Supplemental digital content is available in the text.
Key Words: aripiprazole lauroxil, aripiprazole lauroxil nanocrystal dispersion, long-acting injectable, pharmacokinetics, schizophrenia
Abstract
Background
Aripiprazole lauroxil (AL), a long-acting injectable antipsychotic for the treatment of schizophrenia, requires 21 days of oral aripiprazole supplementation upon initiation. We report findings from a phase 1 study investigating a nanocrystalline milled dispersion of AL (ALNCD) as a potential 1-day initiation regimen. The 1-day initiation regimen is designed to enable rapid achievement of plasma aripiprazole concentrations that are comparable with the 21-day oral initiation regimen. Here, a 6-month pharmacokinetic study compared 2 different initiation regimens for starting AL.
Methods
Patients were randomized 1:1:1:1 to receive 1 of 4 treatments consisting of the 1-day (single ALNCD injection + one 30-mg dose of oral aripiprazole on day 1 only) or the 21-day (15-mg daily dose of oral aripiprazole for 21 days) initiation regimen, each combined with a starting AL dose of either 441 mg or 882 mg.
Results
In total, 133/161 patients completed the study. The pharmacokinetic profile of the 1-day initiation regimen was comparable to the 21-day initiation regimen; both achieved aripiprazole concentrations in the therapeutic range within 4 days and remained in a comparable concentration range during treatment initiation. Common adverse events (≥5.0%) were injection-site pain, headache, increased weight, insomnia, dyspepsia, and anxiety. Nine akathisia events occurred (4 events in 4 patients and 5 events in 2 patients in the 1-day and 21-day initiation regimen groups, respectively).
Conclusions
The 1-day initiation regimen resulted in plasma aripiprazole concentrations consistent with the 21-day initiation regimen. Therefore, a single dose of ALNCD with a single 30-mg oral dose of aripiprazole provides an alternative initiation regimen for starting AL.
Long-acting injectable (LAI) medications provide continuous plasma antipsychotic exposure until the subsequent LAI injection is due. Even in patients with stable schizophrenia, medication gaps for oral antipsychotics as brief as 1 to 10 days increases the risk of hospitalization.1 Long-acting injectables are often started during acute episodes of schizophrenia in the hospital setting with the premise that the patient may be more likely to stay on medication after discharge.2
Aripiprazole lauroxil (AL), a prodrug of the atypical antipsychotic aripiprazole, is available as a long-acting intramuscular (IM) injection indicated for the treatment of schizophrenia.3,4 The conversion of AL to aripiprazole is governed by the slow dissolution of AL and subsequent enzyme-mediated cleavage by esterases, generating N-hydroxymethyl aripiprazole and lauric acid. Aripiprazole is released from a water-based hydrolysis of N-hydroxymethyl aripiprazole.5 Elimination of plasma aripiprazole is primarily hepatic, where the cytochrome P450 3A4 (CYP3A4) and cytochrome P450 2D6 (CYP2D6) enzymes transform aripiprazole to dehydroaripiprazole.6 Genetic polymorphisms in CYP2D6 result in pharmacokinetic (PK) differences among CYP2D6 metabolizer phenotypes after administration of oral aripiprazole.7 The lowest dose strength of AL should be used with a strong CYP2D6 inhibitor. Aripiprazole lauroxil and aripiprazole also have known PK interactions with strong CYP3A4 inhibitors and strong CYP3A4 inducers.8,9
Aripiprazole lauroxil can be initiated at any of the 4 available doses: 441 mg, 662 mg, 882 mg, or 1064 mg. The dissolution properties of AL allow for long dose intervals, namely, an AL dose specifically indicated as a 2-month dose interval option (1064 mg).10 However, the slow dissolution of AL means that plasma concentrations of aripiprazole that are associated with therapeutic effects (as observed in previous studies)11–13 are not immediately achieved after the first injection.5,14 To rapidly achieve target aripiprazole concentrations, the initial AL dose requires 21 days of oral aripiprazole supplementation, after which AL itself provides continuous aripiprazole concentrations.5,14 The clinical effectiveness of this strategy was shown in a randomized, double-blind, 12-week study of AL given at 441 mg or 882 mg every 4 weeks compared with placebo, with the active AL dosage arms receiving 21 days of oral aripiprazole at 15 mg/d.3
We evaluated a nanocrystalline milled dispersion of AL (ALNCD) for use as a single additional injection when starting AL as a way to shorten the oral aripiprazole supplementation from 21 days to 1 day. This clinical study was conducted using a 662-mg dose of ALNCD, as determined in a prior PK study (data not shown). Because dissolution depends, in part, on particle size of the injected drug product, it was expected that the smaller nano-sized particle formulation, ALNCD, would increase the rate of dissolution relative to the current micron-sized AL formulation.15 The PK profile of a single injection of ALNCD was assessed, and the results suggested that, when combined with a single dose of 30-mg oral aripiprazole, ALNCD would provide rapid achievement of aripiprazole concentrations within the therapeutic range in a time frame similar to that of the 21-day oral aripiprazole supplementation and consistent with the 21-day oral regimen used in the pivotal efficacy study of AL.3 The present study tested the PK projection that a single dose of ALNCD, along with a single 30-mg dose of oral aripiprazole, could be used as part of a 1-day initiation regimen that has a PK and safety profile comparable to that of the 21-day oral aripiprazole supplementation with proven efficacy.
MATERIALS AND METHODS
Study Design and Treatment Regimens
The study was conducted in accordance with the Declaration of Helsinki and with Good Clinical Practice Guidelines agreed by the International Conference on Harmonization in 1997. The study protocols, amendments, and informed consent forms were approved by an independent ethics committee/institutional review board for each site. All patients provided written informed consent before entering the study. The institutional review board and study sites are listed in Supplemental Digital Content 1, http://links.lww.com/JCP/A514.
This was a 6-month, double-blind, placebo-controlled, phase 1 study to assess the PK, safety, and tolerability of 2 initiation regimens for starting treatment with AL in patients with schizophrenia (Fig. 1). The 1-day initiation regimen (comprising a single IM 662-mg ALNCD dose, a single 30-mg dose of oral aripiprazole, and either AL 441 or 882 mg) was compared with the 21-day initiation regimen (21 days of 15-mg oral aripiprazole with either AL 441 or 882 mg).3
FIGURE 1.
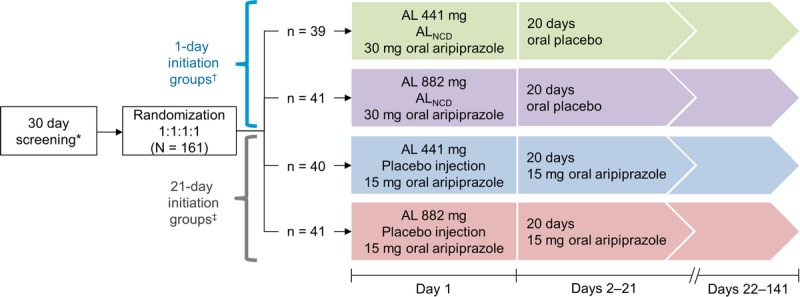
Study design. Patients were admitted to an inpatient facility on day −1 and were discharged after assessments on day 15. After discharge, patients received outpatient follow-up assessments until day 141. *Patients naive to aripiprazole were administered 5-mg test doses of oral aripiprazole on day −30 and day −29. †Patients in the 1-day initiation groups were treated as follows: ALNCD intramuscular (gluteal) plus 30-mg oral aripiprazole plus either intramuscular AL 441 mg (deltoid) or AL 882 mg (contralateral gluteal) on day 1 followed by 20 days of oral placebo. ‡Patients in the 21-day initiation groups were treated as follows: placebo injection (gluteal) plus 15-mg oral aripiprazole plus either intramuscular AL 441 mg (deltoid) or AL 882 mg (contralateral gluteal) on day 1 followed by 20 days of 15-mg oral aripiprazole.
A total of 160 patients were randomized 1:1:1:1 to 1 of 4 treatment groups. In all study groups, the order of administration on day 1 was as follows: oral aripiprazole; IM injection of ALNCD or placebo (<15 minutes after oral aripiprazole); IM injection of AL (<30 minutes after IM injection of ALNCD or placebo). Nanocrystalline milled dispersion of AL or placebo was administered as IM injections in the gluteal muscle. For AL doses, a single 441-mg dose was given in the deltoid muscle or a single 882-mg dose in the gluteal muscle contralateral to the ALNCD (or placebo) injection.
Patients were admitted as inpatients 1 day before their first scheduled dose and were maintained as inpatients for the first 15 days. After discharge, patients returned for outpatient follow-up assessments. Pharmacokinetic samples were taken daily on days 1 to 15, every other day from days 17 to 25, on days 28 and 31, once weekly from days 35 to 85, and on days 113 and 141. On days 1 and 21, samples were collected at multiple time points (as detailed in Pharmacokinetics).
Study Population
Main Inclusion Criteria
Eligible patients were adults aged 18 to 65 years with a diagnosis of chronic schizophrenia or schizoaffective disorder based on the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, and a documented history of tolerability to aripiprazole or a demonstrated tolerability to test doses during screening. In addition, patients were required to have clinically stable schizophrenia, defined as having no hospitalizations for acute psychiatric exacerbations within 3 months before screening and a Clinical Global Impressions-Severity (CGI-S) score of 3 or lower (mild) at screening and study initiation. Patients were on a stable oral antipsychotic medication regimen (excluding aripiprazole and clozapine) for 2 months or more before screening without any medication changes between screening and randomization.
Main Exclusion Criteria
Key exclusion criteria included patients who had received oral aripiprazole for 28 days or less before randomization or any other LAI antipsychotic for 3 months or less before admission and patients who were participating or who had participated in a clinical trial involving any investigational product for 3 months or less before admission. Patients who received AL or IM depot aripiprazole for 6 months or less before inpatient admission were excluded. A history of primary psychopathology other than schizophrenia or schizoaffective disorder or a positive test result for illicit drug use at screening or at admission was not permitted. Patients deemed to be CYP2D6 poor metabolizers were excluded from this study as dose adjustments are required in this patient population (see Table S1, Supplemental Digital Content 2, http://links.lww.com/JCP/A515).
Study Assessments
Pharmacokinetics
Blood samples for liquid chromatography–tandem mass spectrometry were collected for analysis within 1 hour predose and 1, 2, 3, 4, 5, 6, and 8 hours (±15 minutes) postdose on day 1. On postinitiation days 2 to 21, a single sample was collected before oral aripiprazole (or oral placebo) administration. As on day 1, after collection of the predose sample on day 21, additional samples were collected 1, 2, 3, 4, 5, 6, and 8 hours (±15 minutes) postdose. For days 23 to 85, a single sample was collected within ±2 hours of the day 1 oral dosing time, or as close to that time frame as possible. Single PK samples were collected on days 113 and 141. Concentrations of aripiprazole and dehydroaripiprazole were quantified in these plasma samples.
Plasma samples were prepared by a protein-precipitation extraction procedure and analyzed using high-performance liquid chromatography coupled to a tandem mass spectrometry detector (LC/MS/MS). The concentrations of all analytes were calculated using 1/x2 linear regression with a lower limit of quantification of 1.00 ng/mL. Chromatographic separations were performed on a reversed phase column (UPLC SB-C8 1.8 μm, 2.1 × 100 m; from Agilent Technologies, Santa Clara, Calif). The mobile phases were pH unadjusted 0.1% formic acid in 10 mM ammonium acetate (A) and acetonitrile (B). A gradient elution was used, starting at 30% B and ramping to 37% in 0.2 minutes, then increasing to 95% B in 2.9 minutes with a flow rate of 0.4 mL/min, holding at 95% B for 3.1 minutes with a flow rate of 0.8 mL/min, and then lowering back to 30% B in 0.2 minutes with a flow rate of 0.4 mL/min. The total run time was 7.0 minutes. The protonated analytes were quantified by selected reaction monitoring in the positive ionization mode by triple quadrupole mass spectrometer. The method was developed to detect AL, N-hydroxymethyl aripiprazole, aripiprazole, and dehydroaripiprazole, as well as their respective deuterated standards, for analyte transitions of 664.4 to 464.2, 482.2 to 452.3, 446.2 to 285.2, and 452.2 to 289.1, respectively. The interassay variability ranged from −7.0% to 1.8%, and the precision (percentage coefficient of variation) across all analytes was 3.0% to 12.1%.
Safety and Tolerability
All safety analyses were performed using observed data from the safety population, and measurements included adverse events (AEs), vital signs measurements, weight, laboratory test results, electrocardiography findings, Columbia-Suicide Severity Rating Scale responses, movement disorder measures, CGI-S responses, and injection-site evaluation.
Adverse events were assessed daily on days 1 to 15, every other day from days 17 to 25, on days 28 and 31, once weekly from days 35 to 85, and on days 113 and 141. Injection-site evaluations were carried out daily on days 1 to 15, every other day from days 17 to 25, and on day 28. The injection site and surrounding area were evaluated after each injection (separately for the ALNCD or placebo injection site and the AL site). Any observed injection-site reactions (ISRs) were monitored until resolution.
Statistical Analysis
Study populations consisted of the safety population (all patients who received the study drug) and the PK population (all patients who received the study drug and had ≥1 measurable concentration of aripiprazole and dehydroaripiprazole).
The area under the concentration–time curve calculated from day 0 to day 28 (AUC0–28) was computed using the linear trapezoidal rule and included only oral predose concentrations. Actual elapsed time from dosing was used to estimate individual parameters. The 1-day initiation regimen was designed to achieve plasma aripiprazole concentrations in the therapeutic range within 4 days, consistent with the oral initiation regimen indicated in the AL prescribing information.16 Concentrations of aripiprazole and dehydroaripiprazole and AUC0–28 were summarized descriptively by treatment group.
A post hoc evaluation was conducted to compare aripiprazole concentration results from the present study with observed concentrations from the 12-week phase 3 efficacy study (that used the 21-day oral regimen).
Safety and tolerability parameters were estimated in the safety population. Adverse events that were new or that worsened from the time of administration of the first dose of study drug (ALNCD and a single 30-mg oral aripiprazole dose or placebo injection and 21 days of oral aripiprazole 15 mg plus either AL 441 or 882 mg) were summarized using descriptive statistics.
RESULTS
Patient Disposition and Baseline Characteristics
In total, 161 patients were enrolled, received one of the initiation regimens, and were included in the PK and safety populations (Fig. 1). Patients were randomized to receive a 1-day initiation regimen (n = 80) or a 21-day initiation regimen (n = 81), along with an AL starting dose of either 441 or 882 mg. Thirty-nine patients were enrolled in the AL 441 mg/1-day initiation group, and 41 patients were enrolled in the AL 882 mg/1-day initiation group. Of those enrolled in the 21-day initiation regimen groups, 40 patients were assigned to the AL 441 mg/21-day initiation group and 41 patients were assigned to the AL 882 mg/21-day initiation group.
A total of 133 patients (82.6%) completed the study. Among the 28 patients (17.4%) who did not complete the study, reasons for study withdrawal included lost to follow-up and withdrawal by the patient (each n = 10; 6.2%), AE (n = 5; 3.1%), protocol deviation (n = 2; 1.2%), and noncompliance with medication (n = 1; 0.6%).
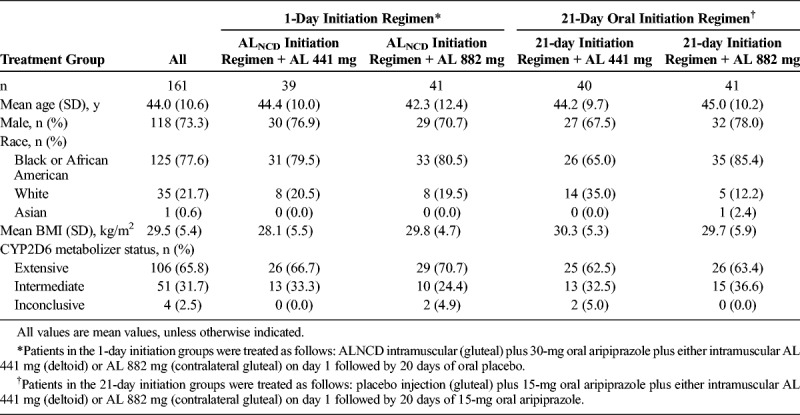
Patient demographics are summarized in Table 1. Mean age and body mass index of the patients were 44 years and 29.5 kg/m2, respectively. All patients were either CYP2D6 extensive or intermediate metabolizers with the exception of 4 patients, whose status was considered to be inconclusive. All patients with an inconclusive result had at least 1 functional allele and were not poor metabolizers. Overall, the treatment groups were well balanced for demographic and baseline characteristics.
TABLE 1.
Patient Baseline Characteristics
Pharmacokinetic Results
Results from the 1-day initiation regimen groups showed mean plasma aripiprazole concentrations and exposures within the first month that were comparable to those of the 21-day initiation regimen groups (Fig. 2). In the first 24 hours after initiation, higher aripiprazole concentrations were observed with the 1-day initiation regimen groups compared with the 21-day initiation regimen groups due to the higher dose of aripiprazole administered on day 1 with the 1-day initiation regimen (30 mg vs 15 mg). Plasma concentrations of postinitiation on day 4 were of particular interest because the 1-day initiation regimen was designed to replicate the 21-day initiation regimen in achieving aripiprazole concentrations in the therapeutic range within 4 days after the first AL dose.16 As shown in Figure 2, the 1-day regimen, like the 21-day initiation regimen, results in achievement of aripiprazole concentrations that are in the therapeutic range within 4 days.
FIGURE 2.
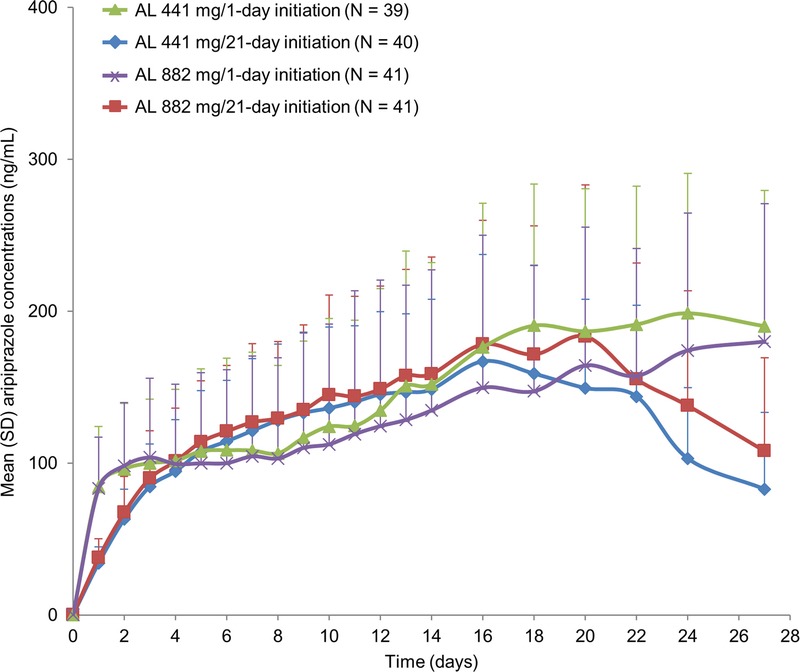
Mean (+SD) aripiprazole concentrations over time (28 days) after treatment initiation with oral aripiprazole (15 mg over 21 days) or ALNCD plus a single dose of oral aripiprazole (30 mg). Data displayed in Figure 2 represents the first 28 days displayed in Figure 3.
As seen in Figure 2, mean concentrations appear visually lower in the 1-day initiation regimen groups than in the 21-day initiation regimen groups from approximately day 4 to day 14; error bars around the plasma concentration means show complete overlap in the range of concentrations across the treatment groups. As expected for the 21-day oral initiation regimen group, aripiprazole concentrations declined after day 21 after discontinuation of the active oral medication. In contrast, for the 1-day initiation regimen groups, plasma aripiprazole concentrations did not show any meaningful changes until after postinitiation on day 30, when mean aripiprazole concentrations began to decline (Fig. 3), indicating that the 1-day initiation regimen provides continuous coverage over a longer period than the 21-day initiation regimen.
FIGURE 3.
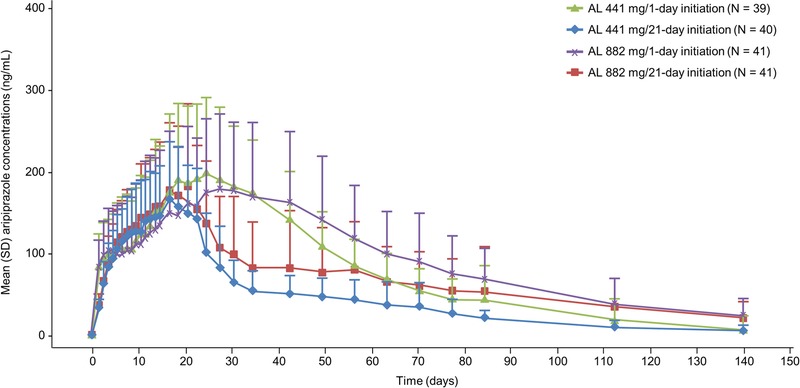
Mean (+SD) aripiprazole concentrations over time (140 days) for treatment initiation with ALNCD plus a single dose of oral aripiprazole (30 mg) or oral aripiprazole (15 mg over 21 days).
Values of AUC0–28 were comparable across the 4 treatment groups (Fig. 4). Comparison of the range of values across groups indicated similar exposure within the first month of treatment regardless of the initiation regimen used.
FIGURE 4.
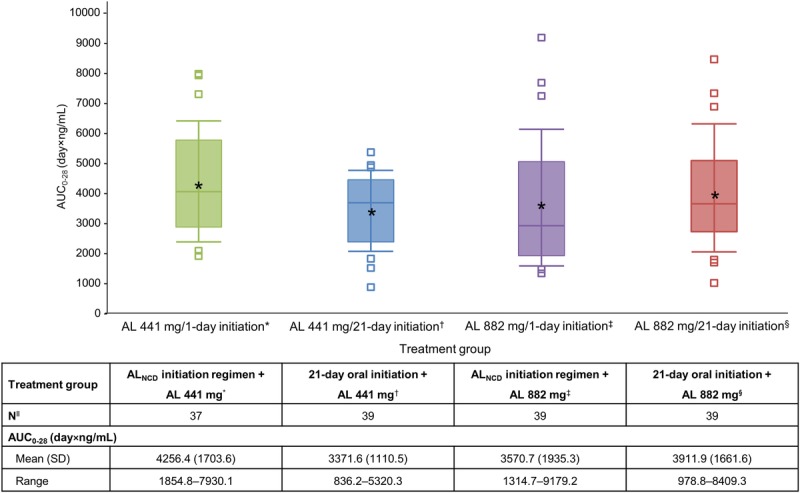
Box plot of AUC0–28 for aripiprazole, by starting AL dose/initiation regimen. The boxes represent the 25th and 75th percentiles of aripiprazole concentration, the line within each box marks the median, the asterisk indicates the mean, and the whiskers indicate the 10th and 90th percentiles. The squares represent individual observations beyond the 10th and 90th percentiles. *AUC0–28 values could not be estimated for all patients because some patients discontinued the study before day 28.
Post hoc comparison of aripiprazole concentrations resulting from the present study with those observed in the 12-week phase 3 efficacy study showed consistent and reproducible results across studies with the 21-day oral aripiprazole initiation regimen (see Fig. S1, Supplemental Digital Content 3, http://links.lww.com/JCP/A516). The 1-day initiation regimen from the present study resulted in aripiprazole concentrations within the concentration range observed with the 21-day oral aripiprazole supplementation used in the phase 3 efficacy study (see Fig. S2, Supplemental Digital Content 4, http://links.lww.com/JCP/A517).
The plasma dehydroaripiprazole concentration–time profile followed that of aripiprazole in each treatment group through day 21 (the last day of oral administration of aripiprazole or placebo). Thereafter, plasma dehydroaripiprazole concentrations continued to persist in the systemic circulation in each 1-day initiation regimen group, whereas concentrations decreased more rapidly in each 21-day initiation regimen group (see Fig. S3, Supplemental Digital Content 5, http://links.lww.com/JCP/A518). Mean ± SD AUC0–28 values were comparable in each treatment group: AL 441 mg/1-day initiation regimen, 1222.4 (455.7) day × ng/mL; AL 882 mg/1-day initiation regimen, 1126.6 (597.4) day × ng/mL; AL 441 mg/21-day initiation regimen, 1435.5 (654.8) day × ng/mL; AL 882 mg/21-day initiation regimen, 1315.9 (439.6) day × ng/mL.
Safety Results
Adverse Events
Throughout the study period, small and similar mean changes (≤0.1) from baseline (score of 3.0, mild) in CGI-S score were seen in each initiation regimen group at all time points, indicating no change in disease severity. All patients had a score of 0 (no suicidal behavior or ideation) for Columbia-Suicide Severity Rating Scale throughout the study.
In the AL 441 mg/1-day initiation and the AL 882 mg/1-day initiation groups, 26 patients (66.7%) and 28 patients (68.3%) experienced AEs, respectively. In addition, 24 patients (60.0%) and 28 patients (68.3%) in the AL 441 mg and the AL 882 mg/21-day initiation groups experienced AEs, respectively (see Table S2, Supplemental Digital Content 6, http://links.lww.com/JCP/A519). Most AEs were mild or moderate in intensity. Serious AEs were reported in 6 patients: 3 each in the 1-day initiation regimen (road traffic accident, status epilepticus, psychotic disorder, and schizoaffective disorder) and the 21-day initiation regimen groups (upper gastrointestinal hemorrhage, cellulitis, road traffic accident, and accidental overdose). Of these, schizoaffective disorder and status epilepticus were assessed as possibly related to treatment. A total of 5 patients discontinued the study because of AEs: 3 in the 1-day initiation regimen (road traffic accident, extrapyramidal disorder, and status epilepticus) and 2 in the 21-day initiation regimen (road traffic accident and nausea). One patient in the 21-day initiation regimen group died as a result of injuries sustained in a road traffic accident (considered not related to study drug). Overall, the most common AEs reported were injection-site pain (23.0%), headache (9.9%), weight increased (7.5%), insomnia (6.2%), dyspepsia (5.6%), and anxiety (5.0%).
Adverse Events of Interest
Injection-site reaction is a common AE associated with LAIs2,17,18 and therefore was evaluated in greater detail. All ISRs associated with the ALNCD, placebo IM, AL 441 mg, and AL 882 mg injections were mild to moderate in severity.
ISRs Associated With ALNCD or Placebo IM Injection
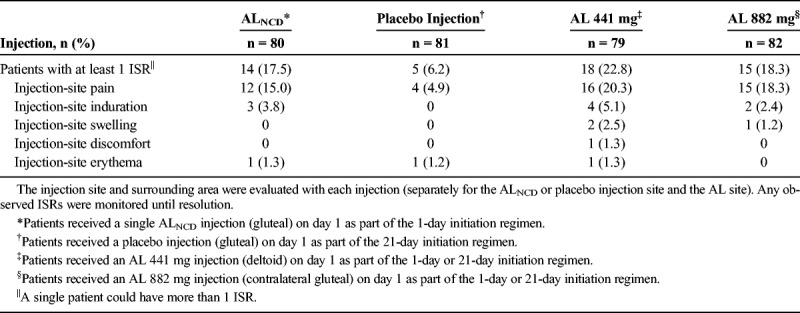
Fourteen (17.5%) of 80 patients who received ALNCD injection experienced ISRs compared with 5 (6.2%) of 81 patients who received placebo injection (Table 2). The most common description of ISRs was injection-site pain, which was reported in 12 (15.0%) of 80 patients who received ALNCD injection compared with 4 (4.9%) of 81 patients who received placebo IM injection.
TABLE 2.
ISRs by Treatment Group Associated With ALNCD or Placebo Injection and Those Associated With AL (441 mg or 882 mg)
ISRs Associated With AL Injection
Eighteen (22.8%) of 79 patients who received AL 441 mg in the deltoid muscle and 15 (18.3%) of 82 patients who received AL 882 mg in the gluteal muscle experienced ISRs (Table 2). The most common description of ISRs was injection-site pain, which was reported in 16 (20.3%) of 79 patients who received AL 441 mg and 15 (18.3%) of 82 patients who received AL 882 mg.
Akathisia
Akathisia was an AE of interest because it is commonly reported in association with oral aripiprazole used for the treatment of patients with schizophrenia.
The overall incidence of akathisia in all groups was low, with a total AE rate of 6 (3.7%) of 161 patients. Among patients treated with the 1-day initiation regimen, akathisia was reported in 4 (5.0%) of 80 patients. Two of these patients reported mild akathisia during the first week of treatment. One was assessed as having mild akathisia probably not related to the study drug and the other was assessed as having mild akathisia definitely related to the study drug. The other 2 patients experienced akathisia in the third week of treatment, one of which was rated as mild and the other as moderate in severity, assessed as probably related and definitely related to treatment, respectively. Among patients treated with the 21-day oral regimen, mild akathisia was reported in 2 (2.5%) of 81 patients. One experienced the first akathisia event in the second week and the other in the third week of treatment, assessed as possibly related and probably related to treatment, respectively.
DISCUSSION
A 1-day initiation regimen was evaluated as an alternative to the current 21-day initiation regimen. In this study, ALNCD in conjunction with 30-mg oral aripiprazole and either a 441-mg or an 882-mg dose of AL resulted in aripiprazole concentrations within the therapeutic range by the fourth day and a range of aripiprazole concentrations that overlapped with those produced by the 21-day period of oral aripiprazole. Results also showed that the 1-day initiation regimen provided continuous exposure within the first month of treatment initiation that was comparable with the 21-day initiation regimen. In addition, total aripiprazole exposure during the first 28 days after treatment initiation was comparable between the 2 initiation regimens. Dehydroaripiprazole concentrations followed a pattern generally similar to that seen for aripiprazole, and total exposure was similar between treatment groups. The 1-day initiation regimen was shown to be suitable as an alternative option to the current 21 days of concomitant oral aripiprazole for starting AL.
Comparison of aripiprazole concentrations for the 21-day initiation regimen groups in the present study with those obtained from the 2 active AL arms in the pivotal 12-week phase 3 efficacy study that used the same dose of oral aripiprazole (21 days of 15 mg/d) (NCT01469039)3 showed that the aripiprazole concentrations achieved were consistent and reproducible. In addition, aripiprazole concentrations resulting from the 1-day initiation regimen in the present study were within the concentration range observed with oral initiation in the phase 3 study. A to-be-marketed dose strength of 675 mg is proposed to distinguish ALNCD from AL in the clinical setting. The dose of 675 mg is within the range of acceptable variance for the 662-mg dose.
Overall, treatment with ALNCD in combination with a single dose of 30-mg oral aripiprazole and either AL 441 mg or AL 882 mg was well tolerated in patients with schizophrenia. The safety profile of the 1-day initiation regimen was generally consistent with the known safety profile of aripiprazole and AL, with most AEs being mild to moderate in intensity and a low rate of discontinuations occurring due to drug-related AEs. A low incidence of akathisia was reported, and analysis of the time to akathisia occurrence and its severity did not show any differences between the 1-day and the 21-day groups.
One patient experienced a serious AE of status epilepticus. This patient experienced peak aripiprazole concentrations on day 7 and by day 17, and when the event occurred, aripiprazole concentrations were declining. The postseizure magnetic resonance imaging findings were consistent with preexisting seizure diathesis and focal structural lesion was excluded. Although these details were revealed through the follow-up information, the causality remained conservatively the same as possibly related. The patient was also prescribed multiple other psychotropic medications. Although rated as “possibly related” to the AL and ALNCD intervention, there are multiple confounders in this case for a plausible relationship to the study drug.
The results demonstrate that a 1-day initiation regimen was comparable to the current 21-day initiation regimen and may offer an alternative for patients starting AL therapy. In addition, a 1-day initiation regimen may be beneficial for patients in whom poor adherence is anticipated, and may offer another option for patients who may prefer a regimen with lower pill burden or who are interested in reducing the duration of the oral initiation regimen from 21 days to 1 day.
In conclusion, a key step in the management of schizophrenia is successful completion of the initiation phase for the LAI antipsychotic treatment. In the present study, we report the development of a 1-day initiation regimen for AL that uses a single injection of ALNCD and a single oral dose of aripiprazole. The proposed 1-day initiation regimen could provide clinicians and patients with the option of an LAI initiation that can be completed in a single day as an alternative to 21 consecutive days of oral aripiprazole in conjunction with the first dose of AL.
Supplementary Material
ACKNOWLEDGMENT
The authors thank all patients and investigators who participated in the clinical trial. Medical writing and editorial support for the preparation of this manuscript (under the guidance of the authors) were provided by Mia Cahill (ApotheCom, United Kingdom).
AUTHOR DISCLOSURE INFORMATION
Dr Hard is a former employee of Alkermes and is presently an employee of Nuventra Pharma Sciences. Drs Wehr, Weiden, and von Moltke are employees of Alkermes. Dr Walling has received grants from Alkermes, Janssen, Otsuka, Forum, Lundbeck, Sunovion, Acadia, Allergan, IntraCellular, Noven, Merck, AbbVie, and Roche.
Lisa von Moltke has declared a relationship with a member of the Editorial Board of the Journal of Clinical Psychopharmacology, which may be considered a possible conflict of interest. An alternative independent review mechanism has been utilized according to the Journal of Clinical Psychopharmacology procedures for dealing with any potential appearance of bias.
The study was conducted in accordance with the Declaration of Helsinki and with Good Clinical Practice Guidelines agreed by the International Conference on Harmonization in 1997. Study protocols, amendments, and informed consent forms were approved by the independent ethics committee/institutional review board for each site.
Informed consent was obtained from all participants included in the study.
Footnotes
Marjie L. Hard is affiliated with Moderna Therapeutics Cambridge, MA.
This study was sponsored by Alkermes, Inc, Waltham, MA. Funding for editorial support for manuscript preparation was provided by Alkermes, Inc, Waltham, MA.
Clinical Trial Registration: The study is based on data from phase 1 clinical trials that have not been registered at any clinical trial registries.
Supplemental digital content is available for this article. Direct URL citation appears in the printed text and is provided in the HTML and PDF versions of this article on the journal’s Web site (www.psychopharmacology.com).
REFERENCES
- 1.Weiden PJ, Kozma C, Grogg A, et al. Partial compliance and risk of rehospitalization among California Medicaid patients with schizophrenia. Psychiatr Serv. 2004;55:886–891. [DOI] [PubMed] [Google Scholar]
- 2.Whyte A, Parker C. A review of the efficacy and tolerability of antipsychotic long-acting injections. Prog Neurol Psychiatry. 2016;20:22–28. [Google Scholar]
- 3.Meltzer HY, Risinger R, Nasrallah HA, et al. A randomized, double-blind, placebo-controlled trial of aripiprazole lauroxil in acute exacerbation of schizophrenia. J Clin Psychiatry. 2015;76:1085–1090. [DOI] [PubMed] [Google Scholar]
- 4.Nasrallah HA AR, Du Y, Stanford AD, et al. Long-term safety and tolerability of aripiprazole lauroxil in patients with schizophrenia. CNS Spectrums. 2018; In Press. [DOI] [PubMed] [Google Scholar]
- 5.Hard ML, Mills RJ, Sadler BM, et al. Aripiprazole lauroxil: Pharmacokinetic profile of this long-acting injectable antipsychotic in persons with schizophrenia. J Clin Psychopharmacol. 2017;37:289–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Swainston Harrison T, Perry CM. Aripiprazole: a review of its use in schizophrenia and schizoaffective disorder. Drugs. 2004;64:1715–1736. [DOI] [PubMed] [Google Scholar]
- 7.Azuma J, Hasunuma T, Kubo M, et al. The relationship between clinical pharmacokinetics of aripiprazole and CYP2D6 genetic polymorphism: effects of CYP enzyme inhibition by coadministration of paroxetine or fluvoxamine. Eur J Clin Pharmacol. 2012;68:29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hard M, Sadler B, Mills R, et al. Aripiprazole lauroxil pharmacokinetics: application of modeling and simulation for dosing considerations of a long-acting injectable antipsychotic in persons with schizophrenia. Paper presented at: American Society of Clinical Psychopharmacology Annual Meeting. May 30–June 3, Scottsdale, Arizona: 2016. [Google Scholar]
- 9.Kubo M, Koue T, Inaba A, et al. Influence of itraconazole co-administration and CYP2D6 genotype on the pharmacokinetics of the new antipsychotic ARIPIPRAZOLE. Drug Metab Pharmacokinet. 2005;20:55–64. [DOI] [PubMed] [Google Scholar]
- 10.Hard ML, Mills RJ, Sadler BM, et al. Pharmacokinetic profile of a 2-month dose regimen of aripiprazole lauroxil: a phase I study and a population pharmacokinetic model. CNS Drugs. 2017;31:617–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raoufinia A, Baker RA, Eramo A, et al. Initiation of aripiprazole once-monthly in patients with schizophrenia. Curr Med Res Opin. 2015;31:583–592. [DOI] [PubMed] [Google Scholar]
- 12.Raoufinia A, Peters-Strickland T, Nylander AG, et al. Aripiprazole once-monthly 400 mg: comparison of pharmacokinetics, tolerability, and safety of deltoid versus gluteal administration. Int J Neuropsychopharmacol. 2017;20:295–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kirschbaum KM, Muller MJ, Malevani J, et al. Serum levels of aripiprazole and dehydroaripiprazole, clinical response and side effects. World J Biol Psychiatry. 2008;9:212–218. [DOI] [PubMed] [Google Scholar]
- 14.Turncliff R, Hard M, Du Y, et al. Relative bioavailability and safety of aripiprazole lauroxil, a novel once-monthly, long-acting injectable atypical antipsychotic, following deltoid and gluteal administration in adult subjects with schizophrenia. Schizophr Res. 2014;159:404–410. [DOI] [PubMed] [Google Scholar]
- 15.Merisko-Liversidge E, Liversidge GG, Cooper ER. Nanosizing: a formulation approach for poorly-water-soluble compounds. Eur J Pharm Sci. 2003;18:113–120. [DOI] [PubMed] [Google Scholar]
- 16.Cruz MP. Aripiprazole lauroxil (Aristada): an extended-release, long-acting injection for the treatment of schizophrenia. P T. 2016;41:556–559. [PMC free article] [PubMed] [Google Scholar]
- 17.Atkins S, Detke HC, McDonnell DP, et al. A pooled analysis of injection site-related adverse events in patients with schizophrenia treated with olanzapine long-acting injection. BMC Psychiatry. 2014;14:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Citrome L. Paliperidone palmitate—review of the efficacy, safety and cost of a new second-generation depot antipsychotic medication. Int J Clin Pract. 2010;64:216–239. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


