Abstract
Deep learning implemented in a collaborative cloud-based platform empowers ophthalmologists in the diagnosis of congenital cataracts.
Rare diseases are often neglected, yet they are a public health concern1. Congenital cataracts (CCs) — a typical rare disease characterized by the clouding of the lens of the eye — can lead to blindness in children, especially in developing countries2. The management of CCs requires accurate diagnosis, timely surgery and rigorous follow-ups. However, it is challenging to integrate the typically scattered and limited medical resources for tackling rare disease that exist in many developing countries. Also, the quality of the healthcare services provided to CC patients, including diagnoses and treatment decisions, often varies across individual centres. Writing in Nature Biomedical Engineering, Yizhi Liu and colleagues report that artificial-intelligence (AI) software can provide diagnostic and treatment suggestions for CC patients, with accuracy comparable to that of individual expert ophthalmologists. They also describe a cloud-based web platform for the collaborative management of CC patients across care centres3.
Liu and co-authors’ machine-learning implementation relies on three deep convolutional neural networks4 (CNNs), trained on ocular images of patients, that identify whether images correspond to healthy or CC patients. For images classified as CCs, the CNNs evaluate the severity of the disease for risk stratification — according to the location (central or peripheral), density (dense or non-dense) and area (extensive or limited) of lens opacity — and provide treatment suggestions (surgery or follow-up). Liu and co-authors used 476 ocular images of healthy eyes and 410 images from patients with CCs with a diverse range of disease severity, collected by the Childhood Cataract Program of the Chinese Ministry of Health (CCPMOH). The authors divided the data randomly into five groups, and conducted a standard cross-validation protocol by training the CNNs with four of the image groups, and by comparing the predictions of the trained CNNs for the remaining group against the assessments provided by a panel of expert ophthalmologists (the process was repeated five times). The authors show that with such a set of images, the AI algorithm identified CC patients with about 99% accuracy, and suggested the right treatment for over 97% of the CC cases. The accuracy of the CNNs for the lens-opacity variables was 94–95%.
Encouraged by the results, Liu and colleagues carried out more stringent, real-world tests. In a phase-I clinical trial that lasted about four years and involved three non-specialized hospitals that recruited 14 CC patients and 43 children with healthy eyes, the accuracies of the CNNs on these new set of 57 ocular images (which had not been used for CNN training) were comparable to those from the CCPMOH data: 98% in the identification of CC patients, 93% in providing the correct treatment suggestion and 93–100% for the lens-opacity indices, all with respect to the assessments of an expert panel that was blind to the CNN predictions. To further validate the performance of the CNNs, Liu and colleagues collected 53 ocular images (40 with CCs and 13 normal) from the Internet. These had significantly diverse quality. The accuracies of the CNNs dropped slightly, yet remained in the 85–95% range. All these tests suggest that AI software ought to be applicable for screening CCs in a variety of real-world settings. Yet the incidence of CCs in the general population is only about 1%. In a simulated test consisting of three independent sets of 100 ocular images of healthy cases and one image from a CC patient, the CNNs successfully identified, stratified and provided the appropriate treatment suggestions for all 303 images (100% accuracy). Moreover, in a test paper with 50 ocular images that included various challenging clinical cases, the CNNs made fewer mistakes (9 false positives and 4 false negatives) than three individual ophthalmologists with varying expertise (with the top-performing practitioner making a total of 16 mistakes). All three ophthalmologists misidentified one particular case with a highly illuminated lens that came immediately after the assessment of a particularly dark yet healthy lens; the CNNs correctly classified the particular ocular image as normal.
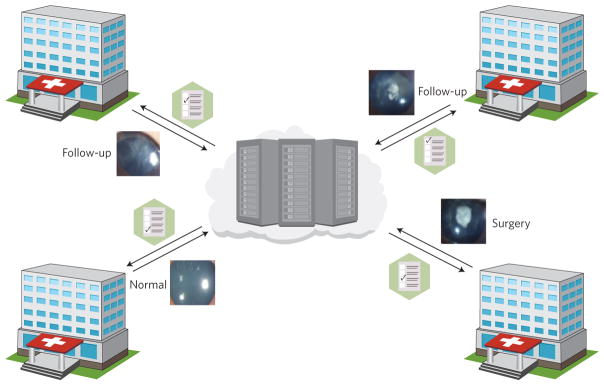
Liu and co-authors’ consistent results across such a battery of simulated and real-world tests should help the eventual implementation of machine learning in the clinic. Towards this goal, the authors integrated the CNN algorithm with a web-based user interface and with cloud computing to help improve the management of CC patients in isolated, non-specialized hospitals. In such a collaborating platform, a registered user can upload the ocular images from a remote site, let the AI software assist with diagnoses and treatment plans, and then check with expert ophthalmologists in a collaborating hospital when necessary (Fig. 1). Importantly, such a platform would facilitate the collection of bigger sets of CC images for further training of the CNNs. This is particularly necessary for rare diseases, as their low prevalence makes it hard for researchers to recruit enough patients to conduct data-driven analyses5.
Figure 1.
A multihospital platform for the collaborative management of congenital cataracts and other rare diseases through artificial intelligence. Ocular images uploaded from collaborating care centres are analysed by deep convolutional neural networks, which provide diagnostic and treatment suggestions (such as follow-up or surgery). Ocular images reproduced from ref. 3, Macmillan Publishers Ltd.
The success of deep learning has been driven by breakthroughs in layer-wise training algorithms, by the maturation of parallel-computing infrastructures and by big data. Neural networks are particularly good at recognizing objects from generic images in large databases, such as ImageNet6. With vastly fewer images available, Liu and colleagues show that, at least for CCs, CNNs can effectively encode disease-related patterns after proper image pre-processing. This is perhaps not surprising in light of a recent report on the use of deep learning to identify diabetic retinopathy — a much more common eye disease — from retinal fundus photographs7. In medical-image analysis more generally, deep learning is becoming a widely used tool for categorizing medical images8,9. Typical applications include medical-image enhancement10, registration11, segmentation12,13 and high-level understanding for diagnosis or clinical evaluation, for example in Alzheimer’s disease14 and cancers15. By pushing CNN technology into clinical settings, and showing that it can perform well for a rare disease, Liu and colleagues’ multihospital collaborative AI platform represents a step towards improving the medical management of rare diseases, in particular in under-resourced areas.
Contributor Information
Qian Wang, Med-X Research Institute, School of Biomedical Engineering, Shanghai Jiao Tong University, Shanghai 200030, China.
Dinggang Shen, Department of Radiology and Biomedical Research Imaging Center, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina 27599, USA.
References
- 1.Schieppati A, Henter JI, Daina E, Aperia A. Lancet. 2008;371:2039–2041. doi: 10.1016/S0140-6736(08)60872-7. [DOI] [PubMed] [Google Scholar]
- 2.Steinkuller PG, et al. J Am Assoc Pediatr Ophthalmol Strab. 1999;3:26–32. [Google Scholar]
- 3.Long E, et al. Nat Biomed Eng. 2017;1:0024. [Google Scholar]
- 4.LeCun Y, Bengio Y, Hinton G. Nature. 2015;521:436–444. doi: 10.1038/nature14539. [DOI] [PubMed] [Google Scholar]
- 5.Andreu-Perez J, Poon CC, Merrifield RD, Wong ST, Yang GZ. IEEE J Biomed Health Inform. 2015;19:1193–1208. doi: 10.1109/JBHI.2015.2450362. [DOI] [PubMed] [Google Scholar]
- 6.Krizhevsky A, Sutskever I, Hinton G. Proc Adv Neural Inf Process Syst. 2012;25:1090–1098. [Google Scholar]
- 7.Gulshan V, et al. JAMA. 2016;316:2402–2410. doi: 10.1001/jama.2016.17216. [DOI] [PubMed] [Google Scholar]
- 8.Zhou SK, Greenspan H, Shen D, editors. Deep Learning for Medical Image Analysis. Academic; 2017. [Google Scholar]
- 9.Shen D, Wu G, Suk HI. Annu Rev Biomed Eng. 2017:19. doi: 10.1146/annurev-bioeng-071516-044442. http://www.annualreviews.org/doi/abs/10.1146/annurev-bioeng-071516-044442. [DOI] [PMC free article] [PubMed]
- 10.Bahrami K, Shi F, Rekik I, Shen D. In: Deep Learning and Data Labeling for Medical Applications. Carneiro G, et al., editors. Vol. 10008. Springer; 2016. pp. 39–47. Lecture Notes in Computer Science. [Google Scholar]
- 11.Wu G, Kim M, Wang Q, Munsell BC, Shen D. IEEE Trans Biomed Eng. 2016;63:1505–1516. doi: 10.1109/TBME.2015.2496253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang W, et al. NeuroImage. 2015;108:214–224. doi: 10.1016/j.neuroimage.2014.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo Y, Gao Y, Shen D. IEEE Trans Med Imag. 2016;35:1077–1089. doi: 10.1109/TMI.2015.2508280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suk HI, Lee SW, Shen D. NeuroImage. 2014;101:569–582. doi: 10.1016/j.neuroimage.2014.06.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng J, et al. Sci Rep. 2016;6:1–13. [Google Scholar]