Abstract
The CuAAC reaction of azides and acetylenic triterpenes was used for synthesis of new triazoles of 3-acetylbetulin and betulone. The triazole derivatives were evaluated for their anticancer activity in vitro against amelanotic melanoma C-32, ductal carcinoma T47D and glioblastoma SNB-19 cell lines. 28-[1-(3’-Deoxythymidine-5’-yl)-1H-1,2,3-triazol-4-yl]carbonylbetulone 6e exhibited a significant IC50 value (0.17 µM) against the human glioblastoma SNB-19 cell line, an almost 5-fold higher potency while compared with reference cisplatin.
Keywords: Betulin; 1,2,3-Triazole; CuAAC; Anticancer activity; Lipophilicity
Introduction
The cycloaddition reaction plays an important role in the synthesis of five-membered heterocyclic structures such as 1,2,3-triazoles. Molecules containing a 1,4-disubstituted 1,2,3-triazole ring are prepared regioselectively from azides and terminal alkynes in the copper-(I)-catalyzed azide-alkyne cycloaddition reaction CuAAC (Wei et al. 2012; Marciniec et al. 2017). CuAAC reactions, described by Sharpless and Meldal groups, give high yields under mild conditions and have been used to obtain drugs, photo stabilizers and dyes. Additionally, this reaction occurs in various organic solvents and in aqueous media, in a wide pH area. In contrast to the CuACC reaction, the ruthenium catalyst azide-alkyne cycloaddition is used in the synthesis of the 1,5-disubstituted triazoles (Rostovtsev et al. 2002; Torne et al. 2002; Bonacorso et al. 2013; Bräse et al. 2008; Totobenazara et al. 2015).
Compounds containing 1,2,3-triazole units exhibit interesting biological activities (antimicrobial, anti-inflammatory, anti-tubercular, and antiviral), which has found numerous applications in bioconjugate chemistry and material science. Additionally, 1,4-disubstitued 1,2,3-triazoles show a significant anticancer activity against human cancer cell lines, which are multidrug-resistant (Wang et al. 2010; Dheer et al. 2017; He et al. 2014).
In the last decades, application of 1,3-dipolar cycloaddiction of naturally occurring triterpenes acquired meaning. Conjugation on azides with various alkyne derivatives of pentacyclic triterpenes is designed for the purposes of introduction of the physiologically stable 1,2,3-triazole group (Spivak et al. 2016; Suman et al. 2016; Yu et al. 2013). Most of the triazole analogs of natural compounds have been investigated for their anticancer activity. Majeed et al. synthesized and tested a series of C-3 aryl-substituted 1,2,3-triazoles of betulinic acid for their cytotoxic activity against various human cancer lines like leukemia (HL-60, THP-1), prostate (DU-145, PC-3), lung (A-549), breast (MCF-7), liver (HEP-2), colon (HCT-15), and neuroblastoma (SF-295). The compounds bearing 2-cyanophenyl and 5-hydroxy-1-naphthyl substituted triazole ring exhibited promising IC50 values against HL-60 cell line of 2.5 and 3.5 µM, respectively, in comparison to betulinic acid (IC50 = 17 µM) (Majeed et al. 2013). In the case of C-28 aryl-substituted 1,2,3-triazoles of betulinic acid, it was observed that compounds containing a 4-fluorophenyl substituted triazole ring had the cytotoxic profile similar to that of betulinic acid. This novel triazole hybrid showed a significant antiproliferative activity in HL-60 (leukemia), MIAPACa2 (pancreas), PC-3 (prostate), and A-549 (lung) cell lines, with IC50 values in the range of 5.0–7.0 µM (Khan et al. 2016).
Previously, we described a synthetic route and evaluation of cytotoxicity of betulin and betulone derivatives with a propynoyl group at the C-28 position (Boryczka et al. 2013; Bębenek et al. 2016). Expanding our interest to propynoyl-substituted triterpenes, we converted those acetylenic derivatives into the corresponding 1,2,3-triazoles. In this work, we presented application of the CuAAC reaction in the synthesis of new triazoles of pentacyclic triterpenes and their anticancer activity, as well as lipophilicity properties.
Material and methods
General
All organic solvents (from Sigma-Aldrich and P.P.H. STANLAB) were dried and used after purification. Melting points (m.p.) were determined in open capillary tubes on an Electrothermal IA 9300 melting point apparatus and are uncorrected. The 1H NMR and 13C NMR spectra were recorded on a Bruker Avance III 600 spectrometer in deuterated-d6 chloroform (CDCl3) or deuterated-d6 dimethyl sulfoxide (DMSO) solution. The chemical shifts were reported in ppm (δ), and coupling constant (J) values—in hertz (Hz). The spin multiplicity was designated as the singlet (s), doublet (d), triplet (t), quartet (q), and multiplet (m). High-resolution mass spectra (HR-MS) were recorded on a Bruker Impact II instrument. Infrared spectra (IR) were recorded on a Shimadzu IRAffinity-1 FTIR spectrophotometer (Shimadzu, Japan) using the KBr pellet method. The progress of the reactions was monitored by thin layer chromatography (TLC) using silica gel 60 254 F plates (Merck, Darmstadt, Germany) and detected by spraying with a solution of 5% sulfuric (VI) acid and heating to 120 °C. Purity of the obtained compounds was confirmed by column chromatography carried out on silica gel 60, <63 μm (Merck). A mixture of CHCl3–EtOH (40:1, 15:1, 5:1 v/v) or CH2Cl2–EtOH (60:1, 40:1, v/v) was used as the mobile phase.
Chemistry
Synthesis of 3-acetyl-28-propynoylbetulin 3 and 28-propynoylbetulone 4
3-Acetyl-28-propynoylbetulin 3 was prepared according to the procedure described by Boryczka et al. (Boryczka et al. 2013).
To an ice-cooled (−10 °C) mixture of 3-acetylbetulin 2 (0.48 g, 1 mmol) and propynoic acid (0.12 g, 1.10 mmol) in dichloromethane (5 mL), a freshly prepared solution of dicyclohexylcarbodiimide (0.23 g, 1.12 mmol) and 4-dimethylaminopyridine (0.01 g, 0.08 mmol) in dichloromethane (1 mL) was added. The mixture was allowed to react under argon atmosphere at −10 °C for 5 h. After warming to room temperature, the mixture was stirred overnight. The reaction was monitored by TLC until completion. The resulting precipitate was filtered and the solvent was removed under reduced pressure. The crude product was purified by silica gel column chromatography (CHCl3–EtOH 40:1, v/v).
3-Acetyl-28-propynoylbetulin (3)
Yield 79%; mp 115–118 °C; Rf 0.44 (CHCl3–EtOH, 40:1, v/v); IR (KBr) νmax 3304, 2946, 2120, 1719, 1457, 1246 cm−1; 1H NMR (600 MHz, CDCl3): δ 0.81 (3H, s, CH3), 0.86 (3H, s, CH3), 0.87 (3H, s, CH3), 0.99 (3H, s, CH3), 1.05 (3H, s, CH3), 2.07 (3H, s, COCH3), 2.45 (1H, m, H-19), 2.91 (1H, s, C≡CH), 4.01 (1H, d, J = 10.8 Hz, H-28), 4.41 (1H, d, J = 10.8 Hz, H-28), 4.48 (1H, m, H-3), 4.62 (1H, s, H-29), 4.71 (1H, s, H-29); 13C NMR (150 MHz, CDCl3): δ 14.7, 16.0, 16.2, 16.5, 18.2, 19.1, 20.8, 21.3, 23.7, 25.1, 27.0, 27.9, 29.5, 29.6, 34.1, 34.4, 37.1, 37.7, 37.8, 38.4, 40.9, 42.7, 46.4, 47.7, 48.8, 50.3, 55.4, 64.9, 74.6, 74.8, 80.9, 110.0, 149.9, 153.3, 171.0; HRAPCIMS m/z: 536.3878 C35H52O4 (calcd. 536.3865).
28-Propynoylbetulone 4 was obtained according to the procedure described by Bębenek et al. The spectra data of acetylenic ester 4 were consistent with those published in the literature (Bębenek et al. 2016).
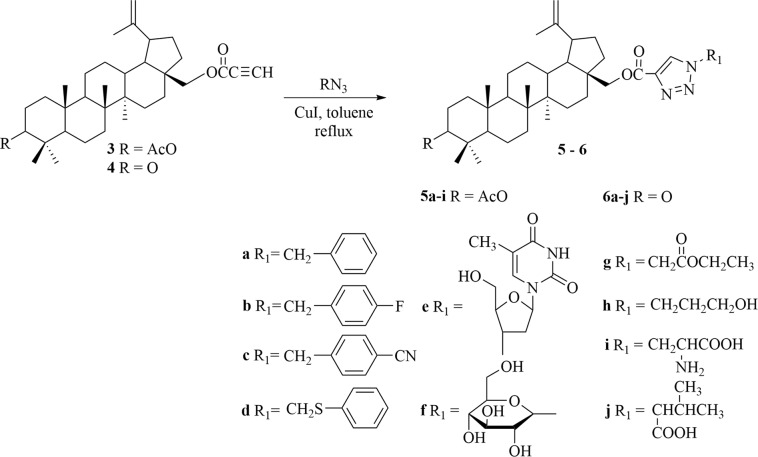
General procedure for the synthesis of triazoles 5a–i and 6a–j
Based on the previously reported method, the acetylenic esters 3–4 were converted into triazoles 5a-i and 6a-j by reactions with organic azides in toluene in the presence of copper(I) iodide (Bębenek et al. 2017). The copper(I) iodide (0.1 eqv, 0.004 g, 0.02 mmol) and the organic azide (1.05 eqv, 0.21 mmol) were added to a solution of propynoylated derivatives 3 or 4 (0.20 mmol) in toluene (4 mL). Next, the reaction mixture was stirred for another 72 h under reflux. The solvent was evaporated. The crude residue was purified by silica gel column chromatography using various mixtures of organic solvents. The same mobile phases were applied for TLC and in column chromatography (Table 1).
Table 1.
The mobile phases used in column chromatography and calculated values of the retention factor
| Compound | Mobile phase | Ratio | Retention factor Rf |
|---|---|---|---|
| 5a | CH2Cl2–EtOH | 60:1 | 0.39 |
| 5b | CH2Cl2–EtOH | 40:1 | 0.55 |
| 5c | CH2Cl2–EtOH | 60:1 | 0.32 |
| 5d | CH2Cl2–EtOH | 60:1 | 0.43 |
| 5e | CHCl3–EtOH | 5:1 | 0.68 |
| 5f | CHCl3–EtOH | 5:1 | 0.18 |
| 5g | CH2Cl2–EtOH | 40:1 | 0.43 |
| 5h | CHCl3–EtOH | 15:1 | 0.45 |
| 5i | CHCl3–EtOH | 5:1 | 0.73 |
| 6a | CH2Cl2–EtOH | 40:1 | 0.43 |
| 6b | CH2Cl2–EtOH | 40:1 | 0.49 |
| 6c | CH2Cl2–EtOH | 60:1 | 0.36 |
| 6d | CH2Cl2–EtOH | 60:1 | 0.37 |
| 6e | CHCl3–EtOH | 15:1 | 0.24 |
| 6f | CHCl3–EtOH | 5:1 | 0.22 |
| 6g | CH2Cl2–EtOH | 60:1 | 0.31 |
| 6h | CHCl3–EtOH | 15:1 | 0.32 |
| 6i | CHCl3–EtOH | 5:1 | 0.74 |
| 6j | CHCl3–EtOH | 5:1 | 0.20 |
3-Acetyl-28-(1-benzyl-1H-1,2,3-triazol-4-yl)carbonylbetulin (5a)
Yield 73%; m.p. 109–111 °C; IR (KBr) νmax 3134, 2947, 1732, 1527, 1456, 1246–1193 cm−1; 1H NMR (600 MHz, CDCl3): δ 0.85 (3H, s, CH3), 0.86 (3H, s, CH3), 0.87 (3H, s, CH3), 0.99 (3H, s, CH3), 1.06 (3H, s, CH3), 1.71 (3H, s, CH3), 2.06 (3H, s, COCH3), 2.51 (1H, m, H-19), 4.13 (1H, d, J = 10.8 Hz, H-28), 4.49 (1H, m, H-3), 4.55 (1H, d, J = 10.8 Hz, H-28), 4.62 (1H, s, H-29), 4.72 (1H, s, H-29), 5.60 (2H, s, CH2), 7.31–7.33 (2H, m, HAr), 7.41–7.44 (3H, m, HAr), 7.97 (1H, s, CH-triazole); 13C NMR (150 MHz, CDCl3): δ 14.2, 14.7, 16.0, 16.2, 16.5, 18.2, 19.1, 20.8, 21.1, 21.3, 23.7, 25.2, 27.1, 27.9, 29.6, 29.8, 34.1, 34.7, 37.1, 37.7, 38.4, 40.9, 42.7, 46.7, 47.7, 48.9, 50.3, 54.5, 55.4, 60.4, 63.6, 80.9, 109.9, 127.1, 128.2, 129.2, 129.3, 133.8, 140.6, 150.1, 161.2, 171.1; HRAPCIMS m/z (neg): 668.4474 C42H58N3O4 (calcd. 668.4427).
3-Acetyl-28-[1-(4-fluorobenzyl)-1H-1,2,3-triazol-4-yl]carbonylbetulin (5b)
Yield 63%; m.p. 113–116 °C; IR (KBr) νmax 3138, 2963, 1734, 1539, 1457, 1226–1193, 802 cm−1; 1H NMR (600 MHz, CDCl3): δ 0.85 (3H, s, CH3), 0.86 (3H, s, CH3), 0.87 (3H, s, CH3), 1.00 (3H, s, CH3), 1.06 (3H, s, CH3), 1.69 (3H, s, CH3), 2.07 (3H, s, COCH3), 2.52 (1H, m, H-19), 4.14 (1H, d, J = 10.8 Hz, H-28), 4.49 (1H, m, H-3), 4.57 (1H, d, J = 10.8 Hz, H-28), 4.62 (1H, s, H-29), 4.72 (1H, s, H-29), 5.57 (2H, s, CH2), 7.10–7.13 (2H, m, HAr), 7.31–7.33 (2H, m, HAr), 7.97 (1H, s, CH-triazole); 13C NMR (150 MHz, CDCl3): δ 13.7, 15.1, 15.2, 15.5, 17.1, 18.1, 19.8, 20.3, 22.7, 24.1, 26.1, 26.9, 28.6, 28.8, 33.1, 33.7, 36.0, 36.6, 36.8, 37.4, 39.9, 41.7, 45.6, 46.7, 47.8, 49.3, 52.7, 54.4, 62.6, 79.9, 108.9, 115.3, 115.4, 125.9, 129.1, 129.2, 139.7, 149.0, 160.0, 161.2, 170.0; HRAPCIMS m/z (neg): 686.4357 C42H57FN3O4 (calcd. 686.4333).
3-Acetyl-28-[1-(4-cyanobenzyl)-1H-1,2,3-triazol-4-yl]carbonylbetulin (5c)
Yield 56%; m.p. 137–140 °C; IR (KBr) νmax 3144, 2949, 2231, 1734, 1540, 1457, 1248–1192 cm−1; 1H NMR (600 MHz, CDCl3): δ 0.83 (3H, s, CH3), 0.86 (3H, s, CH3), 0.87 (3H, s, CH3), 1.00 (3H, s, CH3), 1.07 (3H, s, CH3), 1.70 (3H, s, CH3), 2.07 (3H, s, COCH3), 2.52 (1H, m, H-19), 4.16 (1H, d, J = 10.8 Hz, H-28), 4.49 (1H, m, H-3), 4.58 (1H, d, J = 10.8 Hz, H-28), 4.63 (1H, s, H-29), 4.72 (1H, s, H-29), 5.68 (2H, s, CH2), 7.40 (2H, d, J = 8.4 Hz, HAr), 7.72 (2H, d, J = 8.4 Hz, HAr), 8.04 (1H, s, CH-triazole); 13C NMR (150 MHz, CDCl3): δ 14.8, 16.0, 16.2, 16.5, 18.2, 19.1, 19.3, 20.8, 21.3, 23.7, 25.2, 27.1, 27.9, 29.6, 29.8, 34.1, 34.7, 37.1, 37.7, 37.8, 38.4, 40.9, 42.7, 46.7, 47.7, 48.9, 50.3, 53.7, 55.4, 63.8, 80.9, 110.3, 113.3, 117.9, 124.4, 127.3, 128.5, 133.1, 138.9, 141.0, 150.0, 160.9, 171.0; HRAPCIMS m/z (neg): 693.4352 C43H57N4O4 (calcd. 693.4380).
3-Acetyl-28-(1-phenylthiomethyl-1H-1,2,3-triazol-4-yl)carbonylbetulin (5d)
Yield 60%; m.p. 105–107 °C; IR (KBr) νmax 2945, 1734, 1539,1456, 1247–1194 cm−1; 1H NMR (600 MHz, CDCl3): δ 0.86 (3H, s, CH3), 0.87 (3H, s, CH3), 0.88 (3H, s, CH3), 1.01 (3H, s, CH3), 1.09 (3H, s, CH3), 1.71 (3H, s, CH3), 2.07 (3H, s, COCH3), 2.51 (1H, m, H-19), 4.14 (1H, d, J = 10.8 Hz, H-28), 4.49 (1H, m, H-3), 4.55 (1H, d, J = 10.8 Hz, H-28), 4.63 (1H, s, H-29), 4.73 (1H, s, H-29), 5.68 (2H, s, CH2), 7.35–7.36 (5H, m, HAr), 8.04 (1H, s, CH-triazole); 13C NMR (150 MHz, CDCl3): δ 14.8, 16.1, 16.2, 16.5, 18.2, 19.1, 20.8, 21.3, 23.7, 25.2, 25.6, 27.1, 27.9, 29.6, 29.8, 34.1, 34.7, 37.1, 37.7, 37.8, 38.4, 40.9, 42.7, 46.7, 47.8, 48.9, 50.3, 54.3, 55.4, 63.6, 68.0, 80.9, 110.0, 126.8, 129.1, 129.7, 131.3, 132.4, 140.6, 150.0, 160.9, 171.0; HRAPCIMS m/z (neg): 700.4141 C42H58N3O4S (calcd. 700.4148).
3-Acetyl-28-[1-(3’-deoxythymidine-5’-yl)-1H-1,2,3-triazol-4-yl]carbonylbetulin (5e)
Yield 65%; m.p. 204–207 °C; IR (KBr) νmax 3446, 3068, 2945, 1730, 1541, 1456, 1246–1192 cm−1; 1H NMR (600 MHz, DMSO-d6): δ 0.81 (3H, s, CH3), 0.83 (3H, s, CH3), 0.98 (3H, s, CH3), 1.02 (3H, s, CH3), 1.18 (3H, s, CH3), 1.67 (3H, s, CH3), 1.78 (3H, s, CH3-AZT), 2.03 (3H, s, COCH3), 2.55 (1H, m, AZT), 2.67 (1H, m, H-19), 3.65–3.70 (2H, m, AZT), 4.03 (1H, d, J = 10.8 Hz, H-28), 4.27 (1H, t, J = 4.8 Hz, AZT), 4.38 (1H, m, H-3), 4.55 (1H, d, J = 10.8 Hz, H-28), 4.59 (1H, s, H-29), 4.73 (1H, s, H-29), 5.28 (1H, t, J = 4.8 Hz, AZT), 5.46 (1H, m, AZT), 6.44 (1H, t, J = 6.6 Hz, AZT), 7.82 (1H, s, AZT), 8.32 (1H, s, CH-triazole), 9.01 (1H, s, NH-AZT); 13C NMR (150 MHz, DMSO-d6): δ 12.7, 14.9, 15.9, 16.1, 16.3, 16.9, 18.2, 19.3, 20.8, 21.5, 23.8, 25.3, 27.1, 28.1, 29.4, 29.7, 34.0, 34.6, 37.1, 37.6, 37.7, 37.8, 38.2, 42.8, 46.8, 47.5, 48.7, 49.9, 55.0, 55.6, 60.2, 61.1, 62.7, 79.6, 80.4, 84.6, 110.1, 110.5, 129.3, 136.7, 139.3, 150.9, 160.9, 164.2; 170.6; HRAPCIMS m/z (neg): 802.4768 C45H64N5O8 (calcd. 802.4755).
3-Acetyl-28-[1-(1-deoxy-β-D-glucopyranosyl)-1H-1,2,3-triazol-4-yl]carbonylbetulin (5f)
Yield 82%; m.p. 210–212 °C; IR (KBr) νmax 3419, 2943, 1732, 1543, 1456, 1246–1191 cm−1; 1H NMR (600 MHz, DMSO-d6): δ 0.80 (3H, s, CH3), 0.81 (3H, s, CH3), 0.83 (3H, s, CH3), 0.98 (3H, s, CH3), 1.04 (3H, s, CH3), 1.68 (3H, s, CH3), 1.85 (1H, m, OH), 2.00 (3H, s, COCH3), 2.54 (1H, m, H-19), 3.27 (1H, m, OH), 3.39 (1H, m, OH), 3.44 (1H, m, OH), 3.71 (1H, m, CH-sugar), 3.85 (1H, m, CH-sugar), 4.03 (1H, d, J = 10.8 Hz, H-28), 4.37 (1H, m, H-3), 4.55 (1H, d, J = 10.8 Hz, H-28), 4.59 (1H, s, H-29), 4.63 (1H, m, CH-sugar), 4.73 (1H, s, H-29), 5.20 (1H, d, J = 5.4 Hz, CH-sugar), 5.35 (1H, d, J = 5.4 Hz, CH-sugar), 5.46 (1H, d, J = 5.4 Hz, CH-sugar), 5.61 (1H, d, J = 5.4 Hz, CH-sugar), 9.08 (1H, s, CH-triazole); 13C NMR (150 MHz, DMSO-d6): δ 15.0, 16.1, 16.3, 16.9, 18.2, 19.3, 20.8, 21.5, 23.8, 25.2, 27.1, 28.1, 29.4, 29.6, 34.0, 34.6, 37.1, 37.6, 37.8, 38.2, 39.6, 42.8, 46.8, 47.5, 48.7, 50.0, 55.0, 61.2, 62.6, 69.9, 72.4, 77.2, 79.6, 80.6, 88.3, 110.5, 129.1, 139.2, 150.3, 160.9, 170.6; HRAPCIMS m/z (neg): 740.4480 C41H62N3O9 (calcd. 740.4486).
3-Acetyl-28-(1-ethylacetyl-1H-1,2,3-triazol-4-yl)carbonylbetulin (5g)
Yield 80%; m.p. 221–224 °C; IR (KBr) νmax 2945, 1732, 1544, 1465, 1247–1213 cm−1; 1H NMR (600 MHz, CDCl3): δ 0.85 (3H, s, CH3), 0.86 (3H, s, CH3), 0.88 (3H, s, CH3), 0.99 (3H, s, CH3), 1.08 (3H, s, CH3), 1.34 (3H, t, J = 7.2 Hz, CH3), 1.68 (3H, s, CH3), 2.07 (3H, s, COCH3), 2.53 (1H, m, H-19), 4.18 (1H, d, J = 10.8 Hz, H-28), 4.32 (2H, q, J = 7.2 Hz, OCH2), 4.48 (1H, m, H-3), 4.58 (1H, d, J = 10.8 Hz, H-28), 4.63 (1H, s, H-29), 4.73 (1H, s, H-29), 5.24 (2H, s, CH2), 8.24 (1H, s, CH-triazole); 13C NMR (150 MHz, CDCl3): δ 14.1, 14.8, 16.1, 16.2, 16.5, 18.2, 20.8, 21.3, 23.7, 25.2, 27.1, 27.9, 29.6, 29.8, 34.1, 34.7, 37.1, 37.7, 37.8, 38.4, 40.9, 42.7, 46.7, 47.8, 48.9, 50.3, 51.0, 55.4, 62.8, 63.7, 80.9, 110.0, 128.7, 140.7, 150.1, 160.9, 165.7, 171.1; HRAPCIMS m/z (neg): 664.4329 C39H58N3O6 (calcd. 664.4326).
3-Acetyl-28-[1-(3-hydroxypropyl)-1H-1,2,3-triazol-4-yl]carbonylbetulin (5h)
Yield 83%; m.p. 116–119 °C; IR (KBr) νmax 3425, 2945, 1732, 1543, 1465, 1246–1199 cm−1; 1H NMR (600 MHz, DMSO-d6): δ 0.80 (3H, s, CH3), 0.81 (3H, s, CH3), 0.82 (3H, s, CH3), 0.98 (3H, s, CH3), 1.03 (3H, s, CH3), 1.09 (2H, m, CH2), 1.67 (3H, s, CH3), 2.02 (3H, s, COCH3), 2.51 (1H, m, H-19), 3.34 (2H, m, CH2), 4.01 (1H, d, J = 10.8 Hz, H-28), 4.38 (1H, m, H-3), 4.48 (2H, t, J = 7.2 Hz, CH2), 4.54 (1H, d, J = 10.8 Hz, H-28), 4.59 (1H, s, H-29), 4.73 (1H, s, H-29), 8.81 (1H, s, CH-triazole); 13C NMR (150 MHz, DMSO-d6): δ 14.9, 16.1, 16.3, 16.9, 18.2, 19.2, 20.7, 21.5, 24.8, 25.1, 27.1, 28.1, 29.4, 29.6, 33.1, 34.0, 34.6, 37.1, 37.6, 37.8, 38.2, 40.9, 42.8, 46.8, 47.5, 47.6, 48.7, 50.0, 55.0, 57.8, 62.5, 80.4, 110.4, 129.7, 139.0, 150.3, 161.0, 170.6; HRAPCIMS m/z (neg): 636.4363 C38H58N3O5 (calcd. 636.4376).
2-Amino-3-[4-(3-acetyl-28-betulinylcarbonyl)-1H-1,2,3-triazol-1-yl]propanoic acid (5i)
Yield 45%; oil; IR (KBr) νmax 3444, 2956, 1732, 1602, 1458, 1246–1122 cm−1; 1H NMR (600 MHz, DMSO-d6) δ: 0.80 (3H, s, CH3), 0.82 (3H, s, CH3), 0.86 (3H, s, CH3), 0.92 (3H, s, CH3), 1.01 (3H, s, CH3), 1.06 (1H, t, J = 7.2 Hz, CH), 1.67 (3H, s, CH3), 2.00 (3H, s, COCH3), 2.52 (1H, m, H-19), 4.14 (1H, d, J = 10.8 Hz, H-28), 4.23 (2H, d, J = 7.2 Hz, CH2), 4.37 (1H, m, H-3), 4.55 (1H, d, J = 10.8 Hz, H-28), 4.58 (1H, s, H-29), 4.73 (1H, s, H-29), 8.62 (1H, s, CH-triazole); 13C NMR (150 MHz, DMSO-d6): δ 14.9, 15.8, 16.1, 16.8, 18.3, 19.2, 19.6, 21.2, 22.9, 23.7, 26.8, 28.8, 30.3, 30.6, 30.8, 34.1, 34.5, 36.8, 37.2, 37.5, 38.5, 39.6, 42.8, 46.2, 47.0, 47.2, 48.6, 50.1, 54.3, 56.5, 67.8, 80.2, 110.0, 129.2, 132.1, 150.1, 161.0, 167.5; HRAPCIMS m/z (neg): 665.4269 C38H57N4O6 (calcd. 665.4278).
28-(1-Benzyl-1H-1,2,3-triazol-4-yl)carbonylbetulone (6a)
Yield 81%; m.p. 196–198 °C; IR (KBr) νmax 2963, 1738, 1700, 1539, 1465, 1261–1193 cm−1; 1H NMR (600 MHz, CDCl3) δ: 0.94 (3H, s, CH3), 1.02 (3H, s, CH3), 1.05 (3H, s, CH3), 1.10 (3H, s, CH3), 1.12 (3H, s, CH3), 1.69 (3H, s, CH3), 2.52 (1H, m, H-19), 4.15 (1H, d, J = 10.8 Hz, H-28), 4.57 (1H, d, J = 10.8 Hz, H-28), 4.62 (1H, s, H-29), 4.72 (1H, s, H-29), 5.60 (2H, s, CH2), 7.31–7.33 (2H, m, HAr), 7.41–7.44 (3H, m, HAr), 7.97 (1H, s, CH-triazole);13C NMR (150 MHz, CDCl3): δ 14.7, 15.8, 15.9, 19.1, 19.6, 21.1, 21.3, 25.2, 25.6, 26.6, 27.1, 29.6, 29.8, 33.5, 34.2, 34.7, 36.9, 37.8, 39.6, 40.9, 42.8, 46.7, 47.4, 47.7, 48.8, 49.7, 54.5, 55.0, 63.5, 68.0, 110.0, 127.1, 128.2, 129.2, 129.3, 133.8, 140.6, 150.0, 161.2, 218.0; HRAPCIMS m/z (neg): 624.4171 C40H54N3O3 (calcd. 624.4165).
28-[1-(4-Fluorobenzyl)-1H-1,2,3-triazol-4-yl]carbonylbetulone (6b)
Yield 73%; m.p. 220–223 °C; IR (KBr) νmax 3131, 2957, 1742, 1699, 1539, 1456, 1223–1198, 814 cm−1; 1H NMR (600 MHz, CDCl3): δ 0.86 (3H, s, CH3), 0.91 (3H, s, CH3), 0.92 (3H, s, CH3), 0.95 (3H, s, CH3), 1.02 (3H, s, CH3), 1.68 (3H, s, CH3), 2.43 (1H, m, H-19), 4.05 (1H, d, J = 10.8 Hz, H-28), 4.48 (1H, d, J = 10.8 Hz, H-28), 4.53 (1H, s, H-29), 4.63 (1H, s, H-29), 5.48 (2H, s, CH2), 7.01–7.04 (2H, m, HAr), 7.23–7.36 (2H, m, HAr), 7.88 (1H, s, CH-triazole); 13C NMR (150 MHz, CDCl3): δ 14.7, 15.8, 15.9, 19.2, 19.6, 21.1, 21.3, 25.2, 26.6, 27.1, 29.6, 29.8, 33.5, 34.2, 34.7, 36.9, 37.8, 39.6, 40.9, 42.8, 46.7, 47.4, 47.7, 48.8, 49.7, 53.7, 55.0, 63.6, 68.1, 110.0, 116.3, 116.5, 126.2, 126.3, 130.2, 140.0, 150.0, 161.1, 218.0; HRAPCIMS m/z (neg): 642.4063 C40H53FN3O3 (calcd. 642.4071).
28-[1-(4-Cyanobenzyl)-1H-1,2,3-triazol-4-yl]carbonylbetulone (6c)
Yield 57%; m.p. 211–214 °C; IR (KBr) νmax 3127, 2951, 2229, 1734,1705, 1525, 1457, 1243–1147 cm−1; 1H NMR (600 MHz, CDCl3): δ 0.87 (3H, s, CH3), 0.93 (3H, s, CH3), 0.96 (3H, s, CH3), 1.02 (3H, s, CH3), 1.03 (3H, s, CH3), 1.68 (3H, s, CH3), 2.44 (1H, m, H-19), 4.07 (1H, d, J = 10.8 Hz, H-28), 4.49 (1H, d, J = 10.8 Hz, 1H, H-28), 4.54 (1H, s, H-29), 4.63 (1H, s, H-29), 5.59 (2H, s, CH2), 7.31 (2H, d, J = 8.4 Hz, HAr), 7.63 (2H, d, J = 8.4 Hz, HAr), 7.96 (1H, s, CH-triazole); 13C NMR (150 MHz, CDCl3): δ 14.7, 15.8, 15.9, 19.1, 19.6, 21.1, 21.3, 23.7, 25.2, 26.6, 27.1, 29.6, 29.8, 33.5, 34.2, 34.7, 36.9, 37.8, 39.6, 40.9, 42.8, 46.7, 47.4, 47.7, 48.8, 49.7, 53.7, 55.0, 63.7 110.1, 113.3, 117.9, 127.4, 128.5, 133.1, 138.9, 141.0, 149.9, 160.9, 218.1; HRAPCIMS m/z (neg): 649.4095 C41H53N4O3 (calcd. 649.4118).
28-(1-Phenylthiomethyl-1H-1,2,3-triazol-4-yl)carbonylbetulone (6d)
Yield 87%; m.p. 188–191 °C; IR (KBr) νmax 3132, 2960, 1734, 1705, 1521, 1456, 1241–1196 cm−1; 1H NMR (600 MHz, CDCl3): δ 0.96 (3H, s, CH3), 1.03 (3H, s, CH3), 1.05 (3H, s, CH3), 1.09 (3H, s, CH3), 1.11 (3H, s, CH3), 1.69 (3H, s, CH3), 2.53 (1H, m, H-19), 4.15 (1H, d, J = 10.8 Hz, H-28), 4.57 (1H, d, J = 10.8 Hz, H-28), 4.63 (1H, s, H-29), 4.74 (1H, s, H-29), 5.69 (2H, s, CH2), 7.34-7.37 (5H, m, HAr), 8.06 (1H, s, CH-triazole); 13C NMR (150 MHz, CDCl3): δ 14.7, 15.8, 15.9, 19.1, 19.6, 21.1, 21.3, 25.2, 25.6, 26.6, 27.1, 29.6, 29.8, 33.5, 34.2, 34.7, 36.9, 37.8, 39.6, 40.9, 42.8, 46.7, 47.4, 47.7, 48.8, 49.7, 54.3, 55.0, 63.6, 68.0, 110.0, 126.8, 129.1, 129.7, 131.3, 132.4, 150.0, 160.9, 218.1; HRAPCIMS m/z (neg): 656.3895 C40H54N3O3S (calcd. 656.3886).
28-[1-(3’-Deoxythymidine-5’-yl)-1H-1,2,3-triazol-4-yl]carbonylbetulone (6e)
Yield 73%; m.p. 199–202 °C; IR (KBr) νmax 3447, 3068, 2945, 1729, 1697, 1541, 1458, 1226–1163 cm−1; 1H NMR (600 MHz, DMSO-d6): δ 0.88 (3H, s, CH3), 0.94 (3H, s, CH3), 0.99 (3H, s, CH3), 1.02 (3H, s, CH3), 1.05 (3H, s, CH3), 1.68 (3H, s, CH3), 1.74 (3H, s, CH3-AZT), 2.51 (1H, m, AZT), 2.67 (1H, m, H-19), 3.65–3.70 (2H, m, AZT), 4.04 (1H, d, J = 10.8 Hz, H-28), 4.27 (1H, t, J = 4.8 Hz, AZT), 4.55 (1H, d, J = 10.8 Hz, H-28), 4.58 (1H, s, H-29), 4.74 (1H, s, H-29), 5.27 (1H, t, J = 4.8 Hz, AZT), 5.46 (1H, m, AZT), 6.44 (1H, t, J = 6.6 Hz, AZT), 7.82 (1H, s, AZT), 8.32 (1H, s, CH-triazole), 9.02 (1H, s, NH-AZT); 13C NMR (150 MHz, DMSO-d6): δ 12.7, 14.9, 15.8, 15.9, 16.1, 19.3, 19.6, 21.2, 21.3, 25.3, 26.8, 27.1, 29.4, 29.6, 33.4, 34.1, 34.6, 36.8, 37.6, 37.7, 39.3, 39.6, 42.8, 46.8, 47.0, 47.5, 48.6, 49.4, 54.3, 55.6, 60.2, 61.1, 62.7, 79.6, 84.3, 84.7, 110.1, 110.5, 129.3, 136.7, 139.3, 150.3, 150.9, 160.9, 164.2, 217.0; HRAPCIMS m/z (neg): 758.4484 C43H60N5O7 (calcd. 758.4493).
28-[1-(1-Deoxy-β-D-glucopyranosyl)-1H-1,2,3-triazol-4-yl]carbonylbetulone (6f)
Yield 74%; m.p. 187–189 °C; IR (KBr) νmax 3419, 2939, 1732, 1701, 1541, 1458, 1232–1190 cm−1; 1H NMR (600 MHz, DMSO-d6): δ 0.88 (3H, s, CH3), 0.94 (3H, s, CH3), 0.99 (3H, s, CH3), 1.02 (3H, s, CH3), 1.07 (3H, s, CH3), 1.68 (3H, s, CH3), 1.86 (1H, m, OH), 2.56 (1H, m, H-19), 3.27 (1H, m, OH), 3.40 (1H, m, OH), 3.45 (1H, m, OH), 3.71 (1H, m, CH-sugar), 3.85 (1H, m, CH-sugar), 4.04 (1H, d, J = 10.8 Hz, H-28), 4.56 (1H, d, J = 10.8 Hz, H-28), 4.59 (1H, s, H-29), 4.63 (1H, m, CH-sugar), 4.74 (1H, s, H-29), 5.20 (1H, d, J = 5.4 Hz, CH-sugar), 5.34 (1H, d, J = 5.4 Hz, CH-sugar), 5.45 (1H, d, J = 5.4 Hz, CH-sugar), 5.62 (1H, d, J = 5.4 Hz, CH-sugar), 9.08 (1H, s, CH-triazole); 13C NMR (150 MHz, DMSO-d6): δ 14.9, 15.8, 16.1, 19.3, 19.6, 21.2, 21.3, 25.2, 26.8, 27.1, 29.4, 29.6, 33.4, 34.1, 34.6, 36.8, 37.1, 39.3, 42.8, 46.8, 47.0, 47.5, 48.7, 49.4, 54.3, 61.2, 62.6, 69.9, 72.4, 77.2, 79.6, 80.6, 88.3, 110.5, 129.1, 139.2, 150.3, 160.9, 218.0; HRAPCIMS m/z (neg): 696.4220 C39H58N3O8 (calcd. 696.4224).
28-(1-Ethylacetyl-1H-1,2,3-triazol-4-yl)carbonylbetulone (6g)
Yield 79%; m.p. 97–99 °C; IR (KBr) νmax 3147, 2945, 1755, 1705, 1541, 1458, 1211–1111 cm−1; 1H NMR (600 MHz, CDCl3): δ 0.88 (3H, s, CH3), 0.93 (3H, s, CH3), 0.95 (3H, s, CH3), 1.00 (3H, s, CH3), 1.03 (3H, s, CH3), 1.26 (3H, t, J = 7.2 Hz, CH3), 1.68 (3H, s, CH3), 2.45 (1H, m, H-19), 4.10 (1H, d, J = 10.8 Hz, H-28), 4.23 (2H, q, J = 7.2 Hz, OCH2), 4.51 (1H, d, J = 10.8 Hz, H-28), 4.54 (1H, s, H-29), 4.65 (1H, s, H-29), 5.15 (2H, s, CH2), 8.16 (1H, s, CH-triazole); 13C NMR (150 MHz, CDCl3): δ 14.2, 14.7, 15.9, 19.6, 21.1, 21.3, 25.2, 26.6, 27.1, 29.6, 29.8, 33.5, 34.2, 34.7, 36.9, 37.8, 39.6, 40.9, 42.8, 46.7, 47.4, 47.7, 48.8, 49.7, 51.0, 55.0, 60.4, 62.8, 63.6, 110.0, 128.7, 140.7, 150.1, 160.9, 165.7, 171.2, 218.1; HRAPCIMS m/z (neg): 620.4049 C37H54N3O5 (calcd. 620.4063).
28-[1-(3-Hydroxypropyl)-1H-1,2,3-triazol-4-yl]carbonylbetulone (6h)
Yield 78%; m.p. 197–199 °C; IR (KBr) νmax 3404, 2960, 1735, 1703, 1543, 1458, 1261–1223 cm−1; 1H NMR (600 MHz, DMSO-d6): δ 0.88 (3H, s, CH3), 0.94 (3H, s, CH3), 0.99 (3H, s, CH3), 1.00 (3H, s, CH3), 1.05 (3H, s, CH3), 1.10 (2H, m, CH2), 1.67 (3H, s, CH3), 2.52 (1H, m, H-19), 3.38 (2H, m, CH2), 4.02 (1H, d, J = 10.8 Hz, H-28), 4.48 (2H, t, J = 7.2 Hz, CH2), 4.55 (1H, d, J = 10.8 Hz, H-28), 4.59 (1H, s, H-29), 4.74 (1H, s, H-29), 8.81 (1H, s, CH-triazole); 13C NMR (150 MHz, DMSO-d6): δ 14.9, 15.8, 16.1, 19.2, 19.5, 21.2, 21.3, 25.2, 26.8, 27.1, 29.4, 29.6, 33.1, 33.4, 34.1, 34.6, 36.8, 37.7, 39.3, 42.8, 46.8, 47.0, 47.5, 47.6, 48.6, 49.3, 54.3, 57.8, 62.5, 79.6, 110.5, 129.7, 139.0, 150.3 161.0, 217.0; HRAPCIMS m/z (neg): 592.4131 C36H54N3O4 (calcd. 592.4114).
2-Amino-3-[4-(3-acetyl-28-betulonylcarbonyl)-1H-1,2,3-triazol-1-yl]propanoic acid (6i)
Yield 48%; 163–166 °C; IR (KBr) νmax 3479, 2956, 1732, 1705, 1606, 1456, 1280–1223 cm−1; 1H NMR (600 MHz, DMSO-d6) δ: 0.85 (3 H s, CH3), 0.89 (3H, s, CH3), 0.98 (3H, s, CH3), 1.02 (3H, s, CH3), 1.04 (3H, s, CH3), 1.07 (1H, t, J = 7.2 Hz, CH), 1.67 (3H, s, CH3), 2.51 (1H, m, H-19), 4.12 (1H, d, J = 10.8 Hz, H-28), 4.21 (2H, d, J = 7.2 Hz, CH2), 4.56 (1H, d, J = 10.8 Hz, H-28), 4.59 (1H, s, H-29), 4.74 (1H, s, H-29), 8.62 (1H, s, CH-triazole); 13C NMR (150 MHz, DMSO-d6): δ 14.3, 15.0, 16.1, 16.3, 16.9, 21.5, 22.9, 23.7, 28.1, 28.8, 30.2, 37.1, 37.8, 38.5, 39.5, 40.8, 42.8, 67.9, 80.4, 110.0, 129.1, 132.1, 132.2, 150.1, 167.5, 217.1; HRAPCIMS m/z (neg): 621.4050 C36H53N4O5 (calcd. 621.4015).
3-Methyl-3-[4-(28-betulonylcarbonyl)-1H-1,2,3-triazol-1-yl]butyric acid (6j)
Yield 54%; m.p. 246–249 °C; IR (KBr) νmax 3446, 2945, 1732, 1709, 1616, 1456, 1280–1211 cm−1; 1H NMR (600 MHz, DMSO-d6) δ: 0.72 (3H, s, CH3), 0.87 (3H, s, CH3), 0.96 (3H, s, CH3), 1.00 (3H, d, J = 6.6 Hz, CH3), 1.01 (3H, s, CH3), 1.04 (3H, s, CH3), 1.08 (3H, d, J = 6.6 Hz, CH3), 1.68 (3H, s, CH3), 2.43 (1H, m, CH), 2.53 (1H, m, H-19), 4.03 (1H, d, J = 10.8 Hz, H-28), 4.28 (1H, m, CHCOOH), 4.55 (1H, d, J = 10.8 Hz, H-28), 4.64 (1H, s, H-29), 4.74 (1H, s, H-29), 8.76 (1H, s, CH-triazole); 13C NMR (150 MHz,, DMSO-d6) δ: 14.3, 14.6, 14.9, 15.8, 16.0, 16.1, 18.9, 19.2, 19.6, 19.9, 21.2, 25.2, 26.8, 27.1, 29.5, 29.7, 30.8, 33.4, 34.1, 36.8, 37.7, 39.3, 40.8, 42.8, 46.8, 47.0, 47.5, 48.6, 49.3, 54.3, 62.6, 79.7, 110.5, 129.4, 138.7, 150.3, 161.3, 217.1; HRAPCIMS m/z (neg): 535.3878 C35H51O4 (calcd. 535.3865).
Biological study
Cells
The triterpenes were evaluated for their cytotoxic activity towards three human cancer cell lines: amelanotic melanoma C-32 (ATCC, Rockville, USA), ductal carcinoma T47D (ATCC, Rockville, USA) and glioblastoma SNB-19 (DSMZ, Braunschweig, Germany). The cells were seeded in 96-well plates (Nunc Thermo Fisher Scientific, Waltham, USA) at a density of 5 × 103 cells per well and maintained for 24 h at 37 °C in a humid atmosphere saturated with 5% CO2. All cancer cell lines were cultured in DMEM (Lonza, Basel, Switzerland) growth medium containing 10% fetal bovine serum (FBS) (Biological Industries, Cromwell, USA), penicillin (10,000 U/mL) and streptomycin(10 mg/mL) (Lonza, Basel, Switzerland).
WST-1 assay
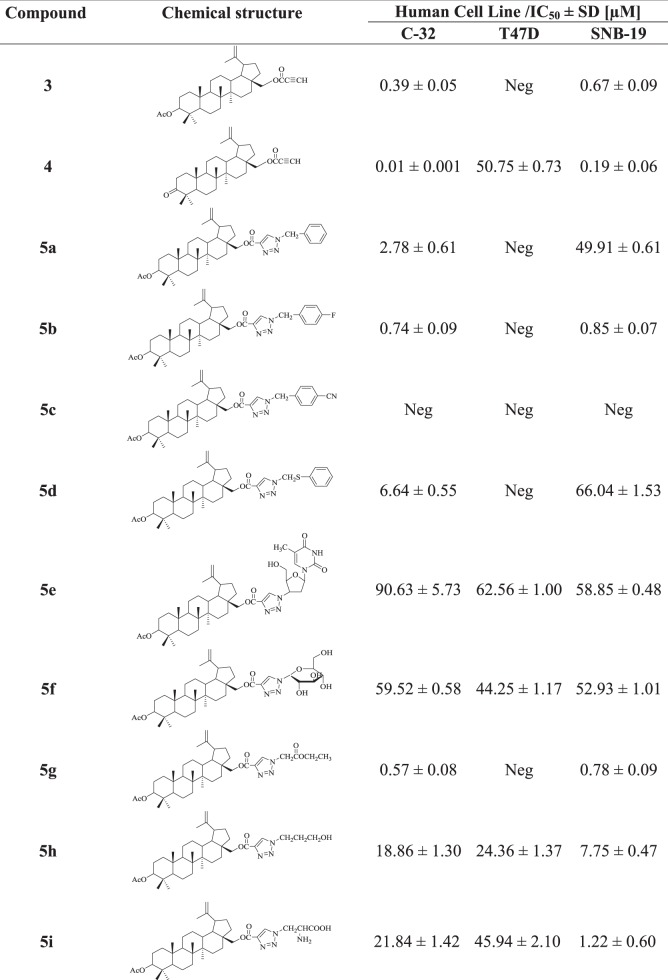
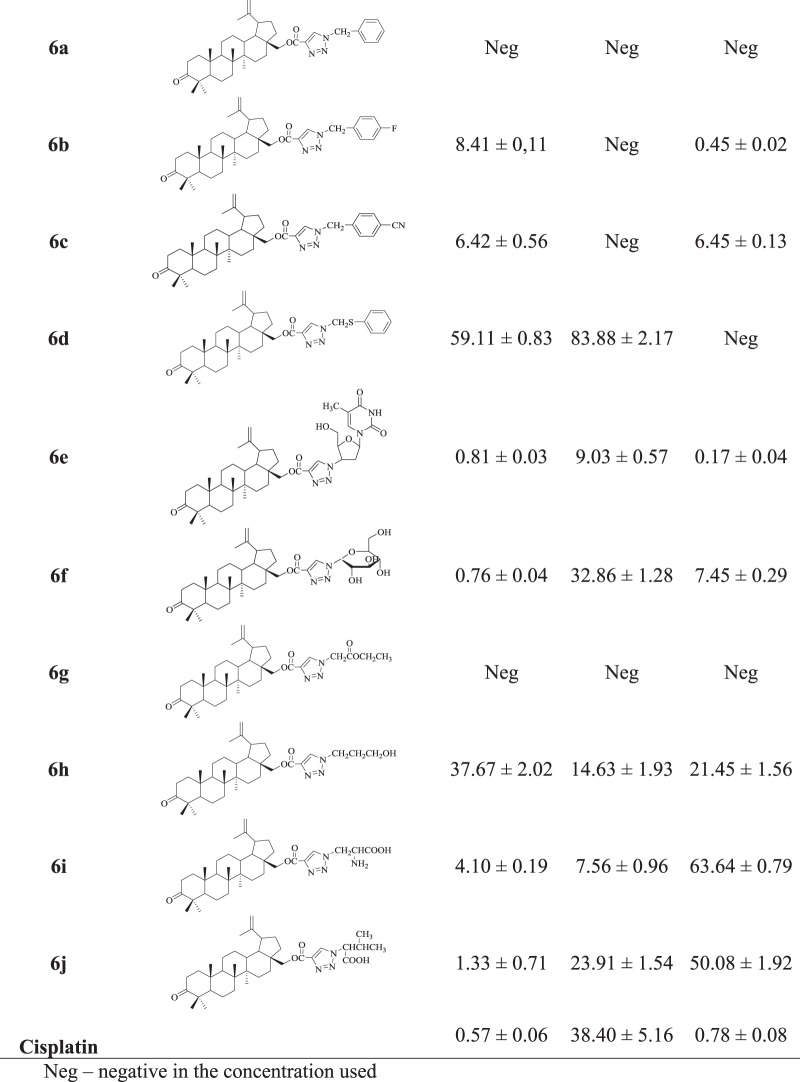
A WST-1 assay (Roche Diagnostics GmbH, Mannheim,Germany) was used for the evaluate of cytotoxicity against the tested human cancer cell lines. The WST-1 assay was carried out after 72 h incubation of the cells with concentrations ranging from 1 to 100 µg/mL of the tested compounds. The WST-1 tetrazolium salt [sodium 2-(4-iodophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium] is reduced by mitochondrial dehydrogenases of viable cells to water-soluble formazan. The amount of formazan produced by viable cells was quantified by measuring the absorbance (λ = 450 nm). The anticancer activity of triterpenes were expressed as an IC50 in µM (Table 2).
Table 2.
Anticancer activity (IC50) of acetylenic esters 3–4, triazoles of triterpenes 5a-i and 6a-j and cisplatin as a reference compound against the tested human cancer cell lines
Lipophilicity studies
The theoretical lipophilicity parameters of triazoles 5a-i and 6a-j were calculated using the commercially available ALOGPS 2.1 software program (Tetko et al. 2005) (Table 3).
Table 3.
The values of calculated lipophilicity parameters of compound 5a–i and 6a–j
| Compound | ALOGPs | AC logP | ALOGP | MLOGP | XLOGP2 | XLOGP3 |
|---|---|---|---|---|---|---|
| 5a | 7.76 | 9.09 | 9.56 | 7.29 | 11.43 | 11.87 |
| 5b | 6.97 | 7.00 | 9.13 | 7.63 | 10.63 | 10.95 |
| 5c | 6.91 | 7.27 | 8.81 | 6.78 | 10.33 | 10.50 |
| 5d | 7.19 | 9.60 | 9.52 | 7.44 | 11.03 | 11.43 |
| 5e | 5.54 | 4.70 | 6.46 | 4.98 | 7.45 | 8.30 |
| 5f | 4.54 | 3.99 | 5.19 | 3.99 | 6.85 | 6.96 |
| 5g | 6.26 | 5.78 | 7.61 | 6.15 | 9.03 | 9.58 |
| 5h | 5.85 | 5.75 | 6.87 | 5.98 | 8.55 | 8.80 |
| 5i | 2.66 | 3.80 | 6.22 | 3.03 | 5.44 | 5.53 |
| 6a | 6.62 | 7.13 | 8.51 | 6.78 | 9.26 | 9.89 |
| 6b | 6.50 | 7.19 | 8.72 | 7.14 | 9.43 | 9.99 |
| 6c | 6.58 | 6.94 | 8.39 | 6.39 | 8.99 | 9.61 |
| 6d | 6.78 | 9.28 | 9.10 | 7.05 | 9.69 | 10.54 |
| 6e | 5.04 | 4.38 | 6.04 | 4.56 | 6.11 | 7.41 |
| 6f | 4.16 | 3.67 | 4.77 | 3.56 | 5.50 | 6.07 |
| 6g | 5.90 | 5.45 | 7.20 | 5.72 | 7.68 | 8.69 |
| 6h | 5.54 | 5.43 | 6.45 | 5.57 | 7.20 | 7.91 |
| 6i | 2.37 | 3.47 | 5.80 | 2.59 | 4.10 | 4.64 |
| 6j | 6.21 | 6.00 | 7.82 | 5.90 | 8.12 | 9.36 |
Results and discussion
Chemistry
The synthesis of triazoles was started from betulin 1 and 3-acetylbetulin 2 (Fig. 1). Acetylation of betulin 1 at the C-3 and C-28 positions with acetic anhydride in the presence of 4-dimethylaminopyridine in pyridine gave betulin 3,28-diacetate. A selective hydrolysis of betulin 3,28-diacetate at C-28 position (MeOH/NaOH/THF) afforded 3-acetylobetulin 2 with a quantitative yield (Thibeault et al. 2007; Santos et al. 2010).
Fig. 1.
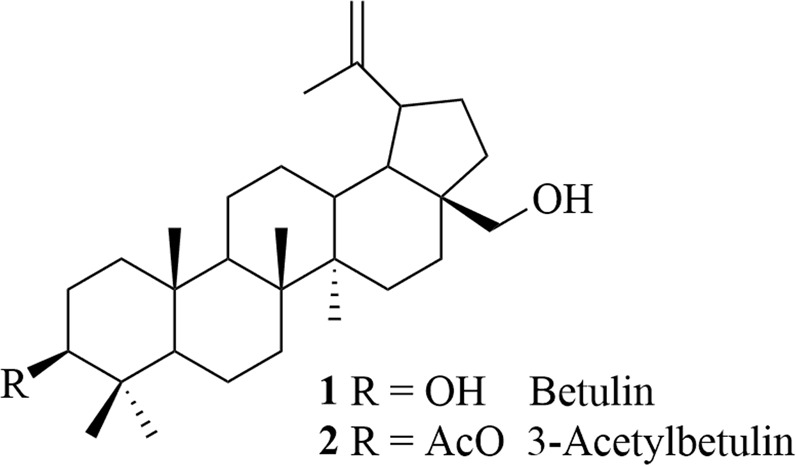
Chemical structure of betulin 1 and 3-acetylbetulin 2
Subsequently, triterpenes 1–2 were used to prepare the propynoylated derivatives 3–4 according to our published procedures (Boryczka et al. 2013). The triazoles 5a-i and 6a-j were obtained by CuAAC reactions of acetylenic esters with various organic azides in toluene with yields in the range of 45–87%. Synthesis of triazoles 5a-i and 6a-j was depicted in Scheme 1. New compounds were purified by column chromatography on silica gel in CHCl3–EtOH or CH2Cl2–EtOH with various ratios. The chemical characterization of all derivatives was carried out by 1H-, 13C-NMR, IR spectroscopies, and HRMS spectra.
Scheme 1.
Synthesis of triazole derivatives 5a-i and 6a-j. Reagents and conditions: organic azide (RN3), CuI, reflux, 72 h
In the 1H NMR spectra of the triazoles 5a-d and 6a-d, singlets of methylene group were observed at δ 5.48–5.69, which suggests the presence of a bond between C-4 (aryl group) and N-1 of the triazole ring. The signals in the range of δ 7.01–7.72 were assigned to the aromatic protons of the aryl group of derivatives 5a-d and 6a-d. Additionally, for all derivatives 5a-i and 6a-j, signals at δ 7.96–9.08 were observed, corresponding to triazolyl protons in the 1,4-disubstituted triazole ring.
Analysis of the 13C NMR spectra of triazoles 5a-i and 6a-j showed that the signals of acetyl and carbonyl groups are located at 167.5–171.1 p.p.m. and 217.0–218.1 p.p.m., respectively.
The IR spectra of new triazoles 5a-i and 6a-j showed characteristic absorption bands at 1527–1616 cm−1 and 1456–1458 cm−1, which were attributed to the C=N and the N=N stretching vibrations of the triazole ring, respectively.
The HRMS negative mode was applied to identify all new compounds. In the mass spectra of triterpenes 3, 5a-i, and 6a-j signals based on ions [M−H]− were observed. These signals were corresponding to the calculated values.
Biological study
The triazole derivatives of 3-acetylbetulin and betulone were evaluated in vitro for their anticancer activity using a WST-1 assay against three human cancer cell lines: amelanotic melanoma C-32, ductal carcinoma T47D and glioblastoma SNB-19. Cisplatin was used as a positive control. The results of the anticancer activity tests of the studied compounds are reported in Table 2 as IC50 (µM).
As shown in Table 2, the lowest anticancer activity (IC50 7.56–83.88 µM) of targeted triazoles was observed in the case of the T47D ductal carcinoma cell line. In the tested group of triazoles, derivative 6i exhibited a highest anticancer activity (IC50 7.56 µM) against the T47D cells, when compared to the positive control.
For triazoles of 3-acetylbetulin 5a-i, the rank order of the anticancer activity towards the C-32 cell line is as follows: 5g > 5b > 5a > 5d > 5h > 5i > 5f > 5e > 5c. The compound 5g containing a 1-ethylacetyl moiety in triazole ring had the same anticancer activity against the C-32 cell line as the reference cisplatin (IC50 0.57 µM). Moreover, triazoles 5c, 6a, and 6g had no anticancer activity towards C-32 cell line in the applied concentration range.
According to our studies, compounds 5b, 5g, 6b, and 6e showed a significant activity against human glioblastoma SNB-19 cell line, with IC50 values from 0.17 to 0.85 µM.
The triazole 6e bearing a 3’-deoxythymidine-5’-yl moiety showed the highest activity in the tested group of compounds against SNB-19 cells, with IC50 value of 0.17 µM.
Our studies suggest, that the introduction of acetyl or carbonyl group at the C-3 position of triazole derivatives of triterpenes afforded compounds having a higher anticancer activity against amelanotic melanoma C-32 cell line. Additionally, the compounds 5f and 6f containing the 1-deoxy-β-D-glucopyranosyl substituted triazole ring had a better activity than their parent 3-hydroxyl substituted analogs against C-32, T47D, and SNB-19 cell lines (Bębenek et al. 2017).
The lipophilicity is one of the important physicochemical parameters in drug development (Andric and Héberger 2015). A lipophilicity study of the tested triazoles was carried out using the ALOGPS 2.1 software program. The predicted log P values were calculated according to the molecular structures of triazoles 5a–i and 6a–j using six computational methods (ALOGPs, AC logP, ALOGP, MLOGP, XLOGP2, and XLOGP3). Considering two triazoles of betulone 6d and 6e, it was observed that their cytotoxic activity increased with the decreasing value of theoretical log P.
Conclusion
In conclusion, on the basis of the CuAAC reaction, a series of new derivatives of 3-acetylbetulin and betulone bearing 1,2,3-triazole moiety has been synthesized. The anticancer activity of the triazoles and cisplatin was tested against the C-32, T47D and SNB-19 cancer cell lines using the WST-1 assay. The triazole 6e with 3’-deoxythymidine-5’-yl substituent proved to be a potent cytotoxic agent with IC50 value of 0.17 µM in the case of the human glioblastoma SNB-19 cell line. Morever, the triazole 6e can be considered as a promising candidate for anticancer therapy.
Acknowledgements
This work was supported by the Medical University of Silesia in Katowice, Poland. Grant No KNW-1-015/K/7/0.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Andri F, Héberger K. Towards better understanding of lipophilicity: assessment of in silico and chromatographic log P measures for pharmaceutically important compounds by nonparametric rankings. J Pharm Biomed Anal. 2015;115:183–191. doi: 10.1016/j.jpba.2015.07.006. [DOI] [PubMed] [Google Scholar]
- Bębenek E, Jastrzębska M, Kadela-Tomanek M, Chrobak E, Orzechowska B, Zwolińska K, Latocha M, Mertas A, Czuba Z, Boryczka S. Novel triazole hybrids of betulin: synthesis and biological activity profile. Molecules. 2017;22:1876–1892. doi: 10.3390/molecules22111876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bębenek E, Kadela-Tomanek M, Chrobak E, Wietrzyk J, Sadowska J, Boryczka New acetylenic derivatives of betulin and betulone, synthesis and cytotoxic activity. Med Chem Res. 2016;26:1–8. doi: 10.1007/s00044-016-1713-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonacorso HG, Moraes MC, Wiethan CW, Luz FM, Meyer AR, Zanatta N, Martins MAP. Synthesis of 1H-1,2,3-triazoles-Rufinamide analogs by 1,3-dipolar cycloaddition and eletrocyclization reactions of trifluoroacetyl enolethers under thermal solventless conditions. J Flu Chem. 2013;156:112–119. doi: 10.1016/j.jfluchem.2013.09.005. [DOI] [Google Scholar]
- Boryczka S, Bębenek E, Wietrzyk J, Kempińska K, Jastrzębska M, Kusz J, Nowak M. Synthesis, structure and cytotoxic activity of new acetylenic derivatives of betulin. Molecules. 2013;18:4526–4543. doi: 10.3390/molecules18044526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bräse S, Friedrich A, Gartner M M, Schröder T. Cycloaddition reactions of azides including bioconjugation. Top Heterocycl Chem. 2008;12:45–115. [Google Scholar]
- Dheer D, Singh V, Shankar R. Medicinal attributes of 1,2,3-triazoles: current developments. Bioorg Chem. 2017;71:30–54. doi: 10.1016/j.bioorg.2017.01.010. [DOI] [PubMed] [Google Scholar]
- He YW, Dong CZ, Zhao JY, Ma LL, Li YH, Aisa HA. 1,2,3-Triazole-containing derivatives of rupestonic acid: click-chemical synthesis and antiviral activities against influenza viruses. Eur J Med Chem. 2014;76:245–255. doi: 10.1016/j.ejmech.2014.02.029. [DOI] [PubMed] [Google Scholar]
- Khan I, Guru SK, Rath SK, Chinthakindi PK, Singh B, Koul S, Bhushan S, Sangwan PL. A novel triazole derivative of betulinic acid induces extrinsic and intrinsic apoptosis in human leukemia HL-60 cells. Eur J Med Chem. 2016;108:104–116. doi: 10.1016/j.ejmech.2015.11.018. [DOI] [PubMed] [Google Scholar]
- Majeed R, Sangwan PL, Chinthakindi PK, Khan I, Dangroo NA, Thota N, Hamid A, Sharma PR, Saxena AK, Koul S. Synthesis of 3-O-propargylated betulinic acid and its 1,2,3-triazoles as potential apoptotic agents. Eur J Med Chem. 2013;63:782–792. doi: 10.1016/j.ejmech.2013.03.028. [DOI] [PubMed] [Google Scholar]
- Marciniec K, Latocha M, Kurczab R, Boryczka S. Synthesis and anticancer activity evaluation of a quinoline-based 1,2,3-triazoles. Med Chem Res. 2017;26:2432–2442. doi: 10.1007/s00044-017-1943-5. [DOI] [Google Scholar]
- Rostovtsev HV, Green LG, Fokin VV, Sharpless KB. A stepwise huisgen cycloaddition process: copper (I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew Chem Int Ed Engl. 2002;41:2596–2599. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Santos RC, Salvador JAR, Marín S, Cascate M, Moreira JN, Dini TCP (2010) Synthesis and structure–activity relationship study of novel cytotoxic carbamate and N-acylheterocyclic bearing derivatives of betulin and betulinic acid. Bioorganic Med Chem 18:4385-4396 [DOI] [PubMed]
- Spivak AY, Gubaidullin RR, Galimshina ZR, Nedopekina DA, Odinokov VN. Effective synthesis of novel C(2)-propargyl derivatives of betulinic and ursolic acids and their conjugation with β-D-glucopyranoside azides via click chemistry. Tetrahedron. 2016;71:1249–1256. doi: 10.1016/j.tet.2016.01.024. [DOI] [Google Scholar]
- Suman P, Patel A, Solano L, Jampana G, Gardner ZS, Holt CM, Jonnalagadda SC. Synthesis and cytotoxicity of Baylis-Hillman template derived betulinic acid-triazole conjugates. Tetrahedron. 2016;73:4214–4226. doi: 10.1016/j.tet.2016.11.056. [DOI] [Google Scholar]
- Tetko IV, Gasteiger J, Todeschini R, Mauri A, Livingstone D, Ertl P, Palyulin VA, Radchenko EV, Zefirov NS, Makarenko AS, Tanchuk VY, Prokopenko VV. Virtual computational chemistry laboratory-design and description. J Comput Aid Mol Des. 2005;19:453–463. doi: 10.1007/s10822-005-8694-y. [DOI] [PubMed] [Google Scholar]
- Thibeault D, Gauthier C, Legault J, Bouchard J, Dufour P, Pichette A. Synthesis and structure-activity relationship study of cytotoxic germanicane - and lupane-type 3β-O-monodesmosidic saponins starting from betulin. Bioorg Med Chem. 2007;15:6144–6157. doi: 10.1016/j.bmc.2007.06.033. [DOI] [PubMed] [Google Scholar]
- Torne CW, Christensen C, Meldal M. Peptidotriazoles on solid phase: [1,2,3]-triazoles by regiospecific copper(I)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J Org Chem. 2002;67:3057–3064. doi: 10.1021/jo011148j. [DOI] [PubMed] [Google Scholar]
- Totobenazara J, Anthony J, Burke AJ. New click-chemistry methods for 1,2,3-triazoles synthesis: recent advances and applications. Tetrahedron Lett. 2015;56:2853–2859. doi: 10.1016/j.tetlet.2015.03.136. [DOI] [Google Scholar]
- Wang XL, Wan K, Zhou CH. Synthesis of novel sulfanilamide-derived 1,2,3-triazoles and their evaluation for antibacterial and antifungal activities. Eur J Med Chem. 2010;45:4631–4639. doi: 10.1016/j.ejmech.2010.07.031. [DOI] [PubMed] [Google Scholar]
- Wei J, Chen J, Xu J, Cao L, Deng H, Sheng W, Zhang H, Cao W. Scope and regioselectivity of the 1,3-dipolar cycloaddition of azides with methyl 2-perfluoroalkynoates for an easy, metal-free route toperfluoroalkylated 1,2, 3-triazoles. J Flu Chem. 2012;133:146–154. doi: 10.1016/j.jfluchem.2011.09.009. [DOI] [Google Scholar]
- Yu F, Wang Q, Zhang Z, Peng Y, Qiu Y, Shi Y, Zheng Y, Xiao S, Wang H, Huang X, Zhu L, Chen K, Zhao C, Zhang C, Yu M, Sun D, Zhang L, Zhou D. Development of oleanane-type triterpenes as a new class of HCV entry inhibitors. J Med Chem. 2013;56:4300–4319. doi: 10.1021/jm301910a. [DOI] [PubMed] [Google Scholar]