Supplemental digital content is available in the text.
Key Words: HIIT, V˙O2peak, ANAEROBIC THRESHOLD, CLINICAL, SHORT TERM
ABSTRACT
Background and Aim
Exercise training regimes can lead to improvements in measures of cardiorespiratory fitness (CRF), improved general health, and reduced morbidity and overall mortality risk. High-intensity interval training (HIIT) offers a time-efficient approach to improve CRF in healthy individuals, but the relative benefits of HIIT compared with traditional training methods are unknown in across different disease cohorts.
Methods
This systematic review and meta-analysis compares CRF gains in randomized controlled trials of short-term (<8 wk) HIIT versus either no exercise control (CON) or moderate continuous training (MCT) within diseased cohorts. Literature searches of the following databases were performed: MEDLINE, EMBASE, CINAHL, AMED, and PubMed (all from inception to December 1, 2017), with further searches of Clinicaltrials.gov and citations via Google Scholar. Primary outcomes were effect on CRF variables: V˙O2peak and anaerobic threshold.
Results
Thirty-nine studies met the inclusion criteria. HIIT resulted in a clinically significant increase in V˙O2peak compared with CON (mean difference [MD] = 3.32 mL·kg−1·min−1, 95% confidence interval [CI] = 2.56–2.08). Overall HIIT provided added benefit to V˙O2peak over MCT (MD = 0.79 mL·kg−1·min−1, 95% CI = 0.20–1.39). The benefit of HIIT was most marked in patients with cardiovascular disease when compared with MCT (V˙O2peak: MD = 1.66 mL·kg−1·min−1, 95% CI = 0.60–2.73; anaerobic threshold: MD = 1.61 mL·kg−1·min−1, 95% CI = 0.33–2.90).
Conclusions
HIIT elicits improvements in objective measures of CRF within 8 wk in diseased cohorts compared with no intervention. When compared with MCT, HIIT imparts statistically significant additional improvements in measures of CRF, with clinically important additional improvements in V˙O2peak in cardiovascular patients. Comparative efficacy of HIIT versus MCT combined with an often reduced time commitment may warrant HIIT’s promotion as a viable clinical exercise intervention.
Objective measures of cardiorespiratory fitness (CRF) (e.g., V˙O2peak and anaerobic threshold [AT]) predict whole-body health, morbidity, and mortality (1–4). These measures of CRF can be altered via participation in exercise training regimens, which in turn may improve general health. Traditionally, endurance-based aerobic activity or “moderate continuous training” (MCT) has been used to improve CRF (5) and exercise tolerance (6).
Despite MCT (150 min of moderate aerobic activity every week) forming the primary basis of almost all public health exercise-based recommendations (7,8), greater attention has recently been paid to the utility of higher intensity exercise (75 min of vigorous activity every week) as an alternative to MCT (7) in the context of “exercise for health” (9) as the latter is more time efficient, which may improve compliance (10).
Patients can have modification of disease risk factors through exercise interventions (e.g., reduction of blood pressure in those at risk of stroke) (11), and exercise can also be used to help optimize patients before a planned intervention (e.g., patients with suspected cancer or those awaiting urgent elective surgery for malignancy) (12). For those having major surgical procedures, perioperative outcome is in large part dependent on preoperative CRF (2). An ability to rapidly improve CRF would therefore be attractive if deliverable in the short time available between the suspicion of cancer and initiation of primary treatment (13).
Often however, there is not an extended period available from clinical suspicion of cancer before first definitive treatment to complete exercise programs: for example, in the United Kingdom, the National Cancer Action Team imposes two cancer waiting time service standards (13). The first is a 62-d target from initial GP referral for suspected cancer or urgent referral from NHS screening program, whereas the second is a 31-d window from the decision to treat to primary treatment (surgery, drug treatment, or radiotherapy) of the cancer (13). These standards have led to increasing interest in novel exercise interventions to improve CRF within truncated time frames. It has been suggested that exercise regimens such as high-intensity interval training (HIIT) may deliver clinically important improvements in CRF within a clinically relevant time frame with minimal time commitment from the patient.
HIIT, defined as brief intermittent bursts of vigorous activity interspersed with periods of rest or low-intensity exercise (14), can bring more pronounced improvements in objective measures of CRF than MCT in healthy individuals over an equivalent number of weeks (15). It is unknown whether individuals with disease will benefit from HIIT in the same way. In any exercise intervention, it is essential that there are high levels of adherence and compliance to maximize benefit, especially given that comorbid patients have been shown to be poor compliers with exercise interventions (16). HIIT has previously been reported to be more enjoyable than MCT (17). Time pressure has been identified as one of the most commonly cited barriers to exercise adherence (10,18). HIIT’s reduced time commitment and training volume makes it an attractive option for rapidly achieving maximal gains in CRF.
Previous reviews in distinct disease groups exploring the efficacy of HIIT over longer time durations (median 12 wk) have reported benefits of HIIT over MCT in cardiometabolic disease (19) and possible improved efficacy in patients with chronic obstructive pulmonary disease (20). However, equal effects on CRF have been seen in HIIT and MCT in patients with coronary artery disease during cardiac rehabilitation (21). In general, within disease groups, 8–16 wk exercise programs involving HIIT have been shown to be as effective as MCT(22), whereas uncontrolled studies have shown large increases in CRF following HIIT across comorbidities as varied as cardiac disease (23), diabetes (24), obesity (25), and asthma (26). HIIT retains the advantage of requiring significantly less time commitment than MCT.
The aim of this review was to compare the effect of HIIT to no exercise control (CON) or MCT on CRF (V˙O2peak/AT) in differing disease states over short time frames (≤8 wk). We also aimed to identify conditions where HIIT might be particularly effective compared with CON or MCT.
METHODS
Study design
This systematic review was prospectively registered with PROSPERO (registration no. CRD42016042299) and performed according to the PRISMA statement (27). Only randomized control trials evaluating HIIT versus CON or HIIT versus MCT were included. Other inclusion criteria were participants >17 yr old with disease, an intervention duration of 8 wk or less, and trials where outcome data were reported pre- and postintervention. Trials involving a drug treatment or dietary supplementation were excluded. We classified trials as delivering HIIT if they satisfied the following criteria: (i) high-intensity efforts interspersed with reduced or no effort recovery periods, (ii) high-intensity bouts >85% predicted heart rate or heart rate reserve, or (iii) high-intensity bouts >85% of peak power output or peak power achieved at baseline exercise test. Studies using “supramaximal” loading of >100% wattage max at cardiopulmonary exercise testing or similar loading criteria were not included.
Literature search
Literature searches were conducted by a research team member (BD) using the following databases: MEDLINE, EMBASE, CINAHL, AMED, and PubMed, all searched from their inception to December 1, 2017, with no language restriction. A detailed search for unpublished studies was conducted on Clinicaltrials.gov. The Cochrane library of systematic reviews was searched for relevant previous reviews, and previous systematic reviews of related topics were also searched for relevant primary studies. References of all identified potentially relevant primary studies were hand searched for further relevant studies. Finally, we searched for studies citing the identified potentially relevant primary studies on Google Scholar to identify any further work potentially meeting the inclusion criteria.
Medical subject headings (MeSH) included the terms “HIIT,” “HIT,” and “EXERCISE.” Free-text words included “exercise,” “high AND intensity,” and “interval.” Abstracts of identified studies were screened by two authors independently (JB and BD). Full text versions of potentially relevant primary studies were then independently screened against the inclusion and exclusion criteria by two authors (JB and SR) and agreement to inclusion reached by consensus.
Data extraction
Study characteristics (authors and year of publication, mean age [yr], % female individuals, training intervention duration (wk), number of planned exercise sessions in total, disease state, individual exercise protocols, and country of origin) were extracted by one author (JB) with outcome data (V˙O2peak, AT, systolic blood pressure [SBP], diastolic blood pressure [DBP], 6-MWT, quality of life [QoL] questionnaires, and adherence data) independently extracted and verified by two authors (JB and SR). Risk of bias for included studies was assessed using the Cochrane Collaboration tool for assessing risk of bias. This was performed independently by two authors (JB and BD), with any disagreement resolved by consensus with a third party author (PH). When outcome data were only reported in graphical form, data were extracted using WebPlotDigitizer (Version 3.12, Austin, TX).
Statistical analysis
To facilitate meta-analysis of change variables when SD values of change were not reported, SD values were imputed using recommended methods described in the Cochrane Handbook (28). First, studies that reported data as SD of the difference between pre- versus postvalues were used to calculate correlation coefficients; these were then averaged for each outcome and used these to calculate change SD from reported baseline and final SD. Outcomes were aggregated using a random-effects model. Changes in V˙O2peak and AT are presented as mean difference (MD) with 95% confidence intervals (CI) in milliliters per kilogram per minute. All other continuous outcomes are also reported as MD. Minimal clinically significant improvements were defined as follows: change in V˙O2peak and AT >1.5 mL·kg−1·min−1 (12), 6-min walk test (6-MWT) >17–23 m (29,30), and SBP/DBP of <10 mm Hg/5 mm Hg (11).
The I2 statistic was used to quantify statistical heterogeneity, with values above 50% taken as evidence of statistical heterogeneity. Publication bias was assessed qualitatively using funnel plots and quantitatively using Egger’s linear regression test (P < 0.05 as evidence of imprecise study effects). We investigated heterogeneity using a random-effects restricted maximum likelihood meta-regression. Covariates included mean age of participants, duration of intervention (wk), and disease cohort. For disease cohorts, we created dummy variables and used the least effective subgroup as the reference category. We report the between-study heterogeneity explained by the model (R2 analog) with a corresponding P value. The Knapp–Hartung modification was used as the variance estimator. To assess the quality of evidence, the GRADE approach (28) was used with evidence downgraded to moderate, low, or very low quality owing to concerns over unexplained heterogeneity, indirectness of evidence, possible publication bias, imprecision in effect estimates, and concerns over risk of bias. All calculations were conducted using STATA 15 (StataCorp, College Station, TX).
RESULTS
Search Results
A total of 2612 abstracts were screened for inclusion, 2570 from the initial literature search and 42 from the reference lists of other identified studies, Google Scholar citations, and other systematic reviews. Of the 2612 abstracts screened, 2559 were excluded as not being relevant or duplicates, leaving 53 studies for full-text review. Of the 53 studies undergoing full text review, 14 were excluded, leaving 39 studies for inclusion in the qualitative analysis and 34 studies for quantitative analysis (Fig. 1, PRISMA Flow Chart [27]) (12,23,31–64).
FIGURE 1.
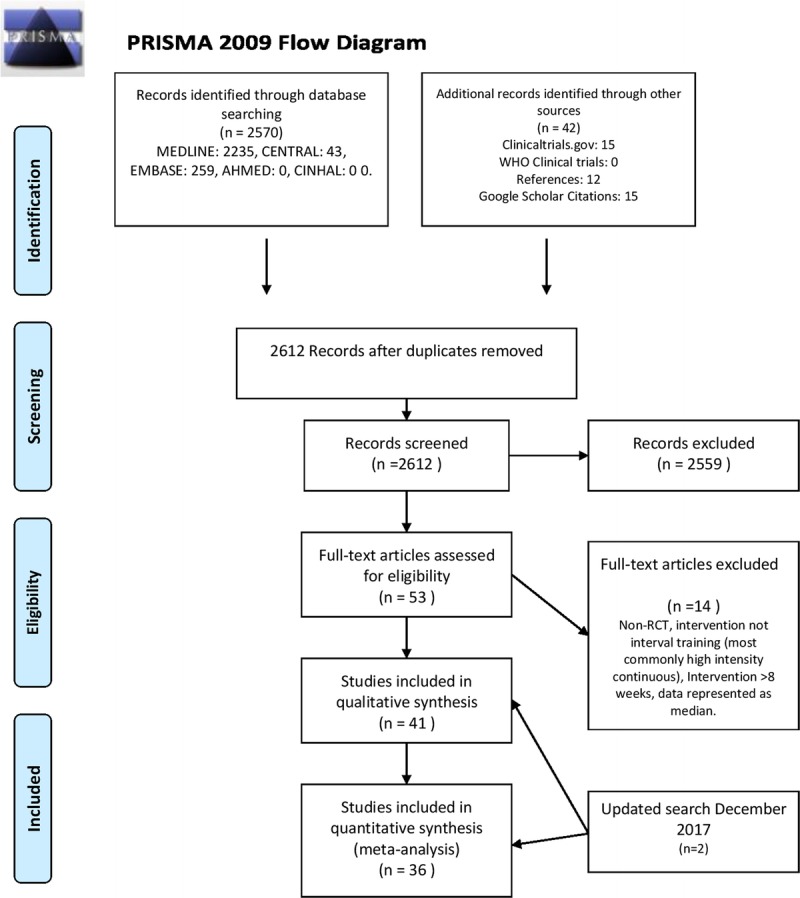
PRISMA flow diagram.
Study Characteristics
The characteristics of the included studies can be found in the online supplementary tables (See Tables, Supplemental Digital Content 1, http://links.lww.com/MSS/B256, Paper Characteristics, HIIT vs CON and Supplemental Digital Content 2, http://links.lww.com/MSS/B257, Paper Characteristics, HIIT vs MCT). The earliest study meeting the inclusion criteria was published in 1999 and the latest in 2016. All studies were published as journal articles. The interventions studied were HIIT versus CON or HIIT versus MCT. Three studies were included in both analyses which compared HIIT versus CON versus MCT (37,38,64).
Risk of Bias
All included studies were at high risk of bias in at least one domain (see Figure, Supplemental Digital Content 3, http://links.lww.com/MSS/B258, which shows risk of bias summary chart). The majority of studies were at high risk of bias due to the innate difficulties in blinding participants to a physical activity intervention. A large number of studies did not describe their random sequence allocation or allocation concealment in sufficient detail to be judged as low risk of bias, and many did not describe blinding of their outcome assessment. Many studies were at risk of reporting bias and some may have suffered from attrition bias.
Data Synthesis
There were sufficient studies to perform independent meta-analysis for V˙O2peak, AT, SBP, and DBP for both HIIT versus CON and HIIT versus MCT interventions.
V˙O2peak
Of 11 study groups from 11 trials analyzed for the comparison of HIIT versus CON, comprising 153 individuals in the HIIT groups and 124 CON participants, HIIT produced a clinically significant increase in V˙O2peak compared with CON (MD = 3.38 mL·kg−1·min−1, 95% CI = 2.7–4.05, I2 = 47.8%) (Fig. 2). Of 25 study groups from 24 trials comparing HIIT to MCT, comprising 359 individuals in the HIIT groups and 341 MCT participants, HIIT provided additional mean increase in V˙O2peak compared with MCT (MD = 0.79 mL·kg−1·min−1, 95% CI = 0.20–1.39, I2 = 50.5%) (Fig. 3). However, this improvement did not meet our a priori target of clinical significance (>1.5 mL·kg−1·min−1). Cardiovascular patients showed the greatest improvement, with clinically significant mean increases in V˙O2peak following HIIT (MD = 1.66 mL·kg−1·min−1, 95% CI = 0.60–2.73, I2 = 43.8%) when compared with MCT (Fig. 3).
FIGURE 2.
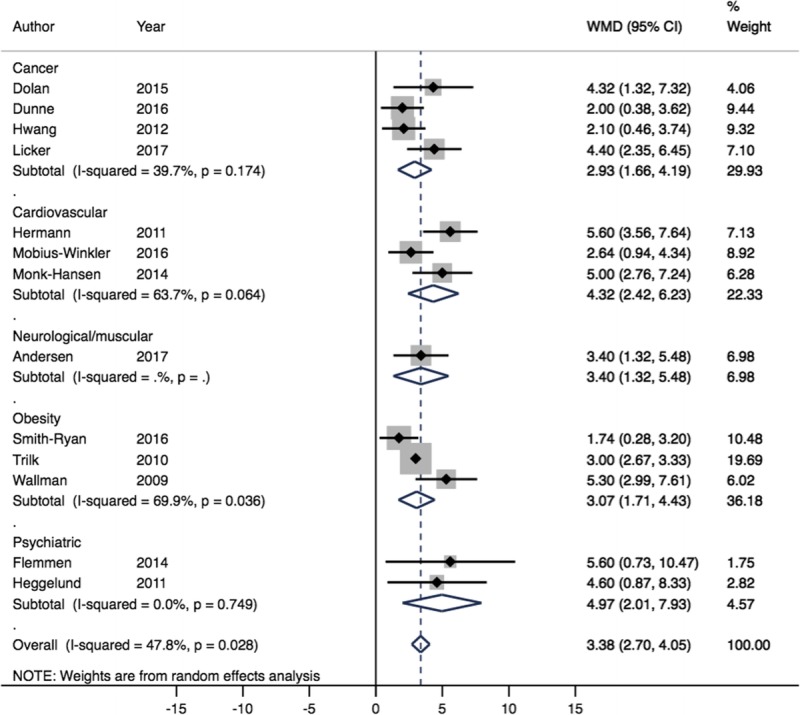
Forest plot showing meta-analysis of V˙O2peak data for HIIT vs CON (WMD mL·kg−1·min−1). Diamonds to the right of the plot show benefit with HIIT.
FIGURE 3.
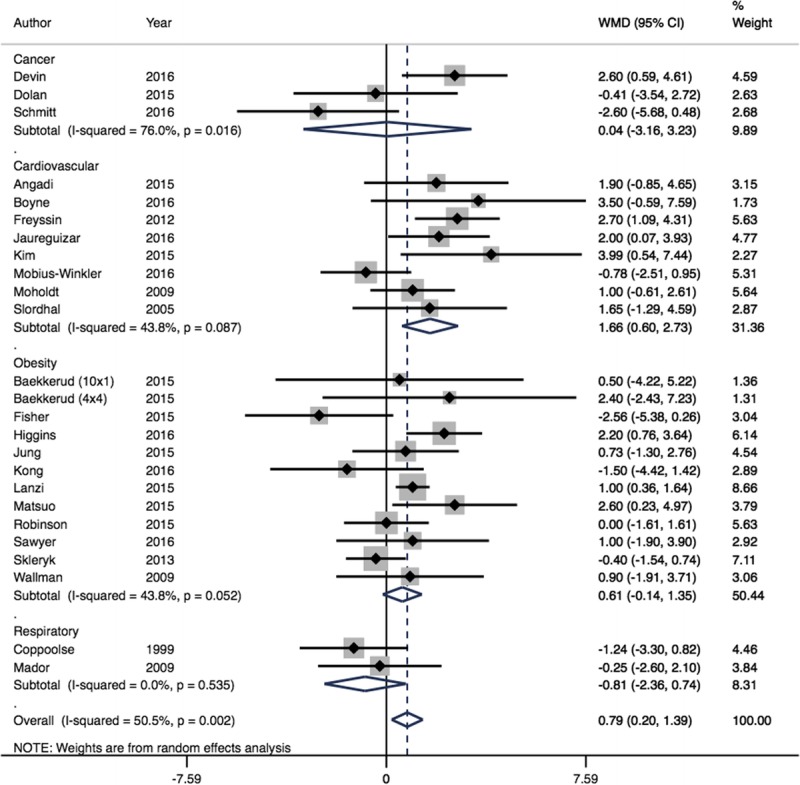
Forest plot showing meta-analysis of V˙O2peak data for HIIT vs MCT (WMD mL·kg−1·min−1). Diamonds to the right of the plot show benefit with HIIT.
On meta-regression analysis, duration of intervention showed significance for HIIT versus CON (R2 = 53.0%, P = 0.04) but nonsignificant for HIIT versus MCT (R2 = 5.54%, P = 0.245). For HIIT versus CON, longer duration of interventions led to larger increases in V˙O2peak. Neither HIIT versus CON nor HIIT versus MCT showed significant interaction for age (R2 = 0%, P = 0.637 and R2 = 0%, P = 0.529, respectively). On meta-regression analysis of HIIT versus MCT, HIIT was more effective in cardiovascular patients (R2 = 4.46%, P = 0.057) than respiratory patients.
There was no evidence of publication bias in either analysis (P = 0.16 and P = 0.91). The quality of evidence of V˙O2peak data was regarded as moderate for HIIT versus CON (downgraded owing to concerns over risk of bias) and low for HIIT versus MCT (downgraded owing to concerns over risk of bias and unexplained heterogeneity) using GRADE criteria (65).
AT
A single study reported AT after HIIT versus CON, showing a mean improvement in AT after HIIT versus CON (MD = 1.5 mL·kg−1·min−1, 95% CI = 0.18–2.82). There was no further data available for meta-analysis to be performed in relation to AT for HIIT versus CON.
HIIT provided additional increase in AT compared with MCT of borderline statistical but not clinical significance (MD = 1.26 mL·kg−1·min−1, 95% CI = −0.02 to 2.54, I2 = 38.3%) in six study groups from five trials, comprising 84 individuals receiving HIIT and 79 MCT. Cardiovascular patients showed the greatest mean improvement in AT after HIIT in comparison with MCT (MD = 1.61 mL·kg−1·min−1, 95% CI = 0.33–2.90, I2 = 39.8%) (Fig. 4). The quality of evidence of AT data for HIIT versus MCT was regarded as low using GRADE criteria (downgraded owing to concerns over risk of bias and imprecision) (65).
FIGURE 4.
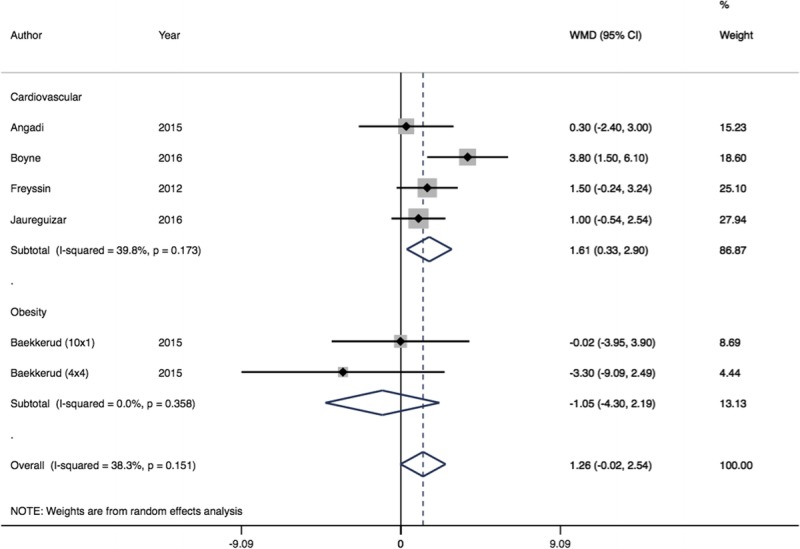
Forest plot showing meta-analysis of AT data for HIIT vs MCT (WMD mL·kg−1·min−1). Diamonds to the right of the plot show benefit with HIIT.
6-MWT
A single study reported 6-MWT outcomes for HIIT versus CON with an effect size of 66 m after HIIT (P = 0.001) (66). For the comparison of HIIT versus MCT, six study groups from 6 trials were analyzed, comprising 151 individuals in the HIIT groups and 149 participants in the MCT group. HIIT delivered an increase in 6-MWT distance compared with MCT (MD = 11.67 m, 95% CI = 1.28–22.06, I2 = 38.9%). Cardiovascular patients showed a greater, yet clinically insignificant improvement (MD = 16.64 m, 95% CI = 5.22–28.07, I2 = 31.9%) compared with respiratory patients (MD = 2.05 m, 95% CI = −12.57 to 16.66, I2 = 0%). The quality of evidence 6-MWT was regarded as low using GRADE criteria (downgraded owing to concerns over risk of bias and imprecision) (65).
Blood pressure
When analyzing blood pressure changes in HIIT versus CON, six study groups from six trials reported SBP results, whereas only five trials presented data for analysis of DBP changes due to unreliable data in one study (47). These studies comprised 79 individuals for SBP in the HIIT groups (DBP 66 individuals) and 67 individuals for SBP in the CON groups (DBP 57 individuals). Compared with CON, HIIT provided a nonsignificant reduction in SBP (MD = −4.48 mm Hg, 95% CI = −11.13 to 2.18, I2 = 58.8%) and a statistically significant reduction in DBP (MD = −3.05 mm Hg, 95% CI = −5.41 to −0.69, I2 = 0%), which however did not meet our a priori target of clinical significance (DBP, 5 mm Hg).
When analyzing BP changes in HIIT versus MCT, for SBP and DBP, eight study groups from eight trials were included. These studies comprised 116 individuals for both SBP and DBP in the HIIT groups and 113 individuals for SBP and DBP in the CON groups. HIIT provided no additional benefit in either SBP (MD = 0.48 mm Hg, 95% CI = −2.01 to 2.97, I2 = 0.0%) or DBP (MD = −0.51 mm Hg, 95% CI = −2.53 to 1.50, P = 0.136, I2 = 36.8%) compared with MCT. The quality of evidence for blood pressure was regarded as moderate to low using GRADE criteria (downgraded owing to concerns over risk of bias and imprecision for some analyses) (65).
QoL
There was marked variation in both instrument selection and reporting of QoL qualitative measures, and questionnaire outcomes were equivocal between both HIIT versus CON and HIIT versus MCT (see Tables, Supplemental Digital Content 4, http://links.lww.com/MSS/B259, HIIT versus CON, and Supplemental Digital Content 5, http://links.lww.com/MSS/B260, HIIT vs MCT, which shows QoL questionnaire outcomes). The most commonly reported QoL questionnaire was SF-36 (67). Studies including SF-36 data did so either with a total score (overall scores) or by domains (summary scores) of the full questionnaire (i.e., Physical Health, Perceived Health, Mental Health). Dunne et al. (12) reported that HIIT prehabilitation was associated with improvements in overall SF-36 QoL and SF-36 mental health scores (change of +11 P = 0.028 and +11 P = 0.037, respectively). Gloeckl et al. (43) reported increased overall SF-36 scores after both HIIT and MCT; however, only the physical health summary score in the MCT group (MD = 4.3 P < 0.05) and the mental health summary score in the HIIT group (MD = 9.7 P < 0.05) improved significantly. Freese et al. (41) reported clinically meaningful improvements in role–physical scores, bodily pain, vitality, social functioning, mental health, and total SF-36 score after 6 wk HIIT. Jaureguizar et al. (48) reported significant increases in the role emotional, mental health, self-reported health status, and mental health index after HIIT only. Other QoL questionnaires used in more than one study are summarized in Tables, Supplemental Digital Content 4, http://links.lww.com/MSS/B259, and Supplemental Digital Content 5, http://links.lww.com/MSS/B260 as above.
Anxiety/mood
Questionnaires used for anxiety and mood can be seen in the supplementary tables (see Tables, Supplemental Digital Content 4, http://links.lww.com/MSS/B259, HIIT vs CON and Supplemental Digital Content 5, http://links.lww.com/MSS/B260, HIIT vs MCT, which shows QoL questionnaires used within studies). The most commonly reported questionnaire to determine anxiety and mood was the Hospital Anxiety and Depression Scale. Again due to paucity of studies reporting values, no meta-analysis was performed across HIIT versus CON or HIIT versus MCT. Flemmen et al. (40) showed a significant reduction in anxiety favoring CON (P < 0.05) and a significant reduction in depression after HIIT (P < 0.05), with no significant difference in reported insomnia. For HIIT versus MCT, both studies showed improvements in the Hospital Anxiety and Depression Scale anxiety and depression domains, however, with no significant benefit between intervention arms (42,57).
Adherence
Because of the widespread lack of reporting and insufficient information included within published papers, we deemed it inappropriate to analyze adherence from the number of dropouts to each intervention, as very few studies reported the direct reason for participants dropping out in HIIT or MCT groups. Disparity in duration of exercise (wk) led to varying numbers of scheduled sessions per study. Overall, adherence to scheduled sessions was high in both groups (see Table, Supplemental Digital Content 6, http://links.lww.com/MSS/B261, which shows reported adherence to HIIT vs MCT protocols).
DISCUSSION
In this review of the current literature exploring the effectiveness of short duration HIIT in disease cohorts, we found that HIIT elicits clinically important improvements (>1.5 mL·kg−1·min−1) in V˙O2peak within 8 wk or less when compared with nonintervention control subjects.
This is in keeping with previous data in both healthy young and older individuals (>60 yr), where HIIT has been shown to improve aspects of fitness. In healthy young individuals completing sprint interval training (4–6 intervals, 30-s all-out sprints), similar adaptations in human skeletal muscle oxidative capacity and exercise performance to those undertaking MCT (90–120 min continuous cycling at 65% V˙O2peak) were seen in as little as 2 wk, despite a vastly reduced time commitment and training volume (approximately 90% lower vs MCT) (68). Similarly, in healthy older individuals, HIIT has been shown to increase V˙O2peak (+8%) and reduce SBP (−9%) in just 6 wk (69). Moreover, in a separate study of healthy older individuals, HIIT has also recently been shown to elicit clinically significant improvements in CRF within just 31 d (70), a time frame that is compliant with the aforementioned UK National Cancer Action Team policy on time from decision to treat to surgery. In addition to the reduced time frame and training volume required by HIIT to elicit improvements in CRF, HIIT may also have the added advantage of rapid adaptation at the level of skeletal muscle, resulting in fewer negative training symptoms (e.g., delayed onset muscle soreness [22]), which is postulated to lead to increased adherence.
HIIT is at least as effective as MCT over short periods across all groups. Subgroup analysis showed additional benefit in cardiovascular patients versus other patient groups following HIIT. To exemplify, cardiovascular patients showed additional increases in V˙O2peak and AT after HIIT when compared with MCT. It is likely that the rapid benefit shown in this review is a result of peripheral adaptations such as mitochondrial oxidative enzyme upregulation and increased buffering capacity (68) as it is only in longer-term training programs (≥12 wk) that improvements in cardiac structure and systolic function have been shown (71). In response to HIIT, the contribution of cardiac change may be underestimated because of the research focus primarily being on mitochondrial upregulation, with potential cardiac changes being understudied.
A small number patients with cancer were included in this review, with varying outcomes. Lung, colon, and breast cancer groups all showed improvement in CRF with HIIT when compared with no exercise. There was no added benefit of HIIT over MCT. Blunted adaptation in these cancer groups (shown as a lack of CRF improvement in response to HIIT compared with the overall effect of HIIT vs CON) may be explained by blunted mitochondrial enzyme activity while cancers remain in situ (72). In addition, colorectal cancer patients presenting for resection have lower CRF than age-matched controls while the cancer is still in situ. However, removal the cancer facilitates a return toward normal CRF (73). Taken together, these studies may lead to a suggestion that tumour presence hinders adaptive capacity to exercise training, at least in this cancer type. Adjuvant chemotherapy has negative effects on CRF preoperatively in colorectal cancer patients (74) and have resulted in higher rates of heart failure and cardiomyopathy after breast cancer chemotherapy (75), as such these confounding drug regimens must be considered when interpreting trainability within these groups.
The beneficial psychological effects of exercise per se are well known, but it is unclear whether HIIT is superior to MCT in improving QoL from this review. This lack of clarity is due to the heterogeneity of tools used, small numbers of studies reporting QoL outcomes, and lack of suitable comparisons for many of the questionnaires.
Beyond mechanistic propositions based on small-scale nonrandomized control trials in distinct disease groups, reasons why certain pathological subgroups might not show CRF improvements with HIIT are far from clear. One possible explanation for certain subgroups is that exercise intervention studies mainly report mean improvements in CRF parameters as milliliters per kilogram per minute, rendering obese patients at a relative disadvantage for demonstrating improvement over short periods; as in the authors’ experience, individuals normally remain weight stable during short-term HIIT protocols (often due to increased lean muscle mass and fat mass reductions). A recent meta-analysis in obesity concluded that HIIT was superior to traditional exercise to improve CRF and reduce body fat percentage. Notably, the median duration of training protocol for this meta-analysis was 12 wk, with a wide range of 2–52 wk (76), which is does not comply with clinical time frames for cancer surgery. By contrast, but in agreement with this review, another recently published meta-analysis found no clinical benefit of HIIT versus MCT in reduction of total body fat or fat mass over shorter training duration (<12 wk) (77).
To achieve benefit from HIIT, it is thought that a minimal dose of exercise expenditure or training load is required to significantly disturb intracellular homeostasis and stimulate mitochondrial biogenesis (14). This may explain why the respiratory patients seem to gain less benefit versus other pathological groups as respiratory limitation may result in low maximal exercise scores and therefore lower training loads, given that most protocols prescribe the training load as a percentage of V˙O2peak or maximal wattage achieved at cardiopulmonary exercise testing.
HIIT can represent a time efficient training method by which to improve CRF, potentially removing the commonly cited “lack of time” as a barrier to exercise (10). Time efficiency can be due to two facets, reduced work duration within a session and/or individual session time. For example, one of the most commonly used HIIT protocols within studies in this review used 10 intervals of 1 min with 1-min rest periods in between (32,49,52,58,59,62,66,78) totaling a session duration of ~20 min. However, another frequently used HIIT protocol used four intervals of 4-min high-intensity work with 3-min rest periods in between each bout, which led to sessions typically lasting >30 min (12,31,32,36,40,44,55,79), including a work duration of 16 min (vs 10 min in the aforementioned example). Herein we show that, excluding warm-up and end-of-session recovery periods, median work duration during a HIIT session was half of that for MCT protocols (16 vs 30 min). In addition, several studies in this review (34,41,42,46,48,49,51,53,54,58–63) used low volume HIIT protocols, involving 10 min (or less) total work duration (80). Indeed, CRF improvements have been shown in as little as 10% of the training volume with HIIT when compared with MCT (81). Taken in combination, reductions in regime duration, total volume of training, and weekly time commitment represent important drivers for enhancing adherence and reducing costs associated with patient training. However, further work is required to elucidate the optimal work-to-rest ratios within HIIT protocols, which may further reduce the total time commitment for the individual. It is also worth noting that although the majority (>90%) of studies within this review used a static cycle ergometer for HIIT, other training modalities (e.g., running) maybe viable. However, further work is needed to assess the efficacy and tolerability when compared with cycle ergometry within certain patient groups.
QoL and mood outcomes analyzed in this review were pre- to posttraining program questionnaires, mostly global QoL scores or disease specific questionnaires. These outcomes are not specific enough to draw conclusions as to whether individuals preferred HIIT or MCT. However, as there were no significant differences in the number of noncompliers, adherence to scheduled sessions (see Table, Supplemental Digital Content 6, http://links.lww.com/MSS/B261, which shows reported adherence to HIIT vs MCT protocols) or reported serious adverse events lead us to believe that neither HIIT nor MCT are inferior for enjoyment, acceptability, or safety when compared.
Limitations
The studies in this review have a high risk of bias, some of which is unavoidable because of the nature of exercise intervention studies and the inability to blind participants (see Figure, Supplemental Digital Content 3, http://links.lww.com/MSS/B258, which shows risk of bias summary chart). There is also a risk of contamination between HIIT and nonintervention controls. In addition, heterogeneity among HIIT protocols, training duration, chronological age, and pathology leads to uncertainty about the true effectiveness of interventions (82) [see Tables, Supplemental Digital Content 1, http://links.lww.com/MSS/B256, Paper Characteristics (HIIT vs CON); Supplemental Digital Content 2, http://links.lww.com/MSS/B257, Paper Characteristics (HIIT vs MCT); Supplemental Digital Content 7, http://links.lww.com/MSS/B262, Training regimes (HIIT vs CON); and Supplemental Digital Content 8, http://links.lww.com/MSS/B263, Training regimes (HIIT vs MCT)].
CONCLUSIONS
We have shown that HIIT leads to clinically significant improvements in CRF within 8 wk in patients with disease, when compared with no intervention. HIIT also resulted in statistically significant improvements in CRF compared with MCT, with clinically significant benefit seen in cardiovascular patients. Because of the reduced exercise volume and improved efficacy (vs MCT) in certain clinical groups, HIIT can be promoted as a viable clinical exercise intervention to rapidly improve CRF.
Supplementary Material
Acknowledgments
This work was supported by the Medical Research Council (grant no. MR/K00414X/1), the Arthritis Research UK (grant no. 19891) awarded to the MRC-ARUK Centre for Musculoskeletal Ageing Research, and the Dunhill Medical Trust (grant no. R468/0216).
The authors declare no conflicts of interest. The results of the present study do not constitute endorsement by the American College of Sports Medicine. The results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.acsm-msse.org).
REFERENCES
- 1.Snowden CP, Prentis J, Jacques B, et al. Cardiorespiratory fitness predicts mortality and hospital length of stay after major elective surgery in older people. Ann Surg. 2013;257(6):999–1004. [DOI] [PubMed] [Google Scholar]
- 2.Older P. Anaerobic threshold, is it a magic number to determine fitness for surgery? Perioper Med. 2013;2(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kodama S, Saito K, Tanaka S, et al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA. 2009;301(19):2024–35. [DOI] [PubMed] [Google Scholar]
- 4.Kokkinos P. Physical activity, health benefits, and mortality risk. ISRN Cardiol. 2012;2012:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chodzko-Zajko WJ, Proctor DN, Fiatarone Singh MA, et al. Exercise and physical activity for older adults. Med Sci Sports Exerc. 2009;41(7):1510–30. [DOI] [PubMed] [Google Scholar]
- 6.G Whyte. Advances in Sport and Exercise Sciences Series: The Physiology of Training. 1st ed Churchill Vilingstone Elsevier; 2006. pp. 68–71. [Google Scholar]
- 7.World Health Organisation. Physical Activity and Adults. 2011. [cited 2017 May 3]; Available from: http://www.who.int/dietphysicalactivity/factsheet_adults/en/.
- 8.Haskell WL, Lee I-M, Pate RR, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116(9):1081. [DOI] [PubMed] [Google Scholar]
- 9.Department of Health Physical Activity Health Improvement and Protection. Start Active, Stay Active: A report on physical activity from the four home countries’ Chief Medical Officers. Report [Internet]. 2011;62 Available from: https://www.gov.uk/government/publications/start-active-stay-active-a-report-on-physical-activity-from-the-four-home-countries-chief-medical-officers. [Google Scholar]
- 10.Trost SG, Owen N, Bauman AE, Sallis JF, Brown W. Correlates of adults’ participation in physical activity: review and update. Med Sci Sports Exerc. 2002;34(12):1996–2001. [DOI] [PubMed] [Google Scholar]
- 11.Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. Br Med J. 2009;338:b1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dunne DF, Jack S, Jones RP, et al. Randomized clinical trial of prehabilitation before planned liver resection. Br J Surg. 2016;103(5):504–12. [DOI] [PubMed] [Google Scholar]
- 13.N.C.I.N. National Cancer Action Team. Cancer Waiting Times: A Guide (Version 7). 2012.
- 14.Gibala MJ, Little JP, Macdonald MJ, Hawley JA. Physiological adaptations to low-volume, high-intensity interval training in health and disease. J Physiol. 2012;590(Pt 5):1077–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Milanović Z, Sporiš G, Weston M. Effectiveness of high-intensity interval training (HIT) and continuous endurance training for V˙O2max improvements: a systematic review and meta-analysis of controlled trials. Sports Med. 2015;45:1469–81. [DOI] [PubMed] [Google Scholar]
- 16.van der Wal MH, Jaarsma T, van Veldhuisen DJ. Non-compliance in patients with heart failure; How can we manage it? Eur J Heart Fail. 2005;7(1):5–17. [DOI] [PubMed] [Google Scholar]
- 17.Kilpatrick M, Jung M, Little J. High-intensity interval training. A review of physiological and psychological responses. Am Coll Sport Med. 2014;18(5):11–6. [Google Scholar]
- 18.Stutts WC. Physical activity determinants in adults. Perceived benefits, barriers, and self efficacy. AAOHN J. 2002;50(11):499–507. [PubMed] [Google Scholar]
- 19.Weston KS, Wisloff U, Coombes JS. High-intensity interval training in patients with lifestyle-induced cardiometabolic disease: a systematic review and meta-analysis. Br J Sport Med. 2014;48(16):1227–34. [DOI] [PubMed] [Google Scholar]
- 20.Beauchamp MK, Nonoyama M, Goldstein RS, Hill K, Dolmage TE, Mathur S. Interval versus continuous training in individuals with chronic obstructive pulmonary disease–a systematic review. Thorax. 2010;65(2):157–64. [DOI] [PubMed] [Google Scholar]
- 21.Tschentscher M, Eichinger J, Egger A, Droese S, Schönfelder M, Niebauer J. High-intensity interval training is not superior to other forms of endurance training during cardiac rehabilitation. Eur J Prev Cardiol. 2016;23(1):14–20. [DOI] [PubMed] [Google Scholar]
- 22.Ross LM, Porter RR, Durstine JL. High-intensity interval training (HIIT) for patients with chronic diseases. J Sport Heal Sci. 2016;5(2):139–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wisloff U, Stoylen A, Loennechen JP, et al. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: a randomized study. Circulation. 2007;115(24):3086–94. [DOI] [PubMed] [Google Scholar]
- 24.Little JP, Gillen JB, Percival ME, et al. Low-volume high-intensity interval training reduces hyperglycemia and increases muscle mitochondrial capacity in patients with type 2 diabetes. J Appl Physiol. 2011;111(6):1554–60. [DOI] [PubMed] [Google Scholar]
- 25.Alahmadi MA. High-intensity interval training and obesity. J Nov Physiother. 2014;4(3). [Google Scholar]
- 26.Emtner M, Herala M, Stålenheim G. High-intensity physical training in adults with asthma. A 10-week rehabilitation program. Chest. 1996;109(2):323–30. [DOI] [PubMed] [Google Scholar]
- 27.Moher D, Liberati A, Tetzlaff J, Altman DG. Academia and clinic annals of internal medicine preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Annu Intern Med. 2009;151(4):264–9. [DOI] [PubMed] [Google Scholar]
- 28.Higgins J, Green S. The Cochrane Collaboration. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2. 2008. 16.1.3.2. [Google Scholar]
- 29.Gremeaux V, Troisgros O, Benaïm S, et al. Determining the minimal clinically important difference for the six-minute walk test and the 200-meter fast-walk test during cardiac rehabilitation program in coronary artery disease patients after acute coronary syndrome. Arch Phys Med Rehabil. 2011;92(4):611–9. [DOI] [PubMed] [Google Scholar]
- 30.Kwok BC, Pua YH, Mamun K, Wong WP. The minimal clinically important difference of six-minute walk in Asian older adults. BMC Geriatr. 2013;13(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Angadi SS, Mookadam F, Lee CD, et al. High-intensity interval training vs. moderate-intensity continuous exercise training in heart failure with preserved ejection fraction: a pilot study. J Appl Physiol. 2015;119(6):753–8. [DOI] [PubMed] [Google Scholar]
- 32.Baekkerud FH, Solberg F, Leinan IM, Wisløff U, Karlsen T, Rognmo Ø. Comparison of three popular exercise modalities on V˙O2max in overweight and obese. Med Sci Sports Exerc. 2016;48(3):491–8. [DOI] [PubMed] [Google Scholar]
- 33.Beale L, McIntosh R, Raju P, Guy L, Brickley G. A comparison of high intensity interval training with circuit training in a short-term cardiac rehabilitation programme for patients with chronic heart failure. Int J Phys Med Rehabil. 2013;1(6):1–7. [Google Scholar]
- 34.Boyne P, Dunning K, Carl D, et al. High-intensity interval training and moderate-intensity continuous training in ambulatory chronic stroke: feasibility study. Phys Ther. 2016;96(10):1533–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coppoolse R, Schols AM, Baarends EM, et al. Interval versus continuous training in patients with severe COPD: a randomized clinical trial. Eur Respir Journal. 1999;14(2):258–63. [DOI] [PubMed] [Google Scholar]
- 36.Devin JL, Sax AT, Hughes GI, et al. The influence of high-intensity compared with moderate-intensity exercise training on cardiorespiratory fitness and body composition in colorectal cancer survivors: a randomised controlled trial. J Cancer Surviv. 2016;10(3):467–79. [DOI] [PubMed] [Google Scholar]
- 37.Dolan LB, Campbell K, Gelmon K, Neil-Sztramko S, Holmes D, McKenzie DC. Interval versus continuous aerobic exercise training in breast cancer survivors—a pilot RCT. Support Care Cancer. 2016;24(1):119–27. [DOI] [PubMed] [Google Scholar]
- 38.Mobius-Winkler S, Uhlemann M, Adams V, et al. Coronary collateral growth induced by physical exercise: results of the impact of intensive exercise training on coronary collateral circulation in patients with stable coronary artery disease (EXCITE) trial. Circulation. 2016;133(15):1438–48. [DOI] [PubMed] [Google Scholar]
- 39.Fisher G, Brown AW, Bohan Brown MM, et al. High intensity interval- vs moderate intensity- training for improving cardiometabolic health in overweight or obese males: a randomized controlled trial. PLoS One. 2015;10(10):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Flemmen G, Unhjem R, Wang E, et al. High-intensity interval training in patients with substance use disorder. Biomed Res Int. 2014;2014:616935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Freese EC, Acitelli RM, Gist NH, Cureton KJ, Evans EM, O’Connor PJ. Effect of six weeks of sprint interval training on mood and perceived health in women at risk for metabolic syndrome. J Sport Exerc Psychol. 2014;36(6):610–8. [DOI] [PubMed] [Google Scholar]
- 42.Freyssin C, Verkindt C, Prieur F, Benaich P, Maunier S, Blanc P. Cardiac rehabilitation in chronic heart failure: effect of an 8-week, high-intensity interval training versus continuous training. Arch Phys Med Rehabil. 2012;93(8):1359–64. [DOI] [PubMed] [Google Scholar]
- 43.Gloeckl R, Halle M, Kenn K. Interval versus continuous training in lung transplant candidates: a randomized trial. J Heart Lung Transplant. 2012;31(9):934–41. [DOI] [PubMed] [Google Scholar]
- 44.Heggelund J, Nilsberg GE, Hoff J, Morken G, Helgerud J. Effects of high aerobic intensity training in patients with schizophrenia: a controlled trial. Nord J Psychiatry. 2011;65(4):269–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hermann TS, Dall CH, Christensen SB, Goetze JP, Prescott E, Gustafsson F. Effect of high intensity exercise on peak oxygen uptake and endothelial function in long-term heart transplant recipients. Am J Transplant. 2011;11(3):536–41. [DOI] [PubMed] [Google Scholar]
- 46.Higgins S, Fedewa MV, Hathaway ED, Schmidt MD, Evans EM. Sprint interval and moderate-intensity cycling training differentially affect adiposity and aerobic capacity in overweight young-adult women. Appl Physiol Nutr Metab. 2016;41(11):1177–83. [DOI] [PubMed] [Google Scholar]
- 47.Hwang CL, Yu CJ, Shih JY, Yang PC, Wu YT. Effects of exercise training on exercise capacity in patients with non-small cell lung cancer receiving targeted therapy. Support Care Cancer. 2012;20(12):3169–77. [DOI] [PubMed] [Google Scholar]
- 48.Jaureguizar KV, Vicente-Campos D, Bautista LR, et al. Effect of high-intensity interval versus continuous exercise training on functional capacity and quality of life in patients with coronary artery disease. J Cardiopulm Rehabil Prev. 2016;36:96–105. [DOI] [PubMed] [Google Scholar]
- 49.Jung ME, Bourne JE, Beauchamp MR, Robinson E, Little JP. High-intensity interval training as an efficacious alternative to moderate-intensity continuous training for adults with prediabetes. J Diabetes Res. 2015;2015:191595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang GH, Ismail H, Murnane A, Kim P, Riedel B. Structured exercise program prior to major cancer surgery improves cardiopulmonary fitness: a retrospective cohort study. Support Care Cancer. 2015;24(5):2277–85. [DOI] [PubMed] [Google Scholar]
- 51.Kong Z, Fan X, Sun S, Song L, Shi Q, Nie J. Comparison of high-intensity interval training and moderate-to-vigorous continuous training for cardiometabolic health and exercise enjoyment in obese young women: a randomized controlled trial. PLoS One. 2016;11(7):e0158589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lanzi S, Codecasa F, Cornacchia M, et al. Short-term HIIT and fat max training increase aerobic and metabolic fitness in men with class II and III obesity. Obes (Silver Spring). 2015;23(10):1987–94. [DOI] [PubMed] [Google Scholar]
- 53.Mador MJ, Krawza M, Alhajhusian A, et al. Interval training versus continuous training in patients with chronic obstructive pulmonary disease. J Cardiopulm Rehabil Prev. 2009;29(2):126–32. [DOI] [PubMed] [Google Scholar]
- 54.Matsuo T, So R, Shimojo N, Tanaka K. Effect of aerobic exercise training followed by a low-calorie diet on metabolic syndrome risk factors in men. Nutr Metab Cardiovasc Dis. 2015;25(9):832–8. [DOI] [PubMed] [Google Scholar]
- 55.Moholdt TT, Amundsen BH, Rustad LA, et al. Aerobic interval training versus continuous moderate exercise after coronary artery bypass surgery: a randomized study of cardiovascular effects and quality of life. Am Heart J. 2009;158(6):1031–7. [DOI] [PubMed] [Google Scholar]
- 56.Monk-Hansen T, Dall CH, Christensen SB, et al. Interval training does not modulate diastolic function in heart transplant recipients. Scand Cardiovasc J. 2014;48(2):91–8. [DOI] [PubMed] [Google Scholar]
- 57.Puhan MA. Interval versus continuous high-intensity exercise in chronic obstructive pulmonary disease. Ann Intern Med. 2006;145:816–25. [DOI] [PubMed] [Google Scholar]
- 58.Robinson E, Durrer C, Simtchouk S, et al. Short-term high-intensity interval and moderate-intensity continuous training reduce leukocyte TLR4 in inactive adults at elevated risk of type 2 diabetes. J Appl Physiol. 2015;119(5):508–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sawyer BJ, Tucker WJ, Bhammar DM, et al. Effects of high-intensity interval training and moderate-intensity continuous training on endothelial function and cardiometabolic risk markers in obese adults. J Appl Physiol. 2016;121(1):279–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schmitt J, Lindner N, Reuss-Borst M, Holmberg HC, Sperlich B. A 3-week multimodal intervention involving high-intensity interval training in female cancer survivors: a randomized controlled trial. Physiol Rep. 2016;4(3):365–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Skleryk JR, Karagounis LG, Hawley JA, Sharman MJ, Laursen PB, Watson G. Two weeks of reduced-volume sprint interval or traditional exercise training does not improve metabolic functioning in sedentary obese men. Diabetes Obes Metab. 2013;15(12):1146–53. [DOI] [PubMed] [Google Scholar]
- 62.Smith-Ryan AE, Trexler ET, Wingfield HL, Blue MN. Effects of high-intensity interval training on cardiometabolic risk factors in overweight/obese women. J Sports Sci. 2016;34(21):2038–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Trilk JL, Singhal A, Bigelman KA, Cureton KJ. Effect of sprint interval training on circulatory function during exercise in sedentary, overweight/obese women. Eur J Appl Physiol. 2011;111(8):1591–7. [DOI] [PubMed] [Google Scholar]
- 64.Wallman K, Plant LA, Rakimov B, Maiorana AJ. The effects of two modes of exercise on aerobic fitness and fat mass in an overweight population. Res Sports Med. 2009;17(3):156–70. [DOI] [PubMed] [Google Scholar]
- 65.Abbasi K, Paterson-brown S. Education and debate. Br Med J. 1998;317(August):401–10.9694761 [Google Scholar]
- 66.Licker M, Karenovics W, Diaper J, et al. Short-term preoperative high-intensity interval training in patients awaiting lung cancer surgery: a randomized controlled trial. J Thorac Oncol. 2017;12(2):323–33. [DOI] [PubMed] [Google Scholar]
- 67.Ware JEJ. SF-36 Health Survey. The use of psychological testing for treatment planning and outcomes assessment. M E Maruish. 1999:1227–46. [Google Scholar]
- 68.Gibala MJ, Little JP, van Essen M, et al. Short-term sprint interval versus traditional endurance training: similar initial adaptations in human skeletal muscle and exercise performance. J Physiol. 2006;575(Pt 3):901–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Adamson SB, Lorimer R, Cobley JN, Babraj JA. Extremely short-duration high-intensity training substantially improves the physical function and self-reported health status of elderly adults. J Am Geriatr Soc. 2014;62(7):1380–1. [DOI] [PubMed] [Google Scholar]
- 70.Boereboom CL, Phillips BE, Williams JP, Lund JN. A 31-day time to surgery compliant exercise training programme improves aerobic health in the elderly. Tech Coloproctol. 2016;20(6):375–82. [DOI] [PubMed] [Google Scholar]
- 71.Cassidy S, Thoma C, Hallsworth K, et al. High intensity intermittent exercise improves cardiac structure and function and reduces liver fat in patients with type 2 diabetes: a randomised controlled trial. Diabetologia. 2016;59(1):56–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Phillips BE, Smith K, Liptrot S, et al. Effect of colon cancer and surgical resection on skeletal muscle mitochondrial enzyme activity in colon cancer patients: a pilot study. J Cachexia Sarcopenia Muscle. 2013;4(1):71–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Williams JP, Nyasavajjala SM, Phillips BE, Chakrabarty M, Lund JN. Surgical resection of primary tumour improves aerobic performance in colorectal cancer. Eur J Surg Oncol. 2014;40(2):220–6. [DOI] [PubMed] [Google Scholar]
- 74.Jack S, West MA, Raw D, et al. The effect of neoadjuvant chemotherapy on physical fitness and survival in patients undergoing oesophagogastric cancer surgery. Eur J Surg Oncol. 2014;40(10):1313–20. [DOI] [PubMed] [Google Scholar]
- 75.Bowles EJ, Wellman R, Feigelson HS, et al. Risk of heart failure in breast cancer patients after anthracycline and trastuzumab treatment: a retrospective cohort study. J Natl Cancer Inst. 2012;104(17):1293–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Türk Y, Theel W, Kasteleyn MJ, et al. High intensity training in obesity: a meta-analysis. Obes Sci Pract. 2017;3(3):258–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Keating SE, Johnson NA, Mielke GI, Coombes JS. A systematic review and meta-analysis of interval training versus moderate-intensity continuous training on body adiposity. Obes Rev. 2017;18(8):943–64. [DOI] [PubMed] [Google Scholar]
- 78.Andersen G, Heje K, Buch AE, Vissing J. High-intensity interval training in facioscapulohumeral muscular dystrophy type 1: a randomized clinical trial. J Neurol. 2017;264(6):1099–106. [DOI] [PubMed] [Google Scholar]
- 79.Kim C, Choi HE, Lim M. Effect of high interval training in acute myocardial infarction patients with drug-eluting stent. Am J Phys Med Rehabil. 2015;94(10):879–86. [DOI] [PubMed] [Google Scholar]
- 80.Gibala MJ, Gillen JB, Percival ME. Physiological and health-related adaptations to low-volume interval training: influences of nutrition and sex. Sports Med. 2014;44:127–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Burgomaster KA, Howarth KR, Phillips SM, et al. Similar metabolic adaptations during exercise after low volume sprint interval and traditional endurance training in humans. J Physiol. 2008;586(1):151–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hijazi Y, Gondal U, Aziz O. A systematic review of prehabilitation programs in abdominal cancer surgery. Int J Surg. 2017;39:156–62. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


