 |
| Month 3 | 12.6 (0.4) | − 4.2 | − 25.0 | 11.6 (0.6) | − 1.5 | − 11.5 | 13.5 (0.5) | − 7.0 | − 34.1 |
| Month 6 | 12.7 (0.4) | − 4.1 | − 24.4 | 12.3 (0.5) | − 0.8 | − 6.1 | 13.0 (0.6) | − 7.5 | − 36.6 |
| Month 12 | 12.4 (0.3) | − 4.4 | − 26.2 | 12.6 (0.4) | − 0.5 | − 3.8 | 12.2 (0.5) | − 8.3 | − 40.5 |
*Reported are least squares means and associated standard errors from a mixed model for repeated measure analysis. Per Bonferroni pairwise comparison, the difference from baseline in mean IOP is statistically significant at each follow-up visit (P < 0.001)
Fig. 2.
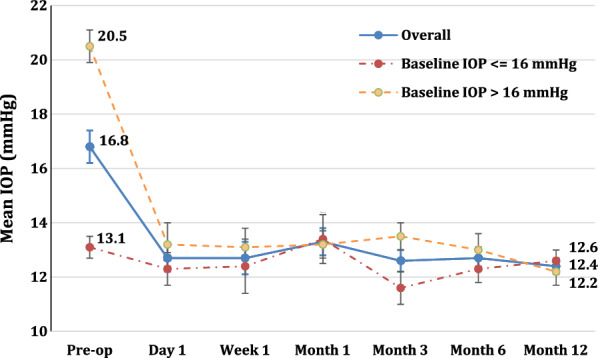
Mean intraocular pressure (± standard error in mmHg) at each time point
Table 2.
Mean medications over time: all eyes and low and high IOP subgroups
| All eyes (n = 52) | Baseline IOP ≤ 16.5 mmHg (n = 26) | Baseline IOP > 16.5 mmHg (n = 26) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean medication (n) ± SE* | Mean difference (n) | Medication change (%) | Mean medication (n) ± SE | Mean difference (n) | Medication change (%) | Mean medication (n) ± SE | Mean difference (n) | Medication change (%) | |
| Baseline | 1.6 (0.2) | – | – | 1.7 (0.2) | – | – | 1.5 (0.3) | – | |
| Day 1 | 0.5 (0.1) | − 1.1 | − 68.8 | 0.7 (0.2) | − 1.0 | − 58.8 | 0.4 (0.2) | − 1.1 | − 73.3 |
| Week 1 | 0.6 (0.2) | − 1.0 | − 62.5 | 0.7 (0.2) | − 1.0 | − 58.8 | 0.6 (0.2) | − 0.9 | − 60.0 |
| Month 1 | 0.7 (0.1) | − 0.9 | − 56.3 | 0.8 (0.2) | − 0.9 | − 52.9 | 0.6 (0.2) | − 0.9 | − 60.0 |
| Month 3 | 1.0 (0.2) | − 0.6 | − 37.5 | 0.8 (0.2) | − 0.9 | − 52.9 | 1.1 (0.3) | − 0.4 | − 26.7 |
| Month 6 | 0.8 (0.1) | − 0.8 | − 50.0 | 0.7 (0.2) | − 1.0 | − 58.8 | 1.0 (0.2) | − 0.5 | − 33.3 |
| Month 12 | 0.8 (0.1) | − 0.8 | − 50.0 | 0.6 (0.2) | − 1.1 | − 64.7 | 1.0 (0.2) | − 0.5 | − 33.3 |
*Reported are least squares means and associated standard errors from a mixed model for repeated measure analysis. Per Bonferroni pairwise comparison, the difference from baseline in mean medications is statistically significant at each follow-up visit (P < 0.05) except at month 3 (P = 0.154)
The proportion of eyes achieving a minimum of 20% IOP reduction from baseline ranged from 54.4% to 67.4% throughout the first year and was 57.7% at the month 12 visit (Table 3). Likewise, the proportion of patients whose topical IOP-lowering regimen was reduced by one or more medications ranged from 56.5% to 64.4%. These data are reported for all time points in Table 3.
Table 3.
Patients who met success criteria over time: all eyes and low and high IOP subgroups
| All eyes (n = 52) | Baseline IOP ≤ 16.5 mmHg (n = 26) | Baseline IOP > 16.5 mmHg (n = 26) | ||||
|---|---|---|---|---|---|---|
| IOP reduction ≥ 20% (%) | Medication reduction ≥ 1 (%) | IOP reduction ≥ 20% (%) | Medication reduction ≥ 1 (%) | IOP reduction ≥ 20% (%) | Medication reduction ≥ 1 (%) | |
| Day 1 | 25 (54.4) | 26 (56.5) | 5 (20.8) | 14 (58.3) | 20 (90.9) | 12 (54.6) |
| Week 1 | 26 (57.8) | 29 (64.4) | 10 (41.7) | 18 (75.0) | 16 (76.2) | 11 (52.4) |
| Month 1 | 27 (55.1) | 31 (63.3) | 5 (20.8) | 18 (75.0) | 22 (88.0) | 13 (52.0) |
| Month 3 | 29 (67.4) | 26 (60.5) | 8 (38.1) | 18 (85.7) | 21 (95.5) | 8 (36.4) |
| Month 6 | 28 (60.9) | 28 (60.9) | 6 (27.3) | 18 (81.8) | 22 (91.7) | 10 (41.7) |
| Month 12 | 30 (57.7) | 33 (63.5) | 4 (15.4) | 22 (84.6) | 26 (100.0) | 11 (42.3) |
To better assess subject-specific goals, the efficacy analysis was repeated in two subgroups: those with baseline IOP ≤ 16.5 mmHg (the median IOP of the full group) and those with baseline IOP > 16.5 mmHg (Tables 1, 2, 3). In the lower IOP group, the primary goal of surgery is assumed to be medication reduction. In this group, medications were reduced by a mean of 0.9–1.1 medications across time points, with a mean medication reduction of 1.1 medications at month 12, at which time 85% of subjects had reduced their medication burden by one or more medications. This was achieved with no compromise of IOP control, as mean IOP remained stable with negligible changes across all study visits (mean changes ranging from 0.3 to − 1.5 mmHg). In the higher IOP group, where IOP reduction was likely the primary goal of surgery, mean IOP reductions ranged from 7.0 to 8.3 mmHg (34.1% to 40.5%), with mean IOP at month 12 being reduced by 8.3 mmHg (40.5%) and 100% of subjects achieving a minimum IOP reduction of 20%. These IOP reductions were accompanied by reductions in medications ranging from 0.4 to 1.1 medications, with subjects using a mean of 0.5 fewer medications at month 12.
Mean visual acuity improved from 0.439 ± 0.041 logMAR preoperatively to 0.137 ± 0.016 logMAR at 12 months postoperatively, representing a 0.302 ± 0.044 logMAR improvement (P < 0.001).
Adverse events were non-sight threatening and self-limited. One eye each (1.9% each) experienced limited iridodialysis that healed without intervention and one peripheral tear in Descemet’s membrane that spontaneously resolved. Sixteen eyes (30.8%) were noted to have intraoperative blood reflux as expected with unroofing of several collector channels. This is common in angle surgeries and is considered an expected observation rather than an adverse event. Generally only trace blood refluxed into the anterior chamber and resolved spontaneously in all cases typically within the 1st postoperative week. Postoperatively, pain/irritation was noted in four eyes (7.7%), posterior capsule opacification in two eyes (3.8%), and IOP spike > 10 mmHg in two eyes (2.8%). No adverse events required secondary interventions (Table 4).
Table 4.
Intraoperative and postoperative adverse events
| Adverse event | Number of eyes (N = 52) | Incidence rate (%) |
|---|---|---|
| Intraoperative | ||
| Difficulty removing strip of trabecular meshwork | 2 | 3.8 |
| Iridodialysis | 1 | 1.9 |
| Tear in Descemet’s membrane | 1 | 1.9 |
| Postoperative | ||
| Pain/irritation | 4 | 7.7 |
| Posterior capsule opacification | 2 | 3.8 |
| Intraocular pressure spike > 10 mmHg | 2 | 3.8 |
| Cystoid macular edema | 1 | 1.9 |
| Choroidal detachment | 1 | 1.9 |
| Floater | 1 | 1.9 |
| Glare | 1 | 1.9 |
| Hazy vision | 1 | 1.9 |
| Hypotony | 1 | 1.9 |
| Peripheral anterior synechiae | 1 | 1.9 |
| Tearing | 1 | 1.9 |
Discussion
This real-world study reflects the early experiences of seven surgeons performing combined cataract extraction and goniotomy with the Kahook Dual Blade. Our study demonstrates that goniotomy performed using the KDB can safely and significantly reduce both IOP (by 26.2%) and the need for IOP-lowering medications (by 50.0%) for at least 12 months when combined with cataract extraction in eyes with OAG and visually significant cataract. In subgroup analysis, 100% of eyes with higher baseline IOP achieved at least a 20% IOP reduction at month 12, and 85% of eyes with lower baseline IOP achieved at least a one medication reduction in treatment burden.
These results compare favorably with other MIGS procedures. In the pivotal trial of the iStent trabecular microbypass device, 66% of eyes undergoing combined surgery with cataract extraction achieved a minimum 20% IOP reduction without medication at 12 months [14]. Similarly, in the pivotal trial of the Cypass suprachoroidal microstent, 82% of eyes undergoing combined surgery with cataract extraction achieved a minimum 20% IOP reduction at 12 months [15]. The pivotal trial of Trabectome surgery did not report the proportion of patients achieving IOP or medication reduction goals, but the mean IOP reductions of about 40% and mean medication reductions of about 0.9 per eye are consistent with our outcomes in higher IOP and lower IOP eyes, respectively [16]. A randomized trial comparing goniotomy with trabeculectomy reported 12-month IOP reductions of approximately 50% in both groups, which compares favorably with the 40.5% 12-month IOP reduction seen in our high-IOP subgroup; this study did not analyze postoperative medication reduction [17]. Of note, the Trabectome ablates a strip of TM and the KDB excises a strip of TM; goniotomy incises but does not remove a strip of TM. The relative risk of surgical failure due to fusing of the residual TM leaflets between these procedures warrants future evaluation. Finally, cataract surgery alone is known to transiently reduce both IOP and the need for IOP-lowering medications in medically treated glaucoma patients. A recent meta-analysis revealed that the average magnitude of these reductions expected at 12 months from cataract surgery alone is 14% for IOP and 0.47 for IOP-lowering medications [18]. In our series, mean reductions of 26.2% and 0.8%, respectively, were observed. Thus, cataract surgery alone is unlikely to account for the results obtained in this surgical series.
The intraoperative reflux of blood into the anterior chamber is common in this procedure and occurs in association with many other MIGS procedures [7]. The reflux occurs when unroofing Schlemm’s canal in the setting of intraoperative hypotony and should be considered an expected event in these surgical procedures. The blood typically resolves quickly and spontaneously without consequence.
This study was intended to describe early real-world experience with the KDB when used to perform goniotomy in combination with cataract surgery. In addition to clinical data, surgeons’ experience with the KDB was assessed and has been reported previously [13]. In 96–98% of cases, surgeons reported that the use of the KDB was straightforward, that entry into the canal with the KDB was uncomplicated, and that advancement of the KDB along the canal was smooth.
Limitations of this study include the small sample size and lack of a control group. While some of the IOP reductions observed may be attributable to cataract surgery alone, our results exceed the outcomes expected from cataract surgery alone and are consistent with outcomes of other MIGS procedures in combination with cataract surgery [14–16].
Conclusions
In summary, this study demonstrates that PE + goniotomy with the KDB performed in eyes with coexisting cataract and OAG provides significant reductions in both IOP and IOP-lowering medication use. The procedure provides a high probability of achieving subject-specific goals of IOP reduction and/or IOP medication reduction. Based on these data, this combined surgical approach is a valid treatment option in medically treated glaucoma patients undergoing cataract surgery in whom reduction of IOP, IOP-lowering medication burden, or both are desired. Ongoing and future studies will further characterize the long-term efficacy and safety of this procedure, compare its performance and cost effectiveness to other glaucoma surgical procedures, and evaluate its performance as a stand-alone procedure.
Acknowledgements
We thank the participants of the study.
Funding
New World Medical, Inc., provided funding for the study, medical writing assistance, and article processing charges and the open access fee. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Editorial Assistance
We would like to thank the following for their substantial support in this study: Suhail Abdullah, MBSc, for significant contributions to the study design and review of manuscript; Mark C. Jasek, PhD, for providing consultations in study design, analysis, and interpretation of data as well as critical review of the article; Khaled Bahjri, MD, MPH, PhD, for assisting in data collection, analysis, and interpretation of findings; and Anthony D. Realini, MD, MPH, for assisting in writing the manuscript. Suhail Abdullah, Mark C. Jasek, and Khaled Bahjri, are employees of New World Medical. Anthony D. Realini, Hypotony Holdings, LLC, is an independent consultant to New World Medical. New World Medical, Inc., provided funding for the medical writing assistance.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Syril Dorairaj declares that he has received consulting fees from New World Medical, was a consultant to New World Medical for the conduct of this study, and has received research support from New World Medical in the past. Leonard Seibold declares that he has received consulting fees from New World Medical, was a consultant to New World Medical for the conduct of this study, and has received research support from New World Medical in the past. Nathan Radcliffe declares that he has received consulting fees from New World Medical, was a consultant to New World Medical for the conduct of this study, and has received research support from New World Medical in the past. Ahmad Aref declares that he has received consulting fees from New World Medical, was a consultant to New World Medical for the conduct of this study, and has received research support from New World Medical in the past. Gabriel Lazcano-Gomez declares that he has received consulting fees from New World Medical, was a consultant to New World Medical for the conduct of this study, and has received research support from New World Medical in the past. Jason Darlington declares that he has received consulting fees from New World Medical, was a consultant to New World Medical for the conduct of this study, and has received research support from New World Medical in the past. Jesus Jimenez-Roman was a consultant to New World Medical for the conduct of this study and has received research support from New World Medical in the past. Kaweh Mansouri declares that he has received consulting fees from Santen, Sensimed, and ImplanData and has received grant support from Topcon, Alcon, Allergan, and Optovue, was a consultant to New World Medical for the conduct of this study, and has received research support from New World Medical in the past. John Berdahl declares that he has received lecture and consulting fees from Alcon, Allergan, Glaukos, consulting fees from AMO, Avedro, Bausch & Lomb, Clarvista, Digisight, Envisia, New World Medical, Ocular Therapeutix, Vittamed, and Mobius, is an equity owner of Equinox, Omega Ophthalmic, Ocular Surgical Data, and Vision 5, has received consulting fees and royalties from Imprimis, and was a consultant to New World Medical for the conduct of this study. He has also received research support from New World Medical in the past. Mayo Clinic does not endorse specific products or services included in this article.
Compliance with Ethics Guidelines
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Data Availability
The data sets during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Enhanced digital features
To view enhanced digital features for this article go to 10.6084/m9.figshare.6756638.
References
- 1.AGIS Study Group. The Advanced Glaucoma Intervention Study (AGIS): 4. Comparison of treatment outcomes within race. Seven-year results. Ophthalmology. 1998;105:1146–64. 10.1016/S0161-6420(98)97013-0 [DOI] [PubMed] [Google Scholar]
- 2.Lichter PR, Musch DC, Gillespie BW, et al. Interim clinical outcomes in the Collaborative Initial Glaucoma Treatment Study comparing initial treatment randomized to medications or surgery. Ophthalmology. 2001;108:1943–53. 10.1016/S0161-6420(01)00873-9 [DOI] [PubMed] [Google Scholar]
- 3.Jay JL, Murray SB. Early trabeculectomy versus conventional management in primary open angle glaucoma. Br J Ophthalmol. 1988;72:881–9. 10.1136/bjo.72.12.881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Migdal C, Hitchings R. Control of chronic simple glaucoma with primary medical, surgical and laser treatment. Trans Ophthalmol Soc UK. 1986;105(Pt 6):653–6. [PubMed] [Google Scholar]
- 5.DeBry PW, Perkins TW, Heatley G, Kaufman P, Brumback LC. Incidence of late-onset bleb-related complications following trabeculectomy with mitomycin. Arch Ophthalmol. 2002;120:297–300. 10.1001/archopht.120.3.297 [DOI] [PubMed] [Google Scholar]
- 6.SooHoo JR, Seibold LK, Radcliffe NM, Kahook MY. Minimally invasive glaucoma surgery: current implants and future innovations. Can J Ophthalmol. 2014;49:528–33. 10.1016/j.jcjo.2014.09.002 [DOI] [PubMed] [Google Scholar]
- 7.Richter GM, Coleman AL. Minimally invasive glaucoma surgery: current status and future prospects. Clin Ophthalmol. 2016;10:189–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sheybani A, Dick HB, Ahmed II. Early clinical results of a novel ab interno gel stent for the surgical treatment of open-angle glaucoma. J Glaucoma. 2016;25:e691–6. 10.1097/IJG.0000000000000352 [DOI] [PubMed] [Google Scholar]
- 9.Batlle JF, Fantes F, Riss I, et al. Three-year follow-up of a novel aqueous humor microshunt. J Glaucoma. 2016;25:e58–65. 10.1097/IJG.0000000000000368 [DOI] [PubMed] [Google Scholar]
- 10.Francis BA, Akil H, Bert BB. Ab interno Schlemm’s canal surgery. Dev Ophthalmol. 2017;59:127–46. 10.1159/000458492 [DOI] [PubMed] [Google Scholar]
- 11.Khouri AS, Wong SH. Ab interno trabeculectomy with a dual blade: surgical technique for childhood glaucoma. J Glaucoma. 2017;26:749–51. 10.1097/IJG.0000000000000701 [DOI] [PubMed] [Google Scholar]
- 12.Seibold LK, Soohoo JR, Ammar DA, Kahook MY. Preclinical investigation of ab interno trabeculectomy using a novel dual-blade device. Am J Ophthalmol. 2013;155(524–9):e2. [DOI] [PubMed] [Google Scholar]
- 13.Greenwood MD, Seibold LK, Radcliffe N, et al. Goniotomy with a single-use dual blade: short-term results. J Cataract Refract Surg. 2017;43(9):1197–201. 10.1016/j.jcrs.2017.06.046 [DOI] [PubMed] [Google Scholar]
- 14.Samuelson TW, Katz LJ, Wells JM, Duh YJ, Giamporcaro JE. Randomized evaluation of the trabecular micro-bypass stent with phacoemulsification in patients with glaucoma and cataract. Ophthalmology. 2011;118:459–67. 10.1016/j.ophtha.2010.07.007 [DOI] [PubMed] [Google Scholar]
- 15.Vold S, Ahmed II, Craven ER, et al. Two-year COMPASS trial results: supraciliary microstenting with phacoemulsification in patients with open-angle glaucoma and cataracts. Ophthalmology. 2016;123:2103–12. 10.1016/j.ophtha.2016.06.032 [DOI] [PubMed] [Google Scholar]
- 16.Minckler DS, Baerveldt G, Alfaro MR, Francis BA. Clinical results with the trabectome for treatment of open-angle glaucoma. Ophthalmology. 2005;112:962–7. 10.1016/j.ophtha.2004.12.043 [DOI] [PubMed] [Google Scholar]
- 17.Quaranta L, Hitchings RA, Quaranta CA. Ab-interno goniotrabeculotomy versus mitomycin C trabeculectomy for adult open-angle glaucoma: a 2-year randomized clinical trial. Ophthalmology. 1999;106:1357–62. 10.1016/S0161-6420(99)00725-3 [DOI] [PubMed] [Google Scholar]
- 18.Armstrong JJ, Wasiuta T, Kiatos E, Malvankar-Mehta M, Hutnik CM. The effects of phacoemulsification on intraocular pressure and topical medication use in patients with glaucoma: a systematic review and meta-analysis of 3-year data. J Glaucoma. 2017;26:511–22. 10.1097/IJG.0000000000000643 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets during and/or analyzed during the current study are available from the corresponding author on reasonable request.


