Abstract
Recently, new criteria for sepsis-induced coagulopathy (SIC) were developed, including the sequential organ failure assessment (SOFA) criteria. The objective of this study was to evaluate the new SIC criteria in patients diagnosed with sepsis 3.0. Data from patients diagnosed with sepsis 3.0 after ICU admission were retrospectively obtained from July 2013 to June 2014. Relevant demographic, clinical, and laboratory parameters were noted. This study included 252 patients. The International Society on Thrombosis and Haemostasis (ISTH) disseminated intravascular coagulation (DIC), modified ISTH-DIC, and SIC scores were higher among nonsurvivors (P < 0.0001). The Acute Physiology and Chronic Health Evaluation II (P < 0.001), ISTH (P = 0.001), modified ISTH (P = 0.001), and SIC scores (P = 0.007) were independent predictors of ICU mortality. Using the receiver operating characteristic curve, SOFA had the greatest power for predicting ICU mortality; ISTH or modified ISTH score had greater predictive power than the SIC score. There were strong correlations between SIC score and ISTH (P < 0.0001), modified ISTH (P < 0.0001), the Acute Physiology and Chronic Health Evaluation II (P = 0.012), and SOFA (P < 0.0001) scores. More nonsurvivors were diagnosed with DIC using the ISTH and modified ISTH criteria (P < 0.001). In contrast, there was no significant difference in the proportion of patients with SIC between both groups (P = 0.055). ISTH score, modified ISTH score, and SIC score were independent risk factors for ICU mortality. Compared with the ISTH and modified ISTH scores, SIC score showed no advantage in diagnosing sepsis-associated coagulopathy or DIC. The application of these three criteria in patients with sepsis 3.0 needs further evaluation.
Keywords: death, disseminated intravascular coagulation, ICU, sepsis
Introduction
Disseminated intravascular coagulation (DIC) is a serious condition resulting from various underlying diseases, including trauma, acute promyelocytic leukemia, and sepsis [1,2]. Furthermore, DIC is an independent predictor of mortality in patients with critical illness [2,3]. There is no single gold-standard diagnostic test for DIC; however, a combination of several conventional coagulation tests may be helpful in diagnosis [4]. Three DIC diagnostic criteria, the Japanese Ministry of Health and Welfare (JMHW) criteria, International Society on Thrombosis and Haemostasis (ISTH) criteria, and Japanese Association for Acute Medicine (JAAM) criteria, are well known [4–7] (Table 1). Each of these criteria has disadvantages; for instance, JMHW criteria and ISTH criteria have poor sensitivity, especially with regard to infectious diseases, and JAAM criteria cannot be applied to DIC complicated by trauma or hematopoietic malignancy [4].
Table 1.
Comparison of existing disseminated intravascular coagulation/coagulopathy diagnostic criteria
| JMHW | ISTH | Modified ISTH | JAAM | SIC | |
| Underlying disease clinical symptoms | 1 p | 0 p (essential) | 0 p (essential) | 0 p (essential) | 0 p |
| Bleeding: 1 p | 0 p | 0 p | SIRS score ≥3: 1 p | 0 p | |
| Organ failure: 1 p | 0 p | 0 p | 0 p | Four items SOFAa | |
| 1 : 1 p | |||||
| ≥2 : 2 p | |||||
| Platelet count (×109/l) | 80<–≤120: 1 p | 50–100: 1 p | 50–100: 1 p | 80–≤120 or >30% reduction/24 h: 1 p | 100–150: 1 p |
| 50<–≤80: 2 p | <50: 2 p | <50: 2 p | <80 or >50% reduction/24 h: 3 p | <100: 2 p | |
| ≤50: 3 p | |||||
| Fibrin-related marker | FDP (μg/ml) | FDP, d-dimer, SF | FDP, d-dimer, SF | FDP (μg/ml) | None |
| 10≤–<20: 1 p | Moderate increase: 2 p | Moderate increase: 2 p | 10≤–<25: 1 p | ||
| 20≤–<40: 2 p | Strong increase: 3 p | Strong increase: 3 p | ≥25: 3 p | ||
| ≥40: 3 p | |||||
| Fibrinogen (g/l) | 1.0<–≤1.5: 1 p | <1.0: 1 p | None | None | None |
| ≤1.0: 2 p | |||||
| PT | PT ratio | Prolonged PT (s) | Prolonged PT (s) | PT ratio | PT ratio |
| 1.25≤–<1.67: 1 p | 3–6: 1 p | 3–6: 1 p | ≥1.2: 1 p | 1.2–1.4: 1 p | |
| ≥1.67: 2 p | >6: 2 p | >6: 2 p | >1.4: 2 p | ||
| None | None | None | None | ||
| Diagnosis of DIC | ≥7 p | ≥5 p | ≥4 p | ≥4 p | ≥4p (coagulopathy) |
DIC, disseminated intravascular coagulation; FDP, fibrin degradation product; ISTH, International Society on Thrombosis and Haemostasis; JAAM, Japanese Association for Acute Medicine; JMHW, Japanese Ministry of Health and Welfare; PT, prothrombin time; SF, soluble fibrin; SIRS, systemic inflammatory response syndrome; SOFA, sequential organ failure assessment.
aFour items SOFA including respiratory SOFA, cardiovascular SOFA, hepatic SOFA, renal SOFA.
Sepsis-associated DIC is characterized by activation of coagulation and an excessive inhibition of fibrinolysis with a high risk of organ dysfunction [8–10]. In contrast with traumatic coagulopathy, hypofibrinogenemia usually occurs at the late stage of sepsis-associated DIC [1,4,11,12]. Thus, in the JAAM criteria there is no ‘fibrinogen’ score, which is the main difference with the ISTH criteria [7] (Table 1). However, some previous studies supported using revised DIC scores without the ‘fibrinogen’ score in patients with sepsis [13–15]. In recent years, JAAM criteria have been widely used for sepsis-induced DIC [14,15]. With the change of sepsis definition from systemic inflammatory response syndrome (SIRS) criteria to sequential organ failure assessment (SOFA) criteria [16], the JAAM criteria, which include the SIRS score, have been challenged. More recently, new criteria for sepsis-induced coagulopathy (SIC) were developed on the basis of logistic regression analyses (Table 1) [17].
The aim of the current study was to evaluate the new SIC criteria in patients diagnosed with sepsis 3.0, as well as to examine the predictive value of the new criteria for prognosis, compared with ISTH DIC criteria and modified ISTH DIC criteria (Table 1).
Methods
Patient selection and data collection
The dataset was obtained from July 2013 to June 2014 at The First Hospital of China Medical University. All patients in this study were admitted to the ICU and were diagnosed with infection or suspected infection. The exclusion criteria were as follows: age less than 18 years, pregnancy, hematopoietic malignancy, cardiopulmonary resuscitation, liver diseases classified as Child-Pugh grade C, major bleeding, chronic renal failure or renal replacement therapy, SOFA score less than 2, or death within 24 h after admission to the ICU. Moreover, the cases with incomplete clinical or laboratory data, unknown prognosis in the ICU, or refusal of treatment were also excluded.
A total of 252 cases were included, all of whom were diagnosed with sepsis 3.0 within 24 h after admission to the ICU. Clinical parameters for these patients were recorded. The laboratory parameters, including the platelet count, prothrombin time (PT), international normalized ratio (INR), activated partial thromboplastin time (APTT), fibrinogen (FIB), fibrin degradation product (FDP), and d-dimer were the ‘initial data’ in the ICU (all blood samples were obtained within 6 h after admission to ICU) and measured in the clinical laboratory of our hospital. The outcome measure was ICU mortality. The ethics committee of our hospital approved this study.
Definitions and organ dysfunction assessments
The severity of illness of the patients was evaluated according to the Acute Physiology and Chronic Health Evaluation (APACHE) II score and the SOFA score, which were determined within 24 h after admission to the ICU. Sepsis 3.0 was defined according to the third international consensus definitions for sepsis and septic shock [16]. The ISTH DIC criteria, modified ISTH DIC criteria, and new SIC criteria are listed in Table 1. Emergency surgery was defined as less than 12 h from the end of the surgical operation to admission to the ICU.
Statistical analysis
Numerical values are presented as the median and interquartile range, and categorical data are presented as counts and frequencies. Between-group comparisons were performed using the Mann–Whitney U test for numerical data and the Chi-squared test or Fisher's exact test for categorical data as appropriate. Variables that had a P value of less than 0.10 in the univariate analysis were used to build the multivariate model. As SOFA criteria are included in the SIC criteria, the APACHE II score was used in the multivariate analysis. The predictive accuracy of APACHE II, SOFA, ISTH, modified ISTH, and SIC scores for mortality was explored by using the receiver operating characteristic (ROC) curve and the relative area under the curve (AUC). For each indicator, different cutoff points were tested for sensitivity and specificity. The correlation of DIC or coagulopathy criteria with severity scores were tested using Pearson's correlation coefficients. A two-tailed P value less than 0.05 was considered to be statistically significant. Statistical analyses were performed using SPSS version 20.0 software (SPSS, Chicago, Illinois, USA).
Results
Baseline characteristics of the patients
Table 2 shows the primary infection site in the study population. Intra-abdominal infection was the main cause of sepsis (174/252; 69%), and 58.3% (147/252) of patients underwent emergency surgery within 12 h prior to admission to the ICU. There were more patients with pneumonia among nonsurvivors (P < 0.001) compared with survivors. The all-cause ICU mortality rate was 43.3% (109/252).
Table 2.
The primary site of infection in study population
| Total, N = 252 (%) | Survivors, N = 143 (%) | Nonsurvivors, N = 109 (%) | P value | ||
| Site of infection | Intra-abdominal | 69.0 | 71.3 | 66.1 | 0.37 |
| Pneumonia | 10.7 | 4.2 | 19.3 | <0.001 | |
| Urinary | 4.4 | 5.6 | 2.8 | 0.274 | |
| Enterogenic | 3.2 | 3.5 | 2.8 | 0.739 | |
| Esophageal rupture Mediastinal abscess | 2.4 | 2.8 | 1.8 | 0.62 | |
| Others | 9.9 | 12.6 | 6.4 | 0.105 | |
| Emergency surgery | 58.3 | 62.2 | 53.2 | 0.15 |
Comparison between survivors and nonsurvivors among septic patients
Table 3 shows the comparison between survivors and nonsurvivors among patients with sepsis. There were no differences in age and sex between the two groups. The initial APACHE II score and SOFA score were significantly higher among nonsurvivors (P < 0.0001). The ISTH-DIC score, modified ISTH-DIC score, and SIC score were all higher among nonsurvivors (P < 0.0001). Regarding hemostatic parameters, only the fibrinogen level was not significantly different between survivors and nonsurvivors (P = 0.371). In contrast, platelet count was lower (P = 0.002), and PT, INR, APTT, FDP, and d-dimer were all higher among nonsurvivors (P < 0.05).
Table 3.
The comparison between survivors and nonsurvivors septic patients
| Total, N = 252 | Survivors, N = 143 | Nonsurvivors, N = 109 | P value | |
| Age (years) | 65 (53.5–76) | 63 (54–78) | 68.00 (53–75) | 0.574 |
| Sex (male/female) | 252 (147/105) | 143 (85/58) | 109 (62/47) | 0.68 |
| APACHE II | 13 (10–17) | 12 (9–15) | 15 (12–20) | 0.000 |
| SOFA | 7 (5–9) | 5 (4–7) | 8 (6–10) | 0.000 |
| PT (s) | 16 (15–19) | 16 (15–18) | 17 (15–20) | 0.014 |
| INR | 1.3 (1.2–1.6) | 1.3 (1.2–1.5) | 1.4 (1.2–1.7) | 0.009 |
| APTT (s) | 46.8 (39.95–55.5) | 45.4 (39.9–52.2) | 51.1 (40.1–60.6) | 0.015 |
| FIB (g/l) | 3.9 (2.55–5.9) | 3.7 (2.7–6.4) | 4 (2.4–5.6) | 0.371 |
| d-Dimer (μg/ml) | 4.15 (2.75–8.25) | 3.7 (2.3–7.1) | 5.1 (3.1–10) | 0.003 |
| FDP (μg/ml) | 18 (10–35) | 15 (8.6–29) | 22 (12–43) | 0.003 |
| Platelet count (×109/l) | 145 (95.5–218) | 170 (114–229) | 119 (78–207) | 0.002 |
| ISTH | 3 (2–4) | 3 (2–4) | 3 (3–5) | 0.000 |
| Modified ISTH | 3 (2–4) | 3 (2–4) | 3 (3–4) | 0.000 |
| SIC | 4 (3–5) | 4 (3–4) | 5 (3–6) | 0.000 |
APACHE, Acute Physiology and Chronic Health Evaluation; APTT, activated partial thromboplastin time; FIB, fibrinogen; FDP, fibrin degradation product; INR, international normalized ratio; ISTH, International Society on Thrombosis and Haemostasis; PT, prothrombin time; SIC, sepsis-induced coagulopathy; SOFA, sequential organ failure assessment.
Univariate and multivariate analyses of ICU mortality
Univariate logistic regression analyses were performed to examine the association between ICU mortality and each variable (Table 4). A multivariate analysis using the enter method of different models showed that the APACHE II score (P < 0.001), ISTH score (P = 0.001), modified ISTH score (P = 0.001), and SIC score (P = 0.007) were all independently associated with ICU mortality (Table 4).
Table 4.
The univariate and multivariable analyses of ICU mortality
| Multivariate | |||||||
| Univariate | Model-1, ISTH | Model-2, modified ISTH | Model-3, SIC | ||||
| Predictor | P value | OR | 95% CI | P value | P value | P value | |
| Age (years) | 0.556 | 1.005 | 0.989 | 1.021 | |||
| Sex | 0.683 | 0.900 | 0.543 | 1.492 | |||
| APACHE II | 0.000 | 1.140 | 1.082 | 1.202 | 0.000 | 0.000 | 0.000 |
| SOFA | 0.000 | 1.322 | 1.199 | 1.458 | |||
| PT (s) | 0.023 | 1.078 | 1.011 | 1.150 | 0.258 | 0.277 | 0.318 |
| INR | 0.025 | 1.873 | 1.081 | 3.247 | 0.351 | 0.347 | 0.369 |
| APTT (s) | 0.018 | 1.010 | 1.002 | 1.018 | 0.215 | 0.208 | 0.303 |
| FIB (g/l) | 0.252 | 0.936 | 0.836 | 1.048 | |||
| d-Dimer (μg/ml) | 0.006 | 1.074 | 1.020 | 1.131 | 0.478 | 0.483 | 0.149 |
| FDP (μg/ml) | 0.017 | 1.011 | 1.002 | 1.020 | 0.337 | 0.350 | 0.327 |
| Platelet count (×109/l) | 0.015 | 0.997 | 0.994 | 0.999 | 0.404 | 0.388 | 0.809 |
| ISTH | 0.000 | 1.609 | 1.332 | 1.943 | 0.001 | ||
| Modified ISTH | 0.000 | 1.614 | 1.333 | 1.953 | 0.001 | ||
| SIC | 0.000 | 1.625 | 1.302 | 2.027 | 0.007 | ||
APACHE, Acute Physiology and Chronic Health Evaluation; APTT, activated partial thromboplastin time; CI, confidence interval; FIB, fibrinogen; FDP, fibrin degradation product; INR, international normalized ratio; ISTH, International Society on Thrombosis and Haemostasis; PT, prothrombin time; SIC, sepsis-induced coagulopathy; SOFA, sequential organ failure assessment.
Value of indicators in predicting ICU mortality
ROC curves were constructed to examine the performance of indicators as predictors of ICU mortality, and then the AUC for each indicator was calculated. The AUC, sensitivity, and specificity of each indicator are given in Fig. 1. SOFA score had the greatest power for predicting ICU mortality, as suggested by the largest AUC of 0.743 ± 0.030. The AUC for SIC score (0.658 ± 0.036) was less than that of ISTH or modified ISTH score (0.684 ± 0.033), as shown in Fig. 1. The sensitivity of SIC score (74.3%) was more than that of ISTH (25.7%) or modified ISTH score (49.5%); however, the specificity for SIC score (37.1%) was less than that of ISTH (91.6%) or modified ISTH score (74.1%).
Fig. 1.
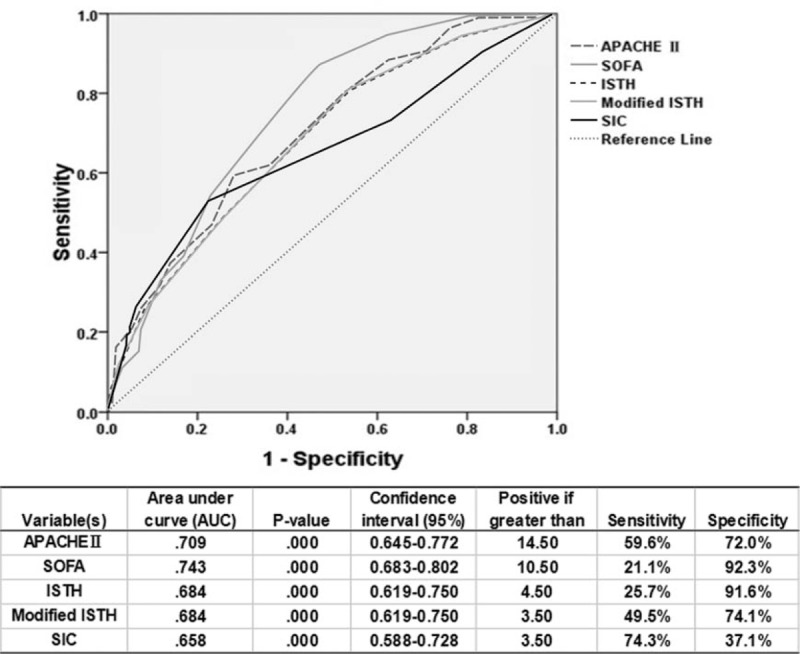
Receiver operating characteristic curve of indicators for prediction of mortality. Receiver operating characteristic curves were constructed to examine the performance of indicators as predictors of ICU mortality, and then the area under the curve for each indicator was calculated. The area under the curve, sensitivity, and specificity of each indicator are given. APACHE, Acute Physiology and Chronic Health Evaluation; ISTH, International Society on Thrombosis and Haemostasis; SIC, sepsis-induced coagulopathy; SOFA, sequential organ failure assessment.
Correlation between different disseminated intravascular coagulation scoring systems and severity of disease
Table 5 shows the correlation between different DIC scoring systems and severity of disease. There were strong correlations between the SIC score and ISTH score (P < 0.0001) and modified ISTH score (P < 0.0001). Moreover, SIC criteria correlated with the APACHE II score (P = 0.012) and SOFA score (P < 0.0001). Both the ISTH score and modified ISTH score demonstrated similar results (Table 5).
Table 5.
The correlation analyses between different scoring systems and severity of disease
| APACHE | SOFA | ISTH score | Modified ISTH score | SIC score | |
| APACHE | 1.000 | 0.540a | 0.224a | 0.223a | 0.158b |
| SOFA | 0.540a | 1.000 | 0.402a | 0.402a | 0.450a |
| ISTH score | 0.224a | 0.402a | 1.000 | 0.998a | 0.631a |
| Modified ISTH score | 0.223a | 0.402a | 0.998a | 1.000 | 0.628a |
| SIC score | 0.158b | 0.450a | 0.631a | 0.628a | 1.000 |
APACHE, Acute Physiology and Chronic Health Evaluation; ISTH, International Society on Thrombosis and Haemostasis; SIC, sepsis-induced coagulopathy; SOFA, sequential organ failure assessment.
aCorrelation is significant at the 0.01 level (two-tailed).
bCorrelation is significant at the 0.05 level (two-tailed).
Comparison of the new sepsis-induced coagulopathy score with the International Society on Thrombosis and Haemostasis disseminated intravascular coagulation score and modified International Society on Thrombosis and Haemostasis disseminated intravascular coagulation score
Table 6 shows the differences in diagnosis of sepsis-associated DIC using the different criteria. A total of 67.9% of patients were diagnosed as having SIC, whereas 15.9 and 36.1% patients met the ISTH criteria and the modified ISTH criteria for DIC, respectively. In ICU survivors, 62.9% were diagnosed as having SIC, whereas 8.4 and 25.9% met the ISTH criteria and the modified ISTH criteria for DIC, respectively. In nonsurvivors, 74.3% were diagnosed as having SIC, whereas 25.7 and 49.5% met the ISTH criteria and the modified ISTH criteria for DIC, respectively. Compared with ICU survivors, more nonsurvivors were diagnosed with DIC using the ISTH criteria and the modified ISTH criteria (P < 0.001). In contrast, there was no significant difference in the proportion of patients with SIC between the two groups (P = 0.055).
Table 6.
The differences of diagnosis of sepsis-associated disseminated intravascular coagulation/coagulophathy using the different criteria
| Variable(s) | Total, N = 252 (%) | Survivors, N = 143 (%) | Nonsurvivors, N = 109 (%) | P value | |
| ISTH | <5 | 84.1 | 91.6 | 74.3 | <0.001 |
| ≥5 | 15.9 | 8.4 | 25.7 | ||
| Modified ISTH | <4 | 63.9 | 74.1 | 50.5 | <0.001 |
| ≥4 | 36.1 | 25.9 | 49.5 | ||
| SIC | <4 | 32.1 | 37.1 | 25.7 | 0.055 |
| ≥4 | 67.9 | 62.9 | 74.3 | ||
ISTH, International Society on Thrombosis and Haemostasis; SIC, sepsis-induced coagulopathy.
Discussion
The current study retrospectively evaluated the SIC, ISTH DIC, and modified ISTH DIC diagnostic criteria in patients with sepsis 3.0. All three diagnostic criteria for sepsis-associated DIC or coagulopathy were related to severity of disease and poor outcome. The SOFA score had the greatest power for predicting ICU mortality, and the SIC score had lower predictive power than the ISTH or modified ISTH score. There were more patients diagnosed as having SIC than there were patients diagnosed as having DIC using the modified ISTH score. More nonsurvivors were diagnosed as having DIC using the ISTH criteria and the modified ISTH criteria. However, there was no significant difference in the proportion of patients with SIC between the survivors and nonsurvivors. A multivariate analysis showed that the APACHE II score, ISTH score, modified ISTH score, and SIC score were all independently associated with ICU mortality. In contrast, no single traditional coagulation index was correlated with outcome.
The SIC score was developed on the basis of logistic regression analyses in a study by Iba et al.[17]. The dataset in that study was obtained from a postmarketing survey performed between 2008 and 2010, when sepsis was defined on the basis of SIRS criteria [18]. The platelet count, PT ratio, and four items in SOFA (respiratory, cardiovascular, hepatic, and renal SOFA) were independent predictors of 28-day mortality [17]. Based on the three variables, SIC was defined as a total score of 4 or more (Table 1) [17]. They also reported that the SIC score performed better than the JAAM-DIC score in predicting 28-day mortality [17]. Similar to the study of Iba et al.[17], we found that the SIC score, as well as the ISTH score and modified ISTH score, were independently associated with ICU mortality in patients with sepsis 3.0.
The FDP criterion was eliminated from SIC criteria, as the FDP level was not significantly different between survivors and nonsurvivors [17]. In contrast, many previous studies showed that fibrinolysis-related markers (FDP or d-dimer) were independently associated with mortality in patients with sepsis [19,20]. In our current study, both FDP and d-dimer were significantly different between ICU survivors and nonsurvivors; however, neither was identified as an independent prognostic factor for patients with sepsis. It is not clear which factors contribute to the difference between studies, but it may be related to the differences in the diseases and populations in each study. Similarly, in the study of Iba et al.[17], the value of PT–INR and the platelet count on admission to the ICU were significantly associated with 28-day mortality, and both were used in the SIC criteria. In the current study, no single traditional coagulation index (e.g., PT, INR, and platelet count) was correlated with ICU mortality of patients with sepsis; nevertheless, combinations of several conventional coagulation tests (e.g., ISTH score, modified ISTH score, and SIC score) were independent predictors of outcome.
In line with a previous study [21], the results of the current study showed that the plasma fibrinogen level was not significantly different between ICU survivors and nonsurvivors, with a fibrinogen level less than 1.0 g/l only occurring in 2.4% (6/252) of patients with sepsis. Unlike traumatic coagulopathy, the fibrinogen level in sepsis, especially in early sepsis, does not decrease and may even increase [12]. However, once the fibrinogen level in patients with sepsis appears to be significantly lower, it may indicate the formation of a large amount of microthrombi in the microcirculation; this occurs when there is a shift from hypercoagulability to consumption coagulopathy and often suggests a poor prognosis. Therefore, fibrinogen level was not incorporated as an indicator in the JAAM standard. Accordingly, we revised the ISTH criteria to remove fibrin level from the ISTH, and the results showed that the modified ISTH score was comparable with that of the original ISTH in predicting ICU mortality; however, the modified ISTH was more sensitive than the original ISTH in the diagnosis of overt DIC (more patients with sepsis met the modified ISTH criteria for DIC compared with the original ISTH criteria: 36.1 vs. 15.9%, respectively). Therefore, we speculate that the modified ISTH criteria may be helpful in the early detection and guidance of anticoagulant therapy for sepsis-associated DIC. We plan to conduct a subsequent prospective clinical study to evaluate further the usefulness of the modified ISTH score.
In the current study, 67.9% of patients with sepsis 3.0 were diagnosed as having SIC; moreover, there was no significant difference in the proportion of patients with SIC between ICU survivors and nonsurvivors (P = 0.055). In contrast, 36.1 and 15.9% of patients met the ISTH criteria and the modified ISTH criteria for DIC, respectively. We considered that SIC criteria might be too sensitive to distinguish which patients could benefit from anticoagulant therapy. There are several reasons why the majority of patients scored as coagulopathy-positive using SIC. First, unlike the modified ISTH score, the SIC and ISTH scores are very different; there are only two laboratory parameters (PT and platelets) that are same in the SIC and ISTH criteria. Second, SIC is sepsis induced ‘coagulopathy’ and ‘coagulopathy’ is common in patients with sepsis [17,22]. DIC is a severe subtype of coagulopathy, and so more patients scored as coagulopathy-positive using SIC, in contrast to ISTH. Third, the SIC criteria includes the SOFA score, and the disease severity of patients included in this study was high. For the diagnosis of sepsis 3.0, SOFA or ΔSOFA more than 2 is acceptable; however, the average SOFA value of the patients included in this study was 7. Taken together, further research needs to identify whether the SIC score or modified ISTH score can guide anticoagulant therapy in sepsis.
As a new diagnostic standard, the important value of SIC criteria is that they introduce SOFA into the diagnostic system of sepsis-associated DIC or coagulopathy. In the previously existing DIC scoring systems, only the JMHW criteria incorporate organ dysfunction, but the weight is not high (Table 1). In contrast, there is no organ function score in the ISTH and JAAM scores. In general, sepsis-associated DIC is the result of an interaction of infection-induced inflammation and coagulation and involves neutrophils, platelets, and endothelial cells [8,9,23]. Activation of the coagulation system, weakening of anticoagulation system, and inhibition of the fibrinolytic system are characteristics of early hypercoagulability in sepsis [8,24,25]. Sepsis later causes a series of abnormal coagulation functions and changes in related molecular markers. Sepsis-associated DIC ultimately causes microthrombosis, microcirculation disorders, and organ dysfunction. Therefore, we believe that the ideal DIC scoring system should include DIC-related molecular biomarkers (endothelial cells, neutrophils, platelets), traditional coagulation-related indicators, novel coagulation-related indicators, and organ function (SOFA without platelet count) (Fig. 2).
Fig. 2.
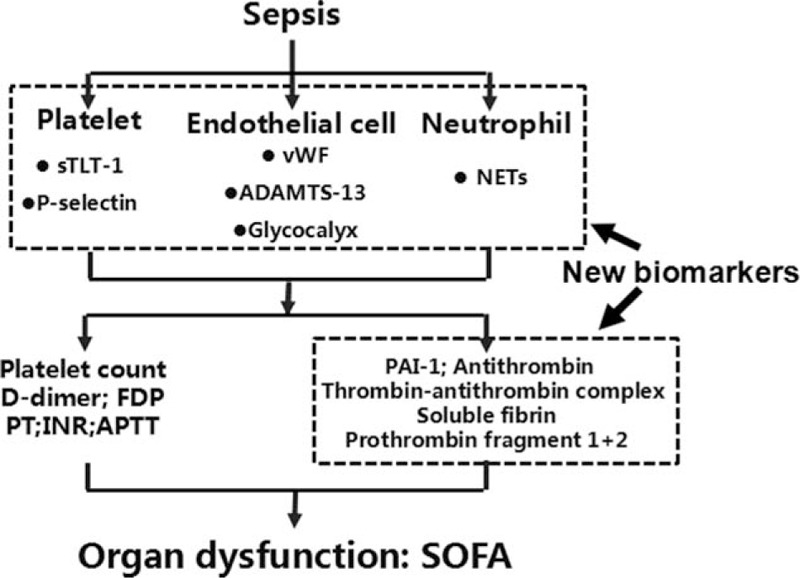
The ideal scoring system for sepsis-associated disseminated intravascular coagulation or coagulopathy. ADAMTS-13, a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13; APTT, activated partial thromboplastin time; DIC, disseminated intravascular coagulopathy; FDP, fibrin degradation product; INR, international normalized ratio; NET, neutrophils form extracellular trap; PAI-1, plasminogen activator inhibitor-1; PT, prothrombin time; SOFA, sequential organ failure assessment; sTLT-1, serum triggering receptor expressed on myeloid cells-like transcript-1; vWF, von Willebrand factor.
From JMHW score to SIC score (Table 1), the DIC or coagulopathy-related scoring systems have been developed on the basis of the traditional coagulation-related indicators (platelet counts, PT, APTT, d-dimer, FDP, etc.). Following a deeper understanding of the coagulation pathway, new markers related to the coagulation–anticoagulation–fibrinolysis system [plasminogen activator inhibitor-1, antithrombin (AT) III, soluble fibrin, thrombin–AT complex, prothrombin fragment 1 + 2, etc.] were developed and gradually implemented to evaluate sepsis DIC or coagulopathy [1,26,27]. In 2016, Japanese scholars proposed another new DIC diagnostic strategy, which classifies DIC according to different diseases and sets different scoring criteria [1]. AT, soluble fibrin, the thrombin–AT complex, and prothrombin fragment 1 + 2 measures were added in the infection-related DIC scoring system [1].
Due to the important role of endothelial cells, platelets, and neutrophils in septic DIC, more and more attention has been focused on cell-related molecular markers in recent years [28,29]. The glycocalyx of endothelial cells is destroyed in sepsis, and the components of the glycocalyx, such as syndecan-1, increase in the plasma. The level of syndecan-1 in plasma may predict the occurrence of DIC, and was significantly associated with mortality of septic patients [30]. In sepsis, von Willebrand factor (vWF) is mainly expressed by endothelial cells and is released from platelets to promote the aggregation and adhesion of platelets, and the generation of thrombosis [29,31]. A disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13 is a metalloproteinase enzyme that cleaves vWF, and its level in plasma is reduced in sepsis, which is also significantly associated with an increased risk of mortality [32,33]. In addition, the specific marker of platelet activation (serum triggering receptor expressed on myeloid cells-like transcript-1) and the expression of P-selectin in platelets and endothelial cells have also been reported to be associated with sepsis DIC [29,34,35]. P-selectin in platelets is also involved in the formation of neutrophils form extracellular traps (NETs) [29]. NETs have a significant effect on promoting coagulation, and the content of NETs-related substances in the plasma of patients with septic DIC is significantly increased [36,37]. However, these markers require further studies to clarify their value in sepsis-associated DIC and prognosis (Fig. 2).
The current study has limitations. It was a retrospective study, and the cases included were mainly patients with abdominal infection and emergency surgery. Moreover, the study included cases with high disease severity (mean SOFA score ∼7) and high ICU mortality (43.3%). These limitations might introduce bias in the results. We plan to conduct a subsequent prospective, multicenter study to further evaluate the value of the three scoring criteria in patients with sepsis 3.0.
In conclusion, the ISTH score, modified ISTH score, and SIC score for sepsis-associated DIC or coagulopathy at the time of ICU admission were related to the severity and poor outcome of disease and were independent risk factors for ICU mortality of patients with sepsis 3.0. However, in this study, compared with ISTH score and modified ISTH score, SIC score showed no advantage in diagnosing sepsis associated coagulopathy or DIC. The application of these three criteria in patients with sepsis 3.0 needs further evaluation.
Acknowledgements
The authors are thankful to Jun Na (Liaoning Province Centers for disease control and prevention) for providing advice regarding the statistical analysis.
The research was supported by the Natural Science Foundation of Liaoning Province of China (2016021819) and the Science and Technology Plan Project of Shenyang of China (17-230-947).
Conflicts of interest
There are no conflicts of interest.
References
- 1.Asakura H, Takahashi H, Uchiyama T, Eguchi Y, Okamoto K, Kawasugi K, et al. DIC Subcommittee of the Japanese Society on Thrombosis and Hemostasis. Thrombosis, hemostasis, proposal for new diagnostic criteria for DIC from the Japanese Society on Thrombosis and Hemostasis. Thromb J 2016; 14:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levi M, van der Poll T. Disseminated intravascular coagulation: a review for the internist. Intern Emerg Med 2013; 8:23–32. [DOI] [PubMed] [Google Scholar]
- 3.Levi M. Disseminated intravascular coagulation. Crit Care Med 2007; 35:2191–2195. [DOI] [PubMed] [Google Scholar]
- 4.Levi M. Another step in improving the diagnosis of disseminated intravascular coagulation in sepsis. Crit Care 2013; 17:448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taylor FB, Jr, Toh CH, Hoots WK, Wada H, Levi M. Scientific Subcommittee on Disseminated Intravascular Coagulation of the International Society on, Haemostasis. Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb Haemost 2001; 86:1327–1330. [PubMed] [Google Scholar]
- 6.Kobayashi N, Maekawa T, Takada M, Tanaka H, Gonmori H. Criteria for diagnosis of DIC based on the analysis of clinical and laboratory findings in 345 DIC patients collected by the Research Committee on DIC in Japan. Bibl Haematol 1983; 49:265–275. [DOI] [PubMed] [Google Scholar]
- 7.Wada H, Gabazza EC, Asakura H, Koike K, Okamoto K, Maruyama I, et al. Comparison of diagnostic criteria for disseminated intravascular coagulation (DIC): diagnostic criteria of the International Society of Thrombosis and Hemostasis and of the Japanese Ministry of Health and Welfare for overt DIC. Am J Hematol 2003; 74:17–22. [DOI] [PubMed] [Google Scholar]
- 8.Okamoto K, Tamura T, Sawatsubashi Y. Sepsis and disseminated intravascular coagulation. J Intensive Care 2016; 4:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iba T, Ito T, Maruyama I, Jilma B, Brenner T, Muller MC, et al. Potential diagnostic markers for disseminated intravascular coagulation of sepsis. Blood Rev 2016; 30:149–155. [DOI] [PubMed] [Google Scholar]
- 10.Madoiwa S. Recent advances in disseminated intravascular coagulation: endothelial cells and fibrinolysis in sepsis-induced DIC. J Intensive Care 2015; 3:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wada H, Matsumoto T, Yamashita Y, Hatada T. Disseminated intravascular coagulation: testing and diagnosis. Clin Chim Acta 2014; 436:130–134. [DOI] [PubMed] [Google Scholar]
- 12.Koami H, Sakamoto Y, Sakurai R, Ohta M, Imahase H, Yahata M, et al. The thromboelastometric discrepancy between septic and trauma induced disseminated intravascular coagulation diagnosed by the scoring system from the Japanese Association for Acute Medicine. Medicine (Baltimore) 2016; 95:e4514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vincent JL, Ramesh MK, Ernest D, LaRosa SP, Pachl J, Aikawa N, et al. A randomized, double-blind, placebo-controlled, Phase 2b study to evaluate the safety and efficacy of recombinant human soluble thrombomodulin, ART-123, in patients with sepsis and suspected disseminated intravascular coagulation. Crit Care Med 2013; 41:2069–2079. [DOI] [PubMed] [Google Scholar]
- 14.Gando S, Iba T, Eguchi Y, Ohtomo Y, Okamoto K, Koseki K, et al. Japanese Association for Acute Medicine Disseminated Intravascular Coagulation Study. A multicenter, prospective validation of disseminated intravascular coagulation diagnostic criteria for critically ill patients: comparing current criteria. Crit Care Med 2006; 34:625–631. [DOI] [PubMed] [Google Scholar]
- 15.Gando S, Saitoh D, Ogura H, Fujishima S, Mayumi T, Araki T, et al. Japanese Association for Acute Medicine Sepsis Registry Study. A multicenter, prospective validation study of the Japanese Association for Acute Medicine disseminated intravascular coagulation scoring system in patients with severe sepsis. Crit Care 2013; 17:R111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 2016; 315:801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iba T, Nisio MD, Levy JH, Kitamura N, Thachil J. New criteria for sepsis-induced coagulopathy (SIC) following the revised sepsis definition: a retrospective analysis of a nationwide survey. BMJ Open 2017; 7:e017046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mimuro J, Takahashi H, Kitajima I, Tsuji H, Eguchi Y, Matsushita T, et al. Impact of recombinant soluble thrombomodulin (thrombomodulin alfa) on disseminated intravascular coagulation. Thromb Res 2013; 131:436–443. [DOI] [PubMed] [Google Scholar]
- 19.Semeraro F, Colucci M, Caironi P, Masson S, Ammollo CT, Teli R, et al. Platelet drop and fibrinolytic shutdown in patients with sepsis. Crit Care Med 2018; 46:e221–e228. [DOI] [PubMed] [Google Scholar]
- 20.Toh JM, Ken-Dror G, Downey C, Abrams ST. The clinical utility of fibrin-related biomarkers in sepsis. Blood Coagul Fibrinolysis 2013; 24:839–843. [DOI] [PubMed] [Google Scholar]
- 21.Sivula M, Tallgren M, Pettila V. Modified score for disseminated intravascular coagulation in the critically ill. Intensive Care Med 2005; 31:1209–1214. [DOI] [PubMed] [Google Scholar]
- 22.Kushimoto S, Gando S, Saitoh D, Ogura H, Mayumi T, Koseki K, et al. Japanese Association for Acute Medicine Disseminated Intravascular Coagulation (JAAM DIC) Study Group. Clinical course and outcome of disseminated intravascular coagulation diagnosed by Japanese Association for Acute Medicine criteria. Thromb Haemost 2008; 100:1099–1105. [PubMed] [Google Scholar]
- 23.Iba T, Levy JH. Inflammation and thrombosis: roles of neutrophils, platelets and endothelial cells and their interactions in thrombus formation during sepsis. J Thromb Haemost 2018; 16:231–241. [DOI] [PubMed] [Google Scholar]
- 24.Levi M, van der Poll T. Coagulation and sepsis. Thromb Res 2017; 149:38–44. [DOI] [PubMed] [Google Scholar]
- 25.Semeraro N, Ammollo CT, Semeraro F, Colucci M. Sepsis, thrombosis and organ dysfunction. Thromb Res 2012; 129:290–295. [DOI] [PubMed] [Google Scholar]
- 26.Koyama K, Madoiwa S, Nunomiya S, Koinuma T, Wada M, Sakata A, et al. Combination of thrombin-antithrombin complex, plasminogen activator inhibitor-1, and protein C activity for early identification of severe coagulopathy in initial phase of sepsis: a prospective observational study. Crit Care 2014; 18:R13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okabayashi K, Wada H, Ohta S, Shiku H, Nobori T, Maruyama K. Hemostatic markers and the sepsis-related organ failure assessment score in patients with disseminated intravascular coagulation in an intensive care unit. Am J Hematol 2004; 76:225–229. [DOI] [PubMed] [Google Scholar]
- 28.Gando S, Levi M, Toh CH. Disseminated intravascular coagulation. Nat Rev Dis Primers 2016; 2:16037. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y, Ouyang Y, Liu B, Ma X, Ding R. Platelet activation and antiplatelet therapy in sepsis: a narrative review. Thromb Res 2018; 166:28–36. [DOI] [PubMed] [Google Scholar]
- 30.Ikeda M, Matsumoto H, Ogura H, Hirose T, Shimizu K, Yamamoto K, et al. Circulating syndecan-1 predicts the development of disseminated intravascular coagulation in patients with sepsis. J Crit Care 2018; 43:48–53. [DOI] [PubMed] [Google Scholar]
- 31.Claus RA, Bockmeyer CL, Budde U, Kentouche K, Sossdorf M, Hilberg T, et al. Variations in the ratio between von Willebrand factor and its cleaving protease during systemic inflammation and association with severity and prognosis of organ failure. Thromb Haemost 2009; 101:239–247. [PubMed] [Google Scholar]
- 32.Peigne V, Azoulay E, Coquet I, Mariotte E, Darmon M, Legendre P, et al. The prognostic value of ADAMTS13 (a disintegrin and metalloprotease with thrombospondin type 1 repeats, member 13) deficiency in septic shock patients involves interleukin-6 and is not dependent on disseminated intravascular coagulation. Crit Care 2013; 17:R273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aibar J, Castro P, Espinosa G, Fernandez S, Hernandez C, Rinaudo M, et al. ADAMTS-13 in critically ill patients with septic syndromes and noninfectious systemic inflammatory response syndrome. Shock 2015; 43:556–562. [DOI] [PubMed] [Google Scholar]
- 34.Esponda O, Morales J, Aguilar A, Gomez M, Washington AV. Clinical studies support a role for trem-like transcript-1 during the progression of sepsis. Bol Asoc Med P R 2010; 102:59–61. [PubMed] [Google Scholar]
- 35.Russwurm S, Vickers J, Meier-Hellmann A, Spangenberg P, Bredle D, Reinhart K, Losche W. Platelet and leukocyte activation correlate with the severity of septic organ dysfunction. Shock 2002; 17:263–268. [DOI] [PubMed] [Google Scholar]
- 36.Alhamdi Y, Toh CH. Recent advances in pathophysiology of disseminated intravascular coagulation: the role of circulating histones and neutrophil extracellular traps. F1000Res 2017; 6:2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Delabranche X, Stiel L, Severac F, Galoisy AC, Mauvieux L, Zobairi F, et al. Evidence of netosis in septic shock-induced disseminated intravascular coagulation. Shock 2017; 47:313–317. [DOI] [PubMed] [Google Scholar]


