Supplemental Digital Content is available in the text
Abstract
BACKGROUND
In contrast to conventional mandatory ventilation, a new ventilation mode, expiratory ventilation assistance (EVA), linearises the expiratory tracheal pressure decline.
OBJECTIVE
We hypothesised that due to a recruiting effect, linearised expiration oxygenates better than volume controlled ventilation (VCV). We compared the EVA with VCV mode with regard to gas exchange, ventilation volumes and pressures and lung aeration in a model of peri-operative mandatory ventilation in healthy pigs.
DESIGN
Controlled interventional trial.
SETTING
Animal operating facility at a university medical centre.
ANIMALS
A total of 16 German Landrace hybrid pigs.
INTERVENTION
The lungs of anaesthetised pigs were ventilated with the EVA mode (n=9) or VCV (control, n=7) for 5 h with positive end-expiratory pressure of 5 cmH2O and tidal volume of 8 ml kg−1. The respiratory rate was adjusted for a target end-tidal CO2 of 4.7 to 6 kPa.
MAIN OUTCOME MEASURES
Tracheal pressure, minute volume and arterial blood gases were recorded repeatedly. Computed thoracic tomography was performed to quantify the percentages of normally and poorly aerated lung tissue.
RESULTS
Two animals in the EVA group were excluded due to unstable ventilation (n=1) or unstable FiO2 delivery (n=1). Mean tracheal pressure and PaO2 were higher in the EVA group compared with control (mean tracheal pressure: 11.6 ± 0.4 versus 9.0 ± 0.3 cmH2O, P < 0.001 and PaO2: 19.2 ± 0.7 versus 17.5 ± 0.4 kPa, P = 0.002) with comparable peak inspiratory tracheal pressure (18.3 ± 0.9 versus 18.0 ± 1.2 cmH2O, P > 0.99). Minute volume was lower in the EVA group compared with control (5.5 ± 0.2 versus 7.0 ± 1.0 l min−1, P = 0.02) with normoventilation in both groups (PaCO2 5.4 ± 0.3 versus 5.5 ± 0.3 kPa, P > 0.99). In the EVA group, the percentage of normally aerated lung tissue was higher (81.0 ± 3.6 versus 75.8 ± 3.0%, P = 0.017) and of poorly aerated lung tissue lower (9.5 ± 3.3 versus 15.7 ± 3.5%, P = 0.002) compared with control.
CONCLUSION
EVA ventilation improves lung aeration via elevated mean tracheal pressure and consequently improves arterial oxygenation at unaltered positive end-expiratory pressure (PEEP) and peak inspiratory pressure (PIP). These findings suggest the EVA mode is a new approach for protective lung ventilation.
Introduction
During conventional mandatory ventilation, expiration is driven only by the passive elastic force of the respiratory system. As a consequence, the airway pressure (Paw) curve shows the well known exponential decrease during expiration. If the Paw decline during expiration is controlled and linearised (flow controlled expiration), a recruiting effect is induced. This has been shown in both animal1 and in human studies.2
The workgroup of Enk3,4 originally developed the new ventilation mode ‘expiratory ventilation assistance’ (EVA) as an emergency ventilation system (Ventrain; Ventinova Medical B.V., Eindhoven, The Netherlands) to restore ventilation via a small bore tracheostomy.5–7 A detailed description of the EVA working principle is found elsewhere.3,4 As a further development, the EVA operating principle was transferred to the prototype of an automated mechanical ventilator (Evone; Ventinova Medical B.V.). This ventilator generates fully controlled expiration by linearising the expiratory flow in a manner comparable with flow-controlled expiration.
We hypothesised that the EVA mode with its recruiting effect due to the linearised expiration would provide better oxygenation compared with volume controlled ventilation (VCV). To test this hypothesis, we conducted a controlled, interventional laboratory study to compare the EVA-based ventilation mode with a commonly applied VCV protocol with regard to gas exchange, ventilation volumes and pressures and lung aeration, the latter assessed via computed tomography (CT), in a model of peri-operative ventilation.
Methods
Ethics
The study was approved by the responsible governmental committee (Regierungspräsidium Freiburg, Bertoldstr. 43, 79098 Freiburg; Ref. G-15/167) on 8 February 2016. The study was performed in accordance with the European and German law on the protection of animals used for scientific purposes (EU-Directive 2010/63; TierSchG, as amended on 3 December 2015) from 16 February 2016 to 28 July 2016.
Study design and animal handling
The study was designed as a controlled interventional trial. Experiments were conducted in two blocks, first the control, and then the interventional group.
German Landrace hybrid pigs with a body weight of 40 to 50 kg were used for this study. All animals were kept for 10 to 14 days in a holding facility prior to the experiment days for adaptation. Animal housing allowed natural circadian light and provided free access to water and food. All experiments began at the same time each morning.
Anaesthesia and instrumentation
After a fasting period of 12 h, the animals were premedicated with an intramuscular injection of ketamine (20 mg kg−1) and midazolam (0.5 mg kg−1). Anaesthesia was induced intravenously with propofol (2 to 4 mg kg−1) and vecuronium (0.4 mg kg−1) and was maintained via continuous infusions of midazolam (0.5 to 1.5 mg kg−1 h−1), ketamine (10 to 30 mg kg−1 h−1), vecuronium (0.2 to 0.4 mg kg−1 h−1) and fentanyl (3 to 6 μg kg−1 h−1). After orotracheal intubation with a standard endotracheal tube (ETT) with an inner diameter (ID) of 8.0 mm, VCV was started (Evita 4; Dräger Medical, Lübeck, Germany) with FiO2 of 0.3, PEEP of 5 cmH2O, tidal volume (VT) of 8 ml kg−1 and inspiration to expiration (I : E) ratio of 1 : 2. The respiratory rate was set to achieve an etCO2 of 4.7 to 6 kPa and was adjusted further as needed. For pulmonary arterial pressure monitoring a pulmonary artery catheter (7F; Edwards Lifesciences, Irvine, California, USA) was inserted via an 8F introducer sheath placed in the left external jugular vein. An arterial catheter (5F; Pulsion Medical Systems, Feldkirchen, Germany) was placed in the femoral artery for blood pressure (BP) measurement and trans-cardiopulmonary thermodilution (PiCCO2; Pulsion Medical Systems). A urinary catheter was inserted into the bladder via a mini laparotomy. No other surgical procedure was performed.
Expiratory ventilation assistance
A special tracheal tube (Tritube; Ventinova Medical B.V.) with an ID of 2.4 mm comes with the EVA-ventilator. The Tritube is equipped with a cuff and a separate lumen for direct tracheal pressure (Ptrach) measurement.8 Its high instrumental flow resistance is actively compensated for by high device pressure during inspiration and negative device pressure during expiration (Fig. 1). It should be noted that negative pressure is exclusively present proximal to the airway tubing. As soon as Ptrach has declined to the set PEEP level, the ventilator switches from expiration to inspiration. Direct and continuous Ptrach measurements represent one of the main control variables of the EVA prototype.
Fig. 1.

Working principle of the expiratory ventilation assistance. (a) Expiration is created by jet entrainment. The gas flows via the inlet (1) through a very narrow nozzle (2) and exhaust pipe (3) to the outside. This flow entrains gas from port (4), which is connected to the endotracheal tube, inducing active expiration. (b) Closing the exhaust pipe (3) results in inspiration through port (4).
Experimental protocol
Following instrumentation and a 15-min equilibration period, the lungs of the pigs were either ventilated with the EVA mode (Evone; Ventinova Medical B.V.) or with VCV (Evita 4; Dräger Medical) as control (Fig. 2). All animals were pre-oxygenated with pure oxygen for 4 min before the ETT was disconnected from the ventilator. For the EVA group, the Tritube was advanced inside the ETT and advanced until its tip just protruded from the distal end of the ETT, and then its cuff was inflated to provide a seal. This procedure lasted approximately 1 min. EVA ventilation was started with FiO2 of 0.3 and PEEP of 5 cmH2O. The peak inspiratory pressure (PIP) and the inspiratory flow rate were set to achieve a VT of 8 ml kg−1 and the respiratory rate adjusted to give an etCO2 of 4.7 to 6 kPa. In the control group, the ETT was reconnected to the ventilator after 1 min of apnoea as a sham procedure. Ventilation was continued as VCV with identical FiO2, PEEP, VT and target etCO2 as in the EVA group. The I : E ratio was maintained at 1 : 2 in the control group, whereas in the EVA group, the ventilation mode determined the expiratory time and produced an I : E ratio of 1 : 1.2.
Fig. 2.

Experimental protocol. For statistical analysis, the mean of the six values T30 to T300 was calculated representing the intervention period. BL, baseline; EQ, equilibration period; CT, computed tomography.
Ventilation was maintained for 5 h in the supine position. Time points for measurements were defined as follows: baseline (before switching to designated ventilation mode), 30, 60, 120, 180, 240 and 300 min (T30 to T300) after the switch to designated ventilation mode.
Respiratory and cardiovascular measurements
At each time point, flow rate was directly recorded from the respective ventilator for 5 min to calculate VT and minute volume. In the EVA group, Ptrach was measured directly, whereas in the control group, Paw was recorded and Ptrach was calculated as described previously.9 In brief, the pressure drop across the ETT was calculated from the nonlinear resistance coefficients and measured flow rate. Ptrach was then calculated as the difference between Paw and the pressure drop across the ETT. Respiratory system compliance, based on Ptrach, was calculated. Arterial partial pressures of oxygen (PaO2) and carbon dioxide (PaCO2) were measured (ABL800 Flex; Radiometer, Brønshøj, Denmark).
Cardiac index (CI) was determined via a three-fold injection of 15 ml of cold saline (8 °C) and was subsequently calculated using the predicted BSA for pigs.10 Further cardiovascular monitoring included heart rate (HR), mean arterial BP (MAP) and mean pulmonal arterial pressure (MPAP).
Computed tomography
After 5 h of ventilation, thoracic CT imaging (Somatom Definition; Siemens, Munich, Germany) was performed under the designated ventilation mode with a respiratory rate of 6 min−1 to reduce movement artefacts. All other ventilation settings remained unchanged. At a fixed thoracic level, 60 sequences of 12 axial cross-sections per 0.75 s at a collimation of 0.75 mm were recorded and reconstructed to images of 9 mm layer thickness thus creating a series of 60 images representing 45 s of real-time ventilation with which to assess lung aeration over time. These multislice series were processed using algorithms created with MatLab (Version R2015b; MathWorks Inc., Natick, Massachusetts, USA) to filter extrapulmonary tissue and to create histograms containing the range from −1000 to 0 Hounsfield units (HU) in steps of 50 HU. According to Gattinoni et al.,11 four ranges were defined: −1000 to −900 HU, representing airways and overdistended lung tissue; −900 to −500 HU, representing normally aerated lung tissue; −500 to −100 HU, representing poorly aerated lung tissue; and −100 to 0 HU, representing nonaerated lung tissue. The relative lung volume of each HU-range was calculated as the median of total lung volume percentages of all frames of a series.
Histopathology
After the experiments, the animals were euthanised with a lethal injection of potassium chloride. Sternotomy was performed and the lower lobe of the right lung was clamped and excised. Representative samples of lung tissue were collected and fixed in 2% paraformaldehyde in PBS for 1 h. Incubation in 30% sucrose overnight at 4 °C and subsequent slow freezing in embedding medium (Tissue-Tek O.C.T.; Sakura Finetek, Alphen van den Rijn, The Netherlands) followed. Cryosections of 6 μm were stained with haematoxylin and eosin. Five images were collected from each tissue sample and alveolar wall thicknesses were measured using AxioVision (ver. 4.8; Carl Zeiss Micro Imaging, Jena, Germany) in five high power fields, randomly assigned to each image.
For determination of the wet-to-dry ratio, superficial fluids were paper-dried and wet weight was recorded immediately. Dry weight was determined after drying at 65 °C for 72 h.
Statistical analysis
An a priori sample size calculation resulted in n=7 for each group for a power of 80%.
Data are presented as mean ± SD, if not declared otherwise. All data were assessed for normal distribution using the Shapiro–Wilk test preceding the following analyses. The mean of the six values of T30 to T300 was calculated, representing the intervention period. For data from baseline and the following intervention periods, repeated measures analysis of variance with group allocation as independent and time as within-group factor were calculated and post hoc Bonferroni multiple comparison tests were performed if appropriate. Nonrepeated measurements (CT, histopathology) were compared using an unpaired two-sided Student's t test. Differences with P less than 0.05 were regarded as significant. GraphPad Prism (ver. 7.01 for Windows; GraphPad Software, La Jolla, California, USA) was used for the statistical analysis.
Results
In total, 16 pigs were included in this study, nine animals in the EVA group and seven animals in the control group. Two animals in the EVA group were excluded due to software errors in the ventilator prototype resulting in unstable ventilation (n=1) or unstable FiO2 delivery (n=1). In one animal in the EVA group, there was an obstruction of the Tritube and it was changed. Ventilator settings and respiratory measurements were unaffected. In one animal in the control group, CT images could not be obtained due to technical failure of the CT scanner. Hence, data from seven animals in each group were available except that for analyses of CT images, data from only six animals in the control group could be used.
Body weight did not differ significantly between the groups (EVA 45.0 ± 2.7 versus control 45.6 ± 2.4 kg, P = 0.68).
Respiratory and cardiovascular measurements
Ptrach curves differed characteristically between EVA-ventilation and VCV (Fig. 3). Mean Ptrach (mPtrach) as well as PaO2 were significantly increased in the EVA group during the intervention period compared with control (mPtrach: 11.6 ± 0.4 versus 9.0 ± 0.3 cmH2O, P < 0.001 and PaO2: 19.2 ± 0.7 versus 17.5 ± 0.4 kPa, P = 0.002; Fig. 4a and b). Peak Ptrach reached comparable levels in both groups (Table 1). PaCO2 showed no significant difference between groups during the intervention period (5.4 ± 0.3 versus 5.5 ± 0.3 kPa, P > 0.99; Fig. 4c), whereas minute volume was significantly lower in the EVA group compared with the control group (5.5 ± 0.2 versus 7.0 ± 1.0 l min−1, P = 0.02; Fig. 4d). A time course of respiratory measurements is provided as supplementary data (refer to Figure, Supplemental Digital Content 1, which displays complete data of mPtrach, PaO2, PaCO2, minute volume, etCO2 and compliance in relation to the experimental protocol for the EVA and control group). VT, respiratory system compliance, HR, MAP, MPAP and CI revealed no significant differences between groups (Table 1).
Fig. 3.
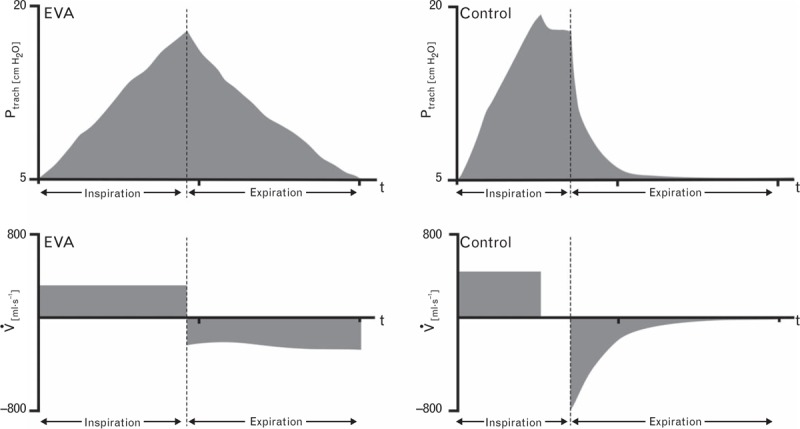
Tracheal pressure and flow during mandatory ventilation. Exemplified tracheal pressure (Ptrach) and flow (V̇) curves of a ventilation cycle for expiratory ventilation assistance group and control group, respectively.
Fig. 4.

Respiratory markers of expiratory ventilation assistance group and control group. (a) Mean tracheal pressure (mPtrach). (b) Arterial partial pressure of oxygen (PaO2). (c) Arterial partial pressure of carbon dioxide (PaCO2). (d) Minute volume. Box and whisker plots indicate median, interquartile range and full range.
Table 1.
Respiratory, cardiovascular and histopathological variables of expiratory ventilation assistance group and control group
| Source of variation (RM-ANOVA) | |||||
| Variable | Time period | EVA | Control | P (time) | P (group) |
| Peak Ptrach (cmH2O) | Baseline | 16.1 ± 0.7 | 17.1 ± 1.2 | <0.0001 | 0.48 |
| Intervention period | 18.3 ± 0.9* | 18.0 ± 1.2* | |||
| VT (ml kg−1) | Baseline | 7.9 ± 0.3 | 8.1 ± 0.2 | 0.06 | 0.87 |
| Intervention period | 8.0 ± 0.3 | 8.0 ± 0.2 | |||
| Compliance (ml cmH2O−1) | Baseline | 31.7 ± 2.3 | 30.2 ± 3.3 | <0.0001 | 0.77 |
| Intervention period | 27.5 ± 1.6* | 28.1 ± 3.0* | |||
| Respiratory rate (min−1) | Baseline | 16.9 ± 1.2 | 19.3 ± 3.0 | 0.048 | <0.01 |
| Intervention period | 15.4 ± 1.1*,** | 19.1 ± 2.0 | |||
| Heart rate (min−1) | Baseline | 82 ± 24 | 82 ± 19 | 0.026 | 0.84 |
| Intervention period | 73 ± 13 | 77 ± 14 | |||
| MAP (mmHg) | Baseline | 74 ± 13 | 88 ± 10 | 0.61 | 0.06 |
| Intervention period | 76 ± 13 | 88 ± 8 | |||
| MPAP (mmHg) | Baseline | 20 ± 7 | 21 ± 5 | <0.01 | 0.97 |
| Intervention period | 23 ± 6* | 22 ± 4 | |||
| CI (l min−1 m−2) | Baseline | 5.4 ± 1.4 | 5.4 ± 1.1 | 0.02 | 0.68 |
| Intervention period | 4.6 ± 0.7* | 5.0 ± 0.8 | |||
| Variable | EVA | Control | P (unpaired t test) |
| Wet-to-dry ratio | 6.0 ± 0.4 | 6.2 ± 0.8 | 0.63 |
| Alveolar wall thickness (μm) | 5.7 ± 0.4 | 5.7 ± 0.3 | 0.96 |
Data presented as mean ± SD. CI, cardiac index; MAP, mean arterial pressure; MPAP, mean pulmonal arterial pressure; Ptrach, tracheal pressure; RM-ANOVA, repeated-measures analysis of variance; VT, tidal volume.
*P < 0.05 versus baseline.
**P < 0.05 versus control (post hoc test Bonferroni adjusted).
Computed tomography
Dynamic CT series showed a characteristic and constant inflation and deflation of the lungs ventilated with the EVA mode, whereas lungs ventilated with VCV showed a more staccato-like movement during inflation and deflation. Representative series are provided as digital content (refer to Animations, Supplemental Digital Content 2 to 5, which display 45 s of real-time ventilation replayed with triple speed for the EVA and control groups, colour coded and native CT, respectively; colour bar indicates corresponding Hounsfield units). CT histograms showed a left shift for the EVA group compared with control (Fig. 5a). The percentage of normally aerated lung volume was significantly higher (81.0 ± 3.6 versus 75.8 ± 3.0%, P = 0.017) and the percentage of poorly aerated lung volume was significantly lower (9.5 ± 3.3 versus 15.7 ± 3.5%, P = 0.007) in the EVA compared with the control group. This finding was more pronounced in the dependent lung regions (normally aerated: 70.2 ± 6.7 versus 59.5 ± 6.6%, P = 0.01 and poorly aerated: 19.2 ± 5.3 versus 27.6 ± 4.8%, P = 0.01). The percentages of overdistended (EVA 4.5 ± 2.5 versus control 3.8 ± 1.4%, P = 0.54) and nonaerated lung volumes (EVA 1.0 ± 0.7 versus control 1.2 ± 0.4%, P = 0.62) were comparable in both groups (Fig. 5b).
Fig. 5.

Analysis of dynamic computed tomography sequence of expiratory ventilation assistance group and control group. (a) Histogram of computed tomography values in steps of 50 HU. (b) Percentages of defined HU ranges: −1000 to −900 HU: airways and overinflated lung tissue; −900 to −500 HU: normally aerated lung tissue; −500 to −100 HU: poorly aerated lung tissue; −100 to 0 HU: nonaerated lung tissue. Box and whisker plots indicate median, interquartile range and full range.
Histopathology
Neither alveolar wall thickness nor wet-to-dry ratio showed significant differences between groups (Table 1).
Discussion
The main results of our study are that EVA ventilation improved lung aeration and oxygenation compared with a commonly applied VCV protocol with identical PEEP, PIP and tidal volume. Furthermore, a lower minute volume was required for achieving normoventilation.
Lung protective ventilation
Improving aeration is of particular interest with regard to intra-operative protective lung ventilation. Although a generally accepted definition is missing, the aim of protective lung ventilation is to keep lung tissue available for effective gas exchange while avoiding excessive pressure levels. Intra-operative strategies to protect the lungs have shown beneficial effects in terms of avoiding postoperative pulmonary complications12 and even reducing mortality.13 With EVA ventilation, aeration of alveolar structures was achieved by an elevated mPtrach which created an increased alveolar surface area for gas exchange and accordingly increased PaO2.14 PEEP and PIP, and consequently the tidal pressure amplitude were not altered. As a further effect, the elevated mPtrach increased the mean alveolar O2 partial pressure gradient, supporting the PaO2 increase.14
With conventional ventilation, this could be achieved by either increasing PEEP or VT, respectively. The dilemma is that both changes contribute to increased peak pressure, thereby adding to the risk for ventilator-induced lung injury15,16 and for postoperative pulmonary complications.13 To limit peak pressure, low tidal volume and low pressure amplitudes have been proposed as part of an intra-operative protective ventilation strategy.13 As EVA ventilation did not alter PIP and PEEP, it offers a complementary option for optimising gas exchange whenever hypoxaemia and/or lung injury is imminent.
A different way to elevate mPtrach is to shorten the expiratory time, which could even result in inverse ratio ventilation. However, an increased I : E ratio yields the risk of incomplete expiration17 and is suspected of aggravating ventilator-induced lung injury.18 EVA ventilation, however, is based on direct measurement of Ptrach and switches from inspiration to expiration if PIP is achieved and from expiration to inspiration if PEEP is achieved. Thus, by its mere controlling principle, incomplete expiration or negative pressure development should be excluded.
The oxygenation increase of 10% in our study might be considered rather small; it is attributed to the limited recruitable lung volume in the healthy lungs of our animals. Quantitative CT scan techniques for lung imaging are well known and are usually performed as static measurements either at the end of inspiration or the end of expiration.19–22 Due to its dynamic nature, intratidal derecruitment might be missed in such static analyses. Our analyses of dynamic sequences revealed an increased fraction of normally aerated and a decreased fraction of poorly aerated lung tissue during EVA ventilation. This can be explained by the shorter time alveolar pressure spends below the closing pressure due to the delayed pressure decrease during expiration. As derecruitment is a matter of seconds,23 we conclude that the EVA mode may result in a diminished intratidal derecruitment. This is in accord with the observed elevated mPtrach and the improved oxygenation in the EVA group.
Histopathology revealed no signs of lung injury or tissue inflammation. As the investigated animals had healthy lungs and both groups were subjected to moderate Paw and VT, development of ventilator-induced lung injury after 5 h of mechanical ventilation seems very unlikely. Consequently, no conclusions can be drawn from this study concerning a direct lung protective effect of the EVA mode. To address these questions, more studies in specific situations like abdominal surgery or acute lung injury are needed.
Dead space ventilation
In general, alveolar gas exchange surface area and shunt volume does not influence PaCO2 as much as PaO2. A relatively high shunt volume of up to 50% of cardiac output is needed before PaCO2 is affected in otherwise normal physiological conditions.24 Nevertheless, in the EVA group, a similar PaCO2 could be generated by a lower minute volume. However, it should be noted that minute volume between baseline and the intervention period in the EVA group was not significantly different. Furthermore, the much greater variance seen in the control group could, in retrospect, be attributed to two animals that required a minute volume of up to 10 l min−1 to achieve normoventilation. Hence, our results on minute volume should be interpreted with care. However, although CO2 homeostasis underpins various balances and effects, it is alveolar ventilation that remains the most influential factor governing PaCO2 in the steady state.25 As alveolar ventilation is increased by decreasing dead space ventilation, the Tritube with its small lumen and reduced instrumental dead space might have contributed to the observed effect.
Cardiovascular changes
An elevation in mPtrach might be expected to increase mean intrathoracic pressure, with an associated reduction in venous return to the heart and consequently a lowering of MAP. This mechanism is still sufficiently controversial to merit discussion.26–29 In fact, MAP was slightly lower in the EVA group, but the difference was not statistically significant, and there was no difference between baseline and the intervention period. There was, however, a time dependent significant difference in MPAP and CI in the EVA group, suggesting a potential influence on cardiovascular stability. Further studies which address these questions explicitly should elucidate if this is clinically relevant.
Limitations of the study
The aim of this study was to compare two ventilation modes that differ mainly during the expiratory phase in a setting of peri-operative ventilation. As control, VCV and its settings were chosen, because it remains the most commonly applied ventilation mode in the peri-operative field.30 In addition, both modes use a constant flow for inspiration; hence comparison of EVA and VCV seemed more appropriate than comparison with pressure controlled ventilation. However, VCV shows the characteristic plateau during inspiration which is missing in EVA ventilation. In this study, plateau time for VCV was not set explicitly; it depended on the inspiratory flow and the respiratory rate. As the plateau phase contributes to an elevation of mPtrach, it seems unlikely that this difference during inspiration accounts for the observed effects. However, the inspiratory differences might have had some influence on the results.
Ptrach equals alveolar pressure when flow is absent.31 As there is no zero-flow period at the end of ventilation phases during EVA ventilation, the pressure distal to the trachea will be slightly overestimated during inspiration and underestimated during expiration. For animals with healthy lungs, we estimate this to be a marginal effect as mean tracheal and alveolar pressures are unaffected.14 However, the ventilator prototype does not offer an end-expiratory occlusion to determine PEEP during zero flow. It is possible that, especially in patients suffering from obstructive or emphysematous disease, dynamic hyperinflation might be generated.
A diminished intratidal derecruitment should lead to improved respiratory system compliance. However, in our study, compliance declined to a similar extent in both groups with the most pronounced change from BL to T30 (see Supplemental Digital Content 1). External recruitment manoeuvres were not applied during the experiment, because this might have masked the effect of the EVA ventilation. However, omitting recruitment of lung tissue directly after the disconnection might have contributed to the observed effect.
The EVA prototype depended on use of a Tritube. In the EVA group, the Tritube was inserted into the ETT already in the trachea to prevent a second laryngoscopy and intubation. As Ptrach is independent of the used airway device, comparison of the two groups seemed appropriate.
Ventilation with narrow bore tubes often is associated with tube obstruction by mucus. The EVA prototype is equipped with an obstruction alarm and responds with a short purge of gas during inspiration. In one animal, the purge did not suffice, so the Tritube was replaced. Ventilator settings and respiratory measurements were not affected. However, tube obstruction could pose a limitation of this ventilation technique.
The analysis of serial measurements as the mean of several time points was described as a valid statistical approach, and our results are appropriately reported.32 However, with this approach temporal changes might be missed. The temporal changes recorded in our data were pronounced from BL to T30, whereas changes from T30 to T300 changed to only a minor extent (see Supplemental Digital Content 1). Hence, a summarised report of T30 to T300 seems justified.
Randomisation of the animals to the two groups was not possible due to limited access to the EVA prototype. Experiments were conducted in two blocks, first the control group, then the EVA group. Random allocation to the experimental groups should avoid differences in basic characteristics between groups. These did not differ significantly in our study, particularly in the use of anaesthetics and neuromuscular blocking drugs between both groups (refer to Table, Supplemental Digital Content 6, which displays body weight and total use of anaesthetics, neuromuscular blocking drugs and analgesics of EVA group and control group). However, other confounding factors could have been present and cannot be completely excluded.
Conclusion
This is the first study to investigate the EVA mode delivered by an automated ventilator. Compared with a commonly applied VCV protocol, by using an expiratory control, the EVA mode enhances aeration of lung tissue and improves oxygenation without changing the minimal and maximal pressure of a ventilation cycle. These findings suggest the EVA mode might represent a promising new approach for protective lung ventilation.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements relating to this article
Assistance with the study: we would like to thank Dietmar Enk, of the University Hospital Münster, inventor of Evone and Tritube, for introducing us to the prototypes used in this study. We would like to thank Bibek Dhital of the University of Freiburg for programming the MATLAB algorithm for CT analyses.
Financial support and sponsorship: this project has received funding from the European Union's Horizon 2020 research and innovation program under grant agreement No. 691519.
Conflicts of interest: none.
Presentation: Presentation: preliminary findings of this study were presented as an oral presentation and as an abstract at the meeting of the Swiss, Austrian and German Societies of Biomedical Engineering in Basel, Switzerland (4 to 6 October 2016). Final data were presented as an oral presentation and as an abstract at the scientific meeting of the German Society of Anaesthesiology and Intensive Care Medicine in Würzburg, Germany (24 to 25 February 2017).
Footnotes
Published online 4 May 2018
References
- 1.Goebel U, Haberstroh J, Foerster K, et al. Flow-controlled expiration: a novel ventilation mode to attenuate experimental porcine lung injury. Br J Anaesth 2014; 113:474–483. [DOI] [PubMed] [Google Scholar]
- 2.Wirth S, Springer S, Spaeth J, et al. Application of the novel ventilation mode FLow-Controlled EXpiration (FLEX): a crossover proof-of-principle study in lung-healthy patients. Anesth Analg 2017; 125:1246–1252. [DOI] [PubMed] [Google Scholar]
- 3.Hamaekers AE, Götz T, Borg PA, et al. Achieving an adequate minute volume through a 2 mm transtracheal catheter in simulated upper airway obstruction using a modified industrial ejector. Br J Anaesth 2010; 104:382–386. [DOI] [PubMed] [Google Scholar]
- 4.Hamaekers AE, Borg PA, Gotz T, et al. Ventilation through a small-bore catheter: optimizing expiratory ventilation assistance. Br J Anaesth 2011; 106:403–409. [DOI] [PubMed] [Google Scholar]
- 5.Willemsen MG, Noppens R, Mulder AL, et al. Ventilation with the Ventrain through a small lumen catheter in the failed paediatric airway: two case reports. Br J Anaesth 2014; 112:946–947. [DOI] [PubMed] [Google Scholar]
- 6.Berry M, Tzeng Y, Marsland C. Percutaneous transtracheal ventilation in an obstructed airway model in postapnoeic sheep. Br J Anaesth 2014; 113:1039–1045. [DOI] [PubMed] [Google Scholar]
- 7.Hamaekers AE, van der Beek T, Theunissen M, et al. Rescue ventilation through a small-bore transtracheal cannula in severe hypoxic pigs using expiratory ventilation assistance. Anesth Analg 2015; 120:890–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kristensen MS, de Wolf MWP, Rasmussen LS. Ventilation via the 2.4 mm internal diameter Tritube(®) with cuff – new possibilities in airway management. Acta Anaesthesiol Scand 2017; 61:580–589. [DOI] [PubMed] [Google Scholar]
- 9.Guttmann J, Eberhard L, Fabry B, et al. Continuous calculation of intratracheal pressure in tracheally intubated patients. Anesthesiology 1993; 79:503–513. [DOI] [PubMed] [Google Scholar]
- 10.Swindle MM, Makin A, Herron AJ, et al. Swine as models in biomedical research and toxicology testing. Vet Pathol 2012; 49:344–356. [DOI] [PubMed] [Google Scholar]
- 11.Gattinoni L, Caironi P, Pelosi P, et al. What has computed tomography taught us about the acute respiratory distress syndrome? Am J Respir Crit Care Med 2001; 164:1701–1711. [DOI] [PubMed] [Google Scholar]
- 12.Futier E, Constantin J-M, Paugam-Burtz C, et al. A trial of intraoperative low-tidal-volume ventilation in abdominal surgery. N Engl J Med 2013; 369:428–437. [DOI] [PubMed] [Google Scholar]
- 13.Ladha K, Vidal Melo MF, McLean DJ, et al. Intraoperative protective mechanical ventilation and risk of postoperative respiratory complications: hospital based registry study. BMJ 2015; 351:h3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marini JJ, Ravenscraft SA. Mean airway pressure: physiologic determinants and clinical importance – Part 2: Clinical implications. Crit Care Med 1992; 20:1604–1616. [PubMed] [Google Scholar]
- 15.Silva PL, Negrini D, Rocco PRM. Mechanisms of ventilator-induced lung injury in healthy lungs. Best Pract Res Clin Anaesthesiol 2015; 29:301–313. [DOI] [PubMed] [Google Scholar]
- 16.Protti A, Andreis DT, Monti M, et al. Lung stress and strain during mechanical ventilation: any difference between statics and dynamics? Crit Care Med 2013; 41:1046–1055. [DOI] [PubMed] [Google Scholar]
- 17.Marini JJ. Dynamic hyperinflation and auto-positive end-expiratory pressure: lessons learned over 30 years. Am J Respir Crit Care Med 2011; 184:756–762. [DOI] [PubMed] [Google Scholar]
- 18.Müller-Redetzky HC, Felten M, Hellwig K, et al. Increasing the inspiratory time and I:E ratio during mechanical ventilation aggravates ventilator-induced lung injury in mice. Crit Care 2015; 19:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gattinoni L, Caironi P, Cressoni M, et al. Lung recruitment in patients with the acute respiratory distress syndrome. N Engl J Med 2006; 354:1775–1786. [DOI] [PubMed] [Google Scholar]
- 20.Rouby JJ, Puybasset L, Nieszkowska A, et al. Acute respiratory distress syndrome: lessons from computed tomography of the whole lung. Crit Care Med 2003; 31:S285–S295. [DOI] [PubMed] [Google Scholar]
- 21.Malbouisson LM, Muller JC, Constantin JM, et al. Computed tomography assessment of positive end-expiratory pressure-induced alveolar recruitment in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 2001; 163:1444–1450. [DOI] [PubMed] [Google Scholar]
- 22.Patroniti N, Bellani G, Maggioni E, et al. Measurement of pulmonary edema in patients with acute respiratory distress syndrome. Crit Care Med 2005; 33:2547–2554. [DOI] [PubMed] [Google Scholar]
- 23.Smith BJ, Grant KA, Bates JHT. Linking the development of ventilator-induced injury to mechanical function in the lung. Ann Biomed Eng 2013; 41:527–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wagner PD. The physiological basis of pulmonary gas exchange: implications for clinical interpretation of arterial blood gases. Eur Respir J 2015; 45:227–243. [DOI] [PubMed] [Google Scholar]
- 25.Lumb AB, Nunn JF. Nunn's applied respiratory physiology. 7th ed.Edinburgh, Churchill Livingstone: Elsevier; 2010. [Google Scholar]
- 26.Gernoth C, Wagner G, Pelosi P, et al. Respiratory and haemodynamic changes during decremental open lung positive end-expiratory pressure titration in patients with acute respiratory distress syndrome. Crit Care 2009; 13:R59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmitt JM, Vieillard-Baron A, Augarde R, et al. Positive end-expiratory pressure titration in acute respiratory distress syndrome patients: impact on right ventricular outflow impedance evaluated by pulmonary artery Doppler flow velocity measurements. Crit Care Med 2001; 29:1154–1158. [DOI] [PubMed] [Google Scholar]
- 28.Mekontso Dessap A, Charron C, Devaquet J, et al. Impact of acute hypercapnia and augmented positive end-expiratory pressure on right ventricle function in severe acute respiratory distress syndrome. Intensive Care Med 2009; 35:1850–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fougeres E, Teboul JL, Richard C, et al. Hemodynamic impact of a positive end-expiratory pressure setting in acute respiratory distress syndrome: importance of the volume status. Crit Care Med 2010; 38:802–807. [DOI] [PubMed] [Google Scholar]
- 30.LAS VEGAS Investigators. Epidemiology, practice of ventilation and outcome for patients at increased risk of postoperative pulmonary complications: LAS VEGAS – an observational study in 29 countries. Eur J Anaesthesiol 2017; 34:492–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marini JJ, Ravenscraft SA. Mean airway pressure: physiologic determinants and clinical importance – Part 1: Physiologic determinants and measurements. Crit Care Med 1992; 20:1461–1472. [PubMed] [Google Scholar]
- 32.Matthews JN, Altman DG, Campbell MJ, et al. Analysis of serial measurements in medical research. BMJ 1990; 300:230–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


