Abstract
Background:
MEDI8897 is a recombinant human monoclonal antibody being developed for prophylaxis of serious respiratory syncytial virus (RSV) disease in all infants.
Methods:
In this phase 1b/2a dose-escalation study, healthy preterm infants with a gestational age of 32–35 weeks were randomized to receive a single intramuscular injection of MEDI8897 (10, 25 or 50 mg) or placebo. Safety, pharmacokinetics, RSV-neutralizing antibody and antidrug antibody (ADA) assessments were performed during the 360-day follow-up period. Infants who experienced medically attended lower respiratory tract infections (LRTIs) were tested for RSV.
Results:
MEDI8897 serum half-life ranged from 62.5–72.9 days. On day 151, 87% of infants in the 50 mg group had serum concentrations above the 90% effective concentration target level of 6.8 µg/mL, and 90% showed a ≥4-fold rise from baseline in serum RSV-neutralizing antibody levels. Adverse events (AEs) were reported in 17 of 18 (94.4%) placebo and 66 of 71 (93.0%) MEDI8897 recipients. Three MEDI8897 recipients experienced 5 serious AEs (3 LRTIs, 2 febrile seizures). ADA was detected at any time postbaseline in 28.2% of MEDI8897 recipients and at day 361 only in 26.5% of subjects. ADA response was not associated with AEs. Five (7%) MEDI8897 recipients experienced medically attended LRTIs through day 150; 1 tested positive for RSV (10 mg group).
Conclusions:
MEDI8897 had a favorable safety profile in healthy preterm infants. The extended half-life of MEDI8897 and demonstrated RSV-neutralizing activity support protection from RSV for the duration of a typical 5-month season after a single 50 mg intramuscular (IM) dose.
Keywords: infants, lower respiratory tract infections, MEDI8897, preterm infants, respiratory syncytial virus
Respiratory syncytial virus (RSV) is the most common cause of lower respiratory tract infections (LRTIs) among infants and children worldwide.1–5 Nearly all children are infected with RSV during the first 2 years of life, with up to 40% experiencing an LRTI.6 In 2005, RSV was responsible for more than 30 million episodes of new LRTIs among children 5 years and younger, resulting in an estimated 66,000–199,000 deaths globally.3
RSV infection is responsible for 50%–90% of pediatric hospitalizations for bronchiolitis and 5%–40% of hospitalizations for pneumonia.7,8 While all children are at risk for severe LRTI during their primary infection, healthy term infants 3 months of age and younger account for more RSV-associated hospitalizations than any other group.2 Severe illness during infancy has the potential to cause both acute and long-term pulmonary sequelae, including recurrent wheezing episodes throughout childhood.9
The health care burden associated with RSV is high. In the United States, illness caused by the virus is responsible for an estimated 1 in 13 pediatric office visits, 1 in 38 emergency department visits and 1 in 334 hospitalizations among children less than 5 years in age.1 Among infants less than 12 months of age enrolled in a Medicaid program between 1995 and 2003, bronchiolitis was responsible for 13.3% of all outpatient visits, 6.2% of all emergency department visits and 5.5% of all hospitalizations or 23-hour hospital stays.10 In the United States, rates of outpatient RSV LRTIs are reported to range from 157.5 to 252.0 per 1000 for children less than 1 year of age, 183.3 to 245.7 per 1000 for late preterm infants (33–36 weeks gestational age) and 128.8 to 171.3 per 1000 for full-term infants.11 High rates of RSV-associated hospitalization, ranging from 10 to 60 per 1000, are reported for infants living in South America, Europe, Asia and Africa.12–15
The high burden of RSV infection among infants and young children highlights the need for safe and effective prevention strategies. Currently, the only approved prophylaxis for RSV disease is palivizumab (Synagis; MedImmune, Gaithersburg, MD). Palivizumab is an RSV fusion (F)-specific immunoglobulin G monoclonal antibody indicated for the prevention of serious lower respiratory tract disease caused by RSV in children at high risk, including preterm infants born at 35 weeks gestational age or less.16 Due in part to the high cost of palivizumab prophylaxis, the most recent guidance from the American Academy of Pediatrics (AAP) does not recommend it for healthy preterm infants born at or after 29 weeks gestational age.17 RSV prophylaxis is not available for use in healthy term infants, and safe and effective active vaccines remain elusive.
MEDI8897 is a recombinant human immunoglobulin G1 kappa monoclonal antibody derived from D25 that targets the prefusion conformation of the RSV F protein. MEDI8897 binds a highly conserved epitope on RSV F and neutralizes a diverse panel of RSV A and B strains with >50-fold higher activity than palivizumab. At similar serum concentrations, prophylactic administration of MEDI8897 is 9-fold more potent than palivizumab at reducing pulmonary viral loads by >3 logs in cotton rats infected with either RSV A or B subtypes.18 The MEDI8897 antibody is engineered with 3 amino acid changes (M257Y/S259T/T261E [YTE]) in the highly conserved fragment crystallizable region. This YTE modification extends the serum half-life (t1/2) of the antibody beyond the typical 21–28 days. In a phase 1, placebo-controlled study of healthy adults, MEDI8897 had a favorable safety profile and an extended mean t1/2 of 85–117 days with increased levels of RSV-neutralizing antibodies detected in serum for more than 150 days.19
Here, we report results from the first infant study to evaluate the safety and pharmacokinetics (PK) of MEDI8897 when administered to healthy preterm infants as a single 10, 25 or 50 mg IM dose.
MATERIALS AND METHODS
Study Design
This phase 1b/2a, randomized, double-blind, placebo-controlled study (ClinicalTrials.gov identifier: NCT02290340) was conducted at 10 sites, 4 each in the United States and South Africa and 2 in Chile. A sequential dose-escalation design was used, in which infants were randomized 4:1 to receive a single dose of 10, 25 or 50 mg of MEDI8897 or placebo (Fig. 1). Escalation from 1 dose cohort to the next was dependent on an acceptable safety profile during the first 14 days after dosing in the preceding dose cohort as determined by the Dose Escalation Committee (DEC). After escalation to, and completion of, the 25 mg escalation cohort, and determination by the DEC that this dose level had an acceptable safety profile, a 25 mg expansion cohort of 30 subjects was enrolled in parallel with the 50 mg escalation cohort of 10 subjects. Similarly, after completion of the 50 mg escalation cohort and review of data by the DEC, a 50 mg expansion cohort of 30 subjects was enrolled. All dose escalations occurred as planned. Infants were followed for approximately 360 days after dosing for safety, PK, RSV-neutralizing antibody levels and antidrug antibody (ADA) assessments.
FIGURE 1.
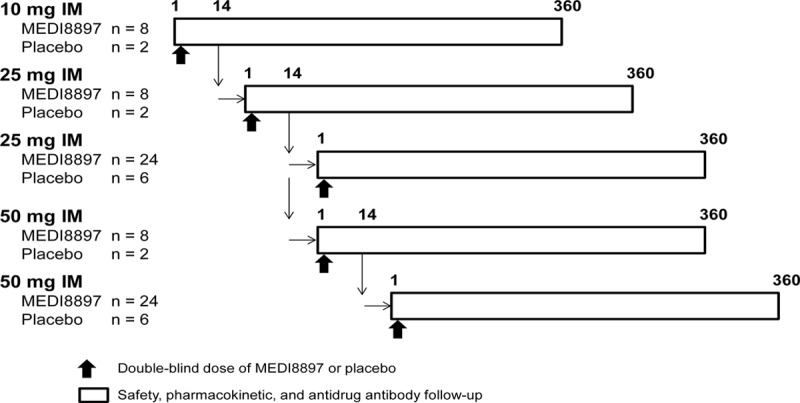
Study design.
The protocol was approved by independent institutional review boards. The trial was conducted in accordance with applicable laws and regulations including the International Conference on Harmonisation Guideline for Good Clinical Practice and the Declaration of Helsinki. Before study start, written informed consent and any locally required authorizations were obtained from the parent(s) or other legal representative of the study subjects.
Study Participants
Healthy preterm infants who were born between 32 weeks, 0 days and 34 weeks, 6 days gestational age and entering their first RSV season at the time of screening were eligible for study enrollment. Only infants who were not recommended to receive palivizumab according to the most recent AAP guidance or by other national or local guidelines were eligible.
Infants were excluded from study enrollment if they had an acute illness at the time of randomization, a fever (defined as ≥38°C) or lower respiratory illness within 7 days before randomization, a known history of RSV infection, hemodynamically significant congenital heart disease, chronic lung disease of prematurity (bronchopulmonary dysplasia) or a history of suspected or actual acute life-threatening events. Infants who had received palivizumab or any other investigational RSV monoclonal antibody and infants who had received an investigational RSV vaccine or were born to a mother who had received an investigational RSV vaccine or any monoclonal or polyclonal antibody other than Rho(D) immune globulin were excluded.
Assessments
PK parameters were evaluated using serum samples obtained before dosing and on days 8, 31, 151 and 361 post-dose administration. MEDI8897 serum concentration was measured using a validated colorimetric enzyme-linked immunosorbent assay (MedImmune, Gaithersburg, MD). Results below the lower limit of quantitation were reported as <0.50 μg/mL. ADA assessments were performed using a validated electrochemiluminescent assay, for which serum samples were obtained before dosing and after dose administration on days 31, 151 and 361. A dilution of 1:50 was the minimum serum dilution that maximized the ability to detect ADA while minimizing interference from serum matrix components. Therefore, ADA titers ≥1:50 were considered positive.
Serum RSV-neutralizing antibody levels were measured before and after dose administration and on days 8, 151 and 361. A validated RSV A2-based microneutralization assay with a lower limit of quantitation of 1:10 or 3.32 log2 was used for this purpose.20 Based on the precision of the assay, there was a <1% chance of reporting a false responder or false seroconversion using a criteria of a 4-fold rise in neutralizing antibodies. Safety was assessed by collecting adverse events (AEs), serious AEs (SAEs), AEs of special interest (AESIs) and new-onset chronic diseases for all study subjects from the time of study enrollment through the end of the study. AESIs for this study included hepatic function abnormalities, anaphylaxis or hypersensitivity reactions, immune complex disease and thrombocytopenia. Investigators were instructed to report any laboratory and vital sign values deemed clinically significant as an AE.
Infants who received inpatient or outpatient medical attention for a respiratory illness were evaluated for the occurrence of an LRTI (eg, bronchiolitis or pneumonia). For study purposes, a medically attended LRTI was defined as physical examination findings of rhonchi, rales, crackles or wheezing with documentation of clinical severity as shown by increased respiratory rate, hypoxemia or other signs of respiratory distress including retractions, grunting or need for assisted ventilation or intravenous fluids to maintain hydration. Infants who met the protocol-defined objective criteria for a medically attended LRTI had nasopharyngeal samples collected for RSV testing. The site investigators reported other respiratory illnesses as AEs based on their clinical assessment. Samples were not collected from infants who had an AE of LRTI that did not meet the protocol definition or from infants who had an upper respiratory infection.
Respiratory secretions were collected within 2 days when possible and up to 14 days after a diagnosis of medically attended LRTI. RSV testing was performed by a central laboratory (BARC USA, Inc, Lake Success, NY) using the Food and Drug Administration cleared and CE-marked Quidel Lyra RSV + human metapneumovirus assay (Quidel Corporation, San Diego, CA). RSV subtypes A and B were identified by sequencing of the RSV G gene and comparing to a reference database (Viracor-IBT Laboratories, Lee’s Summit, MO).
Statistical Analyses
As this phase 1b/2a study did not involve the statistical testing of a hypothesis, a formal sample size was not determined. The planned number of subjects was considered adequate to evaluate PK, ADA and safety and tolerability of MEDI8897 before initiating a phase 2b trial. Baseline values were defined as those observed on day 1 (before dosing). In cases of missing data points, only observed data were analyzed. Data were analyzed using SAS version 9.3 or higher (SAS Institute, Inc., Cary, NC) on a UNIX platform.
PK parameters were estimated by noncompartmental analysis using Phoenix 64 WinNonlin 6.3 (Pharsight, Mountain View, CA). RSV antibody neutralization levels were summarized by mean (standard deviation) log2 half-maximal inhibitory concentration (IC50) values and geometric mean fold rise for each treatment group. The relationship between MEDI8897 PK and RSV-neutralizing antibody present in serum was evaluated using a parametric correlation analysis.
The number and percentage of infants positive for ADA at baseline and positive at any postbaseline time point were summarized. The effect of MEDI8897 ADA on PK was evaluated by visual examination of PK profiles because of the limitations of the currently available data. A model-based approach will be utilized to determine the impact of ADA on PK with additional data from a subsequent study.
RESULTS
Of the 89 infants randomized to receive either MEDI8897 or placebo, 85 (95.5%) completed the study (Fig. 2). Among the 4 infants who discontinued the study, 1 from the MEDI8897 50 mg group was lost to follow-up, 2 were lost to relocation (1 each from MEDI8897 50 mg and placebo groups) and 1 was withdrawn (placebo group).
FIGURE 2.

Subject disposition.
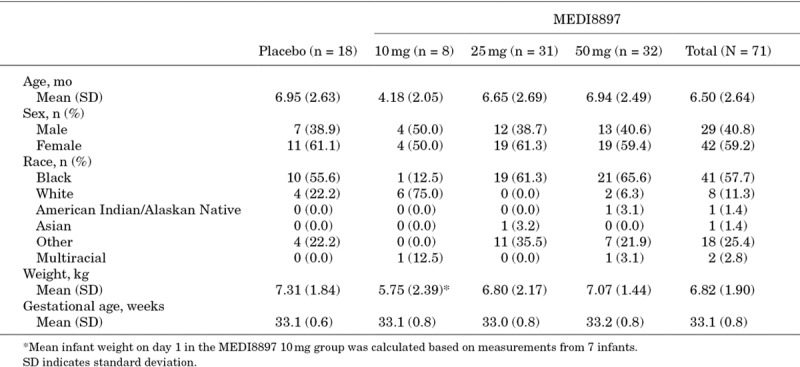
Overall, demographics and baseline characteristics were similar between the MEDI8897 and placebo groups (Table 1), with the exception that in the MEDI8997 10 mg group, more subjects were white and had a lower mean chronologic age and lower mean body weight (BW) at randomization.
TABLE 1.
Demographics and Baseline Characteristics
Pharmacokinetics
Peak concentration of MEDI8897 was observed within 7 days of administration (Table 2). When MEDI8897 dose levels increased from 10 to 25 mg, the exposure levels (maximal observed concentration and area under the curve from time zero to infinity) increased in a less than dose-proportional manner. The increase in exposure was approximately dose proportional when doses increased from 25 to 50 mg (Fig. 3).
TABLE 2.
MEDI8897 Mean Pharmacokinetic Parameters
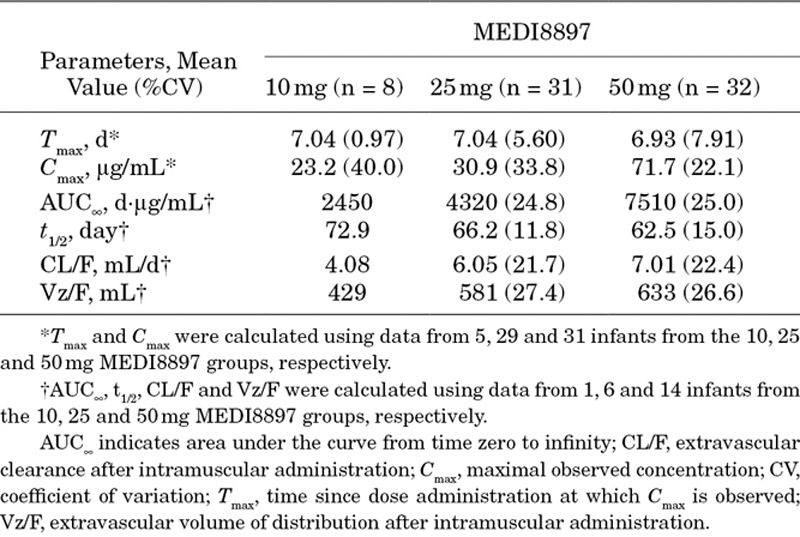
FIGURE 3.
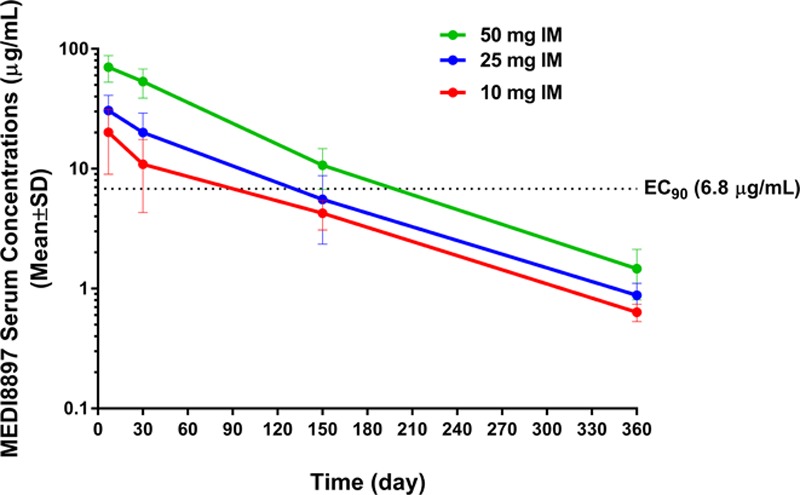
Mean MEDI8897 serum concentrations after a single IM dose. Error bars represent the standard deviations (SDs). EC90 indicates 90% effective concentration.
MEDI8897 serum t1/2 was estimated to be 62.5–72.9 days. On day 151, target serum concentrations above the 90% effective concentration level of 6.8 μg/mL were observed in 87% of infants who received a 50 mg dose of MEDI8897. Because a single fixed IM dose was given to infants, a somewhat negative correlation between PAGE (an integrated parameter for postmenstrual age; PAGE = gestational age + chronologic age) and baseline BW with the MEDI8897 levels on day 151 (C151) was expected. PAGE at the 25 mg dose level (R2 = 0.24; P < 0.01) and BW (R2 = 0.25; P < 0.001) and PAGE (R2 = 0.36; P < 0.001) at the 50 mg dose level were found to be statistically significantly correlated with C151, respectively. The correlations observed between C151 and BW or PAGE at the 10 mg dose level and between C151 and BW at the 25 mg dose level were not statistically significantly different.
RSV-neutralizing Antibody Levels
At baseline, serum RSV-neutralizing antibody mean log2 IC50 levels were 5.46 in the placebo group and 5.03, 5.18 and 4.88 in the 10, 25 and 50 mg MEDI8897 groups, respectively. A dose-dependent increase was seen from baseline to day 8 among those receiving MEDI8897, with peak neutralizing antibody mean log2 IC50 levels of 11.18, 11.85 and 12.31 in the 10, 25 and 50 mg groups, respectively, and at least 95% of infants in each dose group experienced a ≥4-fold rise from baseline (Fig. 4). At day 151, the neutralizing antibody mean log2 IC50 levels were 4.33, 8.88, 9.52 and 10.70 in the placebo and MEDI8897 10, 25 and 50 groups, respectively, and 90.0% of the MEDI8897 50 mg group, but none of the placebo recipients, had a ≥4-fold rise from baseline. At day 361, RSV-neutralizing antibody levels measured from subjects in the placebo group had increased to 8.10, with 73.3% of infants having experienced a ≥4-fold increase from baseline, likely indicating an RSV exposure between days 151 and 361. In contrast, the RSV-neutralizing antibody levels for the MEDI8897-treated infants decreased between days 151 and 361, with only 51.6% of infants having a ≥4-fold increase from baseline at the end of the study. MEDI8897 serum concentrations correlated with serum RSV-neutralizing antibody levels across all dose groups, confirming the neutralizing RSV activity of MEDI8897 (Fig. 5).
FIGURE 4.
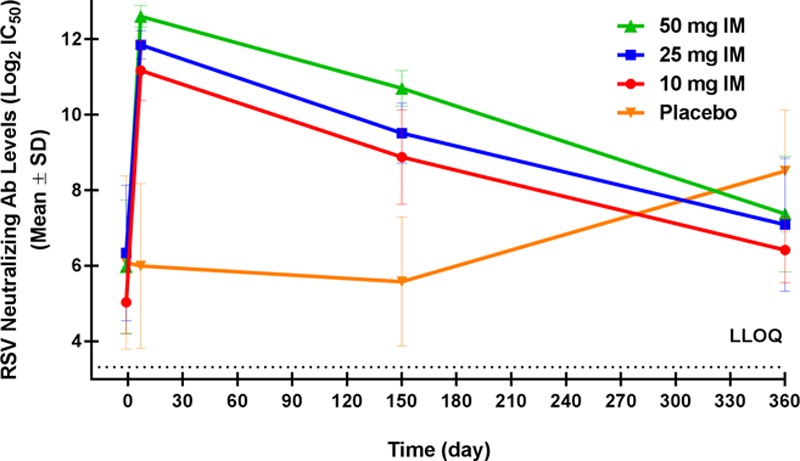
RSV-neutralizing antibody levels after a single IM dose of MEDI8897 or placebo. Data points represent the mean RSV A2 neutralizing antibody levels on a log2 scale. Error bars represent the standard deviations (SDs). LLOQ indicates lower limit of quantitation.
FIGURE 5.

Correlation between serum anti-RSV activity and MEDI8897 serum concentrations.
ADA Response
Overall, postbaseline ADA was detected in 20 of 71 subjects (28.2%) in the total MEDI8897 group and 0 of 17 subjects (0.0%) in the placebo group. None of the MEDI8897 ADA-positive subjects were ADA positive at baseline or had ADA detected at day 151. Two subjects with a transient positive ADA titer at day 50 were ADA negative thereafter. At day 361, ADA was detected in 18 of 68 subjects (26.5%) who had samples available for testing. The highest ADA titer detected was 1:25,600, which was measured in 1 subject in the 25 mg group and 1 subject in the 50 mg group. For all 20 subjects who had ADA detected, the ADA targeted the YTE domain in the fragment crystallizable portion of the antibody. Because there was an observed difference between the first 6 months and the second 6 months of the study with regard to the number of subjects with ADA detected on day 361, the potential impact on PK was assessed. Subjects with detectable and undetectable ADA had similar MEDI8897 serum concentrations for 150 days after dosing, suggesting that the generation of ADA did not impact PK of MEDI8897 for that time period. Because the majority of ADA-positive subjects were detected on day 361, and there were proportionally more concentrations below the limit of quantification in ADA-positive subjects, it is likely that PK exposures were impacted by ADA between days 151 and 361 in some subjects.
Safety
Treatment-emergent AEs (TEAEs) were reported in 66 of 71 infants (93.0%) who received MEDI8897 and 17 of 18 infants (94.4%) who received placebo. The majority of TEAEs were of mild or moderate severity (Table 3). The most frequent TEAEs among infants who received MEDI8897 were upper respiratory tract infection (69.0%), gastroenteritis (29.6%), cough (25.4%), pyrexia (22.5%) and otitis media (21.1%) (Table 4). The most common TEAEs among infants in the placebo group were upper respiratory tract infection (66.7%), anemia (33.3%), gastroenteritis (22.2%), cough (22.2%) and otitis media (22.2%).
TABLE 3.
Safety Summary
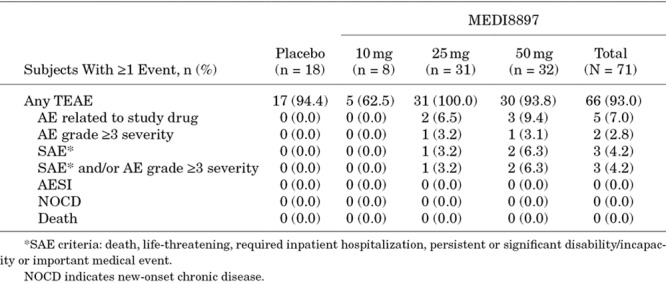
TABLE 4.
TEAEs Occurring in >10% of the MEDI8897 Total Group and With a ≥5% Difference Between Any MEDI8897 Group and Placebo Through Day 361

Five infants (7.0%) who received MEDI8897 experienced TEAEs that were considered related to the investigational product by the study site investigators, including nasal congestion, pyrexia, gastroenteritis, upper respiratory tract infection and wheezing. All treatment-related TEAEs were grade 1 in severity. No infants from the placebo group experienced treatment-related TEAEs.
There were 5 treatment-emergent SAEs that were hospitalizations for LRTIs and febrile seizures in 3 infants (4.2%) who received MEDI8897. One infant in the MEDI8897 25 mg group who was hospitalized for LRTI was found to have human metapneumovirus. None of the hospitalizations for LRTI during the 150 days after dosing were because of RSV. No treatment-emergent SAEs were reported in the placebo group. No deaths, AESIs or new-onset chronic diseases were reported, and no infant was discontinued from the study because of a TEAE (Table 3).
Lower Respiratory Tract Infections
Overall, 10 subjects (14.1%) who received MEDI8897 and 1 subject (5.6%) who received placebo developed an LRTI through day 151. Five MEDI8897 subjects (10 mg group, n = 1; 25 mg group, n = 2; 50 mg group, n = 2) had events that met the objective clinical criteria for a medically attended LRTI through day 151. No placebo recipients met the objective clinical criteria for a medically attended LRTI through day 151. One of the 5 infants with a medically attended LRTI was positive for RSV A (10 mg group) on day 8. Interestingly, that infant’s twin who was also enrolled in the study and randomized to the 10 mg dose group developed an LRTI but did not meet the objective clinical criteria for a medically attended LRTI. A nasopharyngeal sample collected from that twin was also positive for RSV A on day 8. The infants who had RSV LRTI were outpatients and did not require hospitalization.
DISCUSSION
This report summarizes the first experience with administering an antibody with an extended t1/2 to infants. MEDI8897, a fully human anti-RSV prefusion F monoclonal antibody, has greater neutralizing activity and a notably longer serum t1/2 than that reported for palivizumab. As such, it is being developed to prevent RSV disease in term and preterm infants with a single fixed dose rather than a weight-based dose. The t1/2 extension of MEDI8897 has the potential to provide protection for the duration of a typical 5-month RSV season. Currently, there is no approved vaccine or effective treatment for RSV. The only approved prophylaxis is palivizumab, which must be administered monthly and is only indicated for use in high risk infants. The most recent AAP guidance has reassessed the gestational age that determines “high-risk” and currently recommends routine use of palivizumab prophylaxis only for those preterm infants born before 29 weeks gestational age.
Data from this study confirm the extended t1/2 of MEDI8897 in healthy preterm infants and support protection against RSV through 150 days after dosing. The serum t1/2 of MEDI8897 in infants was estimated to be 63–73 days, which is considerably longer than that reported for palivizumab (mean t1/2 of 19–27 days).21 Most importantly, on day 151, target serum concentrations remained above the 90% effective concentration level of 6.8 μg/mL in 87% of infants who received a 50 mg dose of MEDI8897. A detailed population PK model incorporating growth, maturation and other covariates including ADA with additional PK data from a subsequent study will enable a better understanding of all the factors impacting PK in infants.
At day 8 after dosing, more than 95% of infants who received MEDI8897 had a ≥4-fold rise from baseline in RSV-neutralizing antibody levels. This increase persisted at day 151 with 90% of infants who received the 50 mg dose continuing to have a ≥4-fold rise from baseline. In addition, MEDI8897 serum concentrations correlated with RSV-neutralizing antibody across all 3 dose groups, confirming its RSV-neutralizing activity.
ADA, which was detected only transiently at 1 time point in 2 infants at day 50 after dosing, was detected late at day 361 but at no other time point in 18 infants. The reason for the presence of ADA at day 361 is not known. Because MEDI8897 has an extended t1/2, subjects were followed for approximately 1 year after dosing. In general, other RSV monoclonal antibody studies have included a 1-month follow-up period from the administration of the last dose.22–25 One exception was a motavizumab treatment study designed to test efficacy in infants who were hospitalized for RSV. Infants enrolled in that treatment study had a 1-year follow-up period that included serum testing for the presence of ADA.26 At study day 360, 60% and 46% of subjects in the 30 and 100 mg/kg groups, respectively, were found to have detectable serum anti-motavizumab antibodies.26 In that study, there were no safety findings or impact on PK associated with the detection of ADA.26 In our current study, the presence of ADA was not associated with changes in MEDI8897 serum concentrations at day 151; however, by day 361, lower MEDI8897 serum concentrations were noted in subjects with detectable ADA. There is no expected clinical significance of the lower serum concentrations at day 361 because target serum concentrations were maintained in the vast majority of infants for a 5-month period that would be representative of a typical RSV season. As in the previous study of motavizumab, there were no safety findings associated with the presence of ADA. The frequency and impact of ADA will be investigated in future studies.
In healthy preterm infants, MEDI8897 was well tolerated at all study doses tested. PK data demonstrate that a single 50 mg dose maintains antibody concentrations predictive of protection from RSV illness for at least 5 months in the majority of infants. Typical AEs that infants experienced were reported at similar frequencies in both MEDI8897 and placebo groups. Importantly, there were no treatment-emergent SAEs or hypersensitivity events related to MEDI8897. In subjects with ADA detected, the presence of ADA had no effect on MEDI8897 serum concentrations over the first 150 days and no effect on any safety parameters through day 361.
The findings of this study support further clinical development of MEDI8897 as a once-per-RSV-season prophylactic agent for the prevention of RSV-associated LRTI in all infants.
ACKNOWLEDGMENTS
The authors thank the children and families who participated in this study and the clinical investigators and staff at the participating sites. The authors also acknowledge the medical writing support provided by Disha Patel, PhD, of inScience Communications, Springer Healthcare (Philadelphia, PA).
Footnotes
J.B.D. is a clinical trial investigator for GlaxoSmithKline, MedImmune, Novavax, Pfizer and Regeneron. A.A.K., M.T.E., K.J., T.T., T.V., F.D. and M.P.G. are employees of MedImmune and have stock/stock options in AstraZeneca.
MedImmune, a subsidiary of AstraZeneca, funded this study and was involved in the study design, collection, analysis and interpretation of data and the writing of this report. Medical writing support was provided by Disha Patel, PhD, of inScience Communications, Springer Healthcare (Philadelphia, PA) and was supported by MedImmune.
REFERENCES
- 1.Hall CB. The burgeoning burden of respiratory syncytial virus among children. Infect Disord Drug Targets. 2012;12:92–97.. [DOI] [PubMed] [Google Scholar]
- 2.Hall CB, Weinberg GA, Iwane MK, et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med. 2009;360:588–598.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nair H, Nokes DJ, Gessner BD, et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet. 2010;375:1545–1555.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shay DK, Holman RC, Newman RD, et al. Bronchiolitis-associated hospitalizations among US children, 1980-1996. JAMA. 1999;282:1440–1446.. [DOI] [PubMed] [Google Scholar]
- 5.Stockman LJ, Curns AT, Anderson LJ, et al. Respiratory syncytial virus-associated hospitalizations among infants and young children in the United States, 1997-2006. Pediatr Infect Dis J. 2012;31:5–9.. [DOI] [PubMed] [Google Scholar]
- 6.American Academy of Pediatrics Subcommittee on Diagnosis and Management of Bronchiolitis. Diagnosis and management of bronchiolitis. Pediatrics. 2006;118:1774–1793.. [DOI] [PubMed] [Google Scholar]
- 7.Hall CB. Respiratory syncytial virus and parainfluenza virus. N Engl J Med. 2001;344:1917–1928.. [DOI] [PubMed] [Google Scholar]
- 8.Leader S, Kohlhase K. Respiratory syncytial virus-coded pediatric hospitalizations, 1997 to 1999. Pediatr Infect Dis J. 2002;21:629–632.. [DOI] [PubMed] [Google Scholar]
- 9.Blanken MO, Rovers MM, Molenaar JM, et al. ; Dutch RSV Neonatal Network. Respiratory syncytial virus and recurrent wheeze in healthy preterm infants. N Engl J Med. 2013;368:1791–1799.. [DOI] [PubMed] [Google Scholar]
- 10.Carroll KN, Gebretsadik T, Griffin MR, et al. Increasing burden and risk factors for bronchiolitis-related medical visits in infants enrolled in a state health care insurance plan. Pediatrics. 2008;122:58–64.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paramore LC, Mahadevia PJ, Piedra PA. Outpatient RSV lower respiratory infections among high-risk infants and other pediatric populations. Pediatr Pulmonol. 2010;45:578–584.. [DOI] [PubMed] [Google Scholar]
- 12.Jansen AG, Sanders EA, Hoes AW, et al. Influenza- and respiratory syncytial virus-associated mortality and hospitalisations. Eur Respir J. 2007;30:1158–1166.. [DOI] [PubMed] [Google Scholar]
- 13.Madhi SA, Kuwanda L, Cutland C, et al. Five-year cohort study of hospitalization for respiratory syncytial virus associated lower respiratory tract infection in African children. J Clin Virol. 2006;36:215–221.. [DOI] [PubMed] [Google Scholar]
- 14.Weigl JA, Puppe W, Schmitt HJ. Incidence of respiratory syncytial virus-positive hospitalizations in Germany. Eur J Clin Microbiol Infect Dis. 2001;20:452–459.. [DOI] [PubMed] [Google Scholar]
- 15.Jonnalagadda S, Rodríguez O, Estrella B, et al. Etiology of severe pneumonia in Ecuadorian children. PLoS One. 2017;12:e0171687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Synagis (Palivizumab): US Prescribing Information. 2014Gaithersburg, MD: MedImmune. [Google Scholar]
- 17.Brady MT, Byington CL, Davies HD, et al. Updated guidance for palivizumab prophylaxis among infants and young children at increased risk of hospitalization for respiratory syncytial virus infection. Pediatrics. 2014;134:415–420.. [DOI] [PubMed] [Google Scholar]
- 18.Zhu Q, McLellan JS, Kallewaard NL, et al. A highly potent extended half-life antibody as a potential RSV vaccine surrogate for all infants. Sci Transl Med. 2017;9:pii: . [DOI] [PubMed] [Google Scholar]
- 19.Griffin MP, Khan AA, Esser MT, et al. Safety, tolerability, and pharmacokinetics of the respiratory syncytial virus-prefusion F-targeting monoclonal antibody with an extended half-life, MEDI8897, in healthy adults. Antimicrob Agents Chemother. 2016;61:e01714–e01716.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patton K, Aslam S, Shambaugh C, et al. Enhanced immunogenicity of a respiratory syncytial virus (RSV) F subunit vaccine formulated with the adjuvant GLA-SE in cynomolgus macaques. Vaccine. 2015;33:4472–4478.. [DOI] [PubMed] [Google Scholar]
- 21.Robbie GJ, Zhao L, Mondick J, et al. Population pharmacokinetics of palivizumab, a humanized anti-respiratory syncytial virus monoclonal antibody, in adults and children. Antimicrob Agents Chemother. 2012;56:4927–4936.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.The IMpact-RSV Study Group. Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics. 1998;102:531–537.. [PubMed] [Google Scholar]
- 23.Carbonell-Estrany X, Simões EA, Dagan R, et al. ; Motavizumab Study Group. Motavizumab for prophylaxis of respiratory syncytial virus in high-risk children: a noninferiority trial. Pediatrics. 2010;125:e35–e51.. [DOI] [PubMed] [Google Scholar]
- 24.Feltes TF, Sondheimer HM, Tulloh RM, et al. ; Motavizumab Cardiac Study Group. A randomized controlled trial of motavizumab versus palivizumab for the prophylaxis of serious respiratory syncytial virus disease in children with hemodynamically significant congenital heart disease. Pediatr Res. 2011;70:186–191.. [DOI] [PubMed] [Google Scholar]
- 25.O’Brien KL, Chandran A, Weatherholtz R, et al. ; Respiratory Syncytial Virus (RSV) Prevention study group. Efficacy of motavizumab for the prevention of respiratory syncytial virus disease in healthy Native American infants: a phase 3 randomised double-blind placebo-controlled trial. Lancet Infect Dis. 2015;15:1398–1408.. [DOI] [PubMed] [Google Scholar]
- 26.Ramilo O, Lagos R, Sáez-Llorens X, et al. ; Motavizumab Study Group. Motavizumab treatment of infants hospitalized with respiratory syncytial virus infection does not decrease viral load or severity of illness. Pediatr Infect Dis J. 2014;33:703–709.. [DOI] [PubMed] [Google Scholar]


