Abstract
The vector mosquitoes Aedes aegypti (L.), native to Africa, and Aedes albopictus (Skuse), native to Asia, are widespread invasives whose spatial distributions frequently overlap. Predictive models of their distributions are typically correlative rather than mechanistic, and based on only abiotic variables describing putative environmental requirements despite extensive evidence of competitive interactions leading to displacements. Here we review putative roles of competition contributing to distribution changes where the two species meet. The strongest evidence for competitive displacements comes from multiple examples of habitat segregation where the two species co-occur and massive reductions in the range and abundance of A. aegypti attributable to A. albopictus invasions in the southeastern U.S.A. and Bermuda (U.K). We summarize evidence to support the primacy of asymmetric reproductive interference, or satyrization, and larval resource competition, both favoring A. albopictus, as displacement mechanisms. Where evidence of satyrization or interspecific resource competition is weak, differences in local environments or alternative ecologies or behaviors of these Aedes spp. may explain local variation in the outcomes of invasions. Predictive distribution modeling for both these major disease vectors needs to incorporate species interactions between them as an important process that is likely to limit their realized niches and future distributions. Experimental tests of satyrization and resource competition are needed across the broad ranges of these species, as are models that incorporate both reproductive interference and resource competition to evaluate interaction strengths and mechanisms. These vectors exemplify how fundamental principles of community ecology may influence distributions of invasive species.
Keywords: Aedes aegypti, Aedes albopictus, resource competition, distribution modeling, reproductive interference, realized/fundamental niche
Introduction
Theory in community ecology is central to understanding biological invasions, and the ecological niche of the organisms and the niche opportunities present in the invaded community are central to this theory (Shea & Chesson 2002). The concept of ecological niche has a long history in ecology and has been defined by different authors in varied ways (reviewed by Whitaker et al. 1973, Hutchinson 1978, Giller 1984, Chase & Leibold 2003, Cadotte 2006, Soberón 2007). The link between biological invasions and niche theory reflects the influence of Charles Elton on the field of ecology. Elton’s (1958) monograph on invasions continues to foster both academic discussion and research about the processes involved in invasion biology (e.g., Richardson 2011), and his early conception of the niche (Elton 1927) continues to influence modern theory of the niche that is the basis for community ecology (Chase & Leibold 2003, Soberón 2007). One purpose of this synthesis is to highlight the importance of a mechanistic understanding of fundamental and realized niches for understanding invasions (Hutchinson 1957). The fundamental niche is the set of environmental conditions in which a species’ population could persist in the absence of species interactions (Hutchinson 1957, Chase & Leibold 2003). In contrast, the realized niche is those environmental conditions in which a species’ population persists despite the constraints imposed by interacting species including competitors (Hutchinson 1957) and predators (Chase & Leibold 2003). A second, related purpose of this synthesis is to emphasize the importance of species interactions and the impacts organisms have on one another for understanding both success and effects of invasive species. Throughout, we rely on newer, synthetic theory of the ecological niche that treats quantitatively both species environmental needs (niche in the sense of Grinnell), which largely consist of abiotic conditions that define suitable environments for that species and species impacts on mostly biotic elements of their environments (niche in the sense of Elton) (Soberón 2007). Those impacts are central to understanding species interactions and coexistence of interacting species in nature, and figure prominently in modern theory of the ecological niche (Chase & Leibold 2003), which combines elements of both Grinnellian and Eltonian niche concepts (Soberón 2007).
Environmental niche modeling or species distribution modeling (terms used synonymously) is commonly used to predict likely geographic distributions of actually or potentially introduced nonnative species (Elith & Leathwick 2009), including mosquitoes (e.g., Kraemer et al. 2015). The same statistical tools and approaches are also used to predict future geographic ranges under climate change scenarios (Elith & Leathwick 2009). This approach predicts potential geographic distributions based on statistical models with predictor variables (e.g., climate variables, vegetation, other macro-habitat features, land use, etc.) based on records from focal species’ native range or current geographic distribution. Rarely does environmental niche modeling include any measure of interactions with predators, competitors, and mutualists among the predictors. Because current distribution data usually confound abiotic variables and occurrence of interacting species, and because the list of potentially interacting species is both long and likely incomplete, most authors have used abiotic predictors alone (Elith & Leathwick 2009, Wisz et al. 2013). This leads to an important and difficult question: Does environmental niche modeling describe the fundamental niche or the realized niche of the focal species? At one level it seems the distribution data must describe the realized niche, because they are data on where a species actually occurs (e.g., in its native range), presumably after it interacts with other species in the native range (Vázquez 2006, Wisz et al. 2013). At another level, the predictors used in the model (virtually all abiotic) are more consistent with the concept of the fundamental niche (and the Grinnellian niche), and species interactions and their effects on the focal species are totally absent from the statistical model. This absence of species interactions from environmental niche models is often cited as a problem limiting the utility of the approach (Davis et al. 1998, Kearney 2006, Elith & Leathwick 2009, Wisz et al. 2013). It may be argued that species interactions are implicitly included in environmental niche modeling, to the extent that those interactions have influenced current distributions in the native range and to the extent that the abiotic environmental predictors are predictive of the distribution of any interacting species. This argument focuses on the real difficulty with environmental niche models: They are correlative and lack mechanisms (Davis et al. 1998, Kearney 2006, Kearney & Porter 2009, Elith & Leathwick 2009, Pellisier et al. 2012). This is obviously true for effects of species interactions and is true for any predictive ability of abiotic variables. Like simpler multiple regression approaches, environmental niche models are designed to be predictive, and not to describe cause and effect.
The lack of mechanistic inclusion of species interactions likely means that predictive ability of the models for the ranges of invasive species entering novel communities is uncertain at best, and wrong at worst. Consider this thought experiment: Build a predictive model for the distribution of a focal species in its native range, using geographic occurrence data and abiotic predictors. Across that native range, there are competitors and predators that impact the distribution and abundance of the focal species, but the correlative model is built without including these interacting species as predictors. Now imagine using that model to predict the range of the focal species as an invader in a new area. Table 1’s column for interacting species ‘Present; not used as predictors’ gives a sense of what might result. The model would only provide an accurate prediction of the invasive range when the same interacting species (or one that is functionally equivalent) is present (Table 1, Cell 3). If no such similar species is present in the nonnative range, then the invasive range is likely to be much greater than predicted (Table 1, Cell 1). When predators affect the native range, this situation describes enemy release, a common hypothesis for success and impact of invaders (Colautti et al. 2004). When competitors affect the native range, this situation describes the empty niche hypothesis, another common hypothesis for success and impact of an invader, which can be traced back to Elton (Elton 1958, Mitchell et al. 2006, MacDougall et al. 2009). If instead, a functionally different interacting species is present in the nonnative range, the predicted invasive range is likely to be wrong in unpredictable ways (Table 1, Cell 5). In short, the realized niche in one environment, affected by one set of interacting species, is unlikely to be the same in a different environment, with a different set of interacting species (Vázquez 2006). Consider next building a model based only on physiological measures of tolerances and performance of focal species (the mechanistic model of the fundamental niche described by Kearney & Porter 2009), purposely excluding any effects of species interactions (Table 1, interacting species ‘Absent or excluded from model’). This model is only likely to yield accurate predictions of the invasive range in the event no interacting species are present (Table 1, Cell 2). If instead the nonnative range is home to interacting species, the model overestimates the invasive range (Table 1, Cells 4, 6) which is actually limited by the biotic resistance of the resident community (Elton 1958, Mitchell et al. 2006). Thus for both types of models, only two rather unlikely scenarios will lead to accurate predictions of the invasive range of our focal species, and the reason is a failure to account for mechanistic interactions that are important parts of the niche biology of focal species.
Table 1.
Effects of failure to include species interactions in models of species potential distributions in an introduced range.
| Model built based on native range 1 | ||
|---|---|---|
| Potential introduced range | Interacting species 2 | |
| Interacting species | Present; not used as predictors | Absent or excluded from model |
| Absent | Cell 1. PREDICTED RANGE TOO NARROW [enemy release/empty niche] | Cell 2. ACCURATE PREDICTION |
| Same or functionally similar to those in native range | Cell 3. ACCURATE PREDICTION | Cell 4. PREDICTED RANGE TOO BROAD [biotic resistance] |
| Functionally dissimilar to those in native range | Cell 5. PREDICTED RANGE TOO BROAD OR TOO NARROW | Cell 6. PREDICTED RANGE TOO BROAD [biotic resistance] |
Environmental niche models are assumed to be predictive, correlative models based on conditions in the native range that are used to predict distributions in the introduced range.
Interacting species (e.g., predators, parasites, competitors) are assumed to constrain the focal species to a realized niche that is narrower than its fundamental (pre-interactive) niche. A similarly structured table could be made assuming interacting species that render the realized niche broader than the fundamental niche (e.g., mutualists, ecosystem engineers); however, deviation of predicted from actual introduced ranges would likely be reversed.
Thus, we argue, as have others (Davis et al. 1998, Shea & Chesson 2002, Kearney 2006, Elith & Leathwick 2009, Wisz et al. 2013), that including effects of interacting species is vital for understanding invasions. We illustrate the complexity of this problem with a case study of two well-known and important invasive species that often meet and interact. Current invasive ranges of the world’s two most invasive mosquito species, Aedes aegypti and Aedes albopictus, overlap extensively, especially in tropical and mild temperate regions. Aedes aegypti, native to Africa, were dispersed intercontinentally on sailing vessels between the 15th–19th centuries (Tabachnick 1991, Lounibos 2002). Major diasporas of A. albopictus, native to Asia, occurred mostly in the 20th century, facilitated by transport on container ships (Reiter 1988, Lounibos 2002). Diapause in the egg stage of temperate A. albopictus, which has favored the spread of this species into colder seasonal environments, is an adaptation (Urbanski et al. 2012) that does not occur in A. aegypti.
For this case study, we first use distributional data that show regional changes in relative abundances of one or the other species. We then interpret these distribution patterns in the context of known competitive interactions and displacement, and the ecology, behavior, and genetics of the individual species. Finally, we suggest that interactions between them could be a model for other, broadly invasive species that meet and influence one another’s distributions outside and within their native ranges. Thus, beyond the epidemiological consequences of the distributions of these important vectors, these species represent one of the best examples of how fundamental principles of community ecology, particularly considerations of ecological niche, are central to understanding invasions (Shea & Chesson 2002)
Owing to their competence and importance in transmitting arboviruses of significant health concerns, currently dengue, chikungunya, and Zika, to humans, understanding the factors that control the ranges and abundances of these species is critical for evaluating health risks. Most range maps and projections for these species are based solely on environmental niche modeling using abiotic predictors for these species expanded to global (e.g. Benedict et al. 2007, Medley 2010, Kraemer et al. 2015, Ding et al. 2018) or regional and local scales (e.g., Rochlin et al. 2013, Hahn et al. 2016, Monaghan et al. 2016). Despite this, documented or strongly suspected interspecific competitive displacement of one or the other of these species has occurred where their distributions overlap (e.g., Gilotra et al. 1967, Craig 1993, Kaplan et al. 2010). Recent use of a range map of A. aegypti predicted by the environmental model of Kraemer et al. (2015) overstates Zika risk in Florida (Grubaugh et al 2017) because of this model’s failure to account for competitive displacements that have reduced the distribution of this species in the southeastern USA (Lounibos et al. 2016).
Geographical Patterns
In Sympatry on Tropical Islands
The importance of islands for examining biogeographical patterns has been recognized since Darwin (MacArthur & Wilson 1973). Here we take advantage of mosquito surveillance information from islands or archipelagos where the two species have been sympatric, in some instances for >100 years, and that have been sampled over time, usually because of disease transmission by these invasive Aedes. Although interspecific competition has been inferred based on distribution changes, it has not been conclusively demonstrated in any of the five following examples.
Hawaiian Islands
Both A. aegypti and A. albopictus have been present for over a century in Hawaii, which has no native mosquito species (Winchester & Kapan 2003). Dengue in Honolulu was vectored by A. aegypti during the first decade of the 20th century, but in the subsequent decade this species was displaced by A. albopictus, which is now present on all islands of this archipelago (Winchester & Kapan 2003). Insecticides applied for mosquito control are believed to have facilitated local reductions of A. aegypti, both during displacement early in the 20th century and during WWII, when this species disappeared from the island of Oahu and the city of Hilo. Before the A. aegypti eradication campaign began in the USA, this species was present on only four islands but by 2002 was found only on the Big Island, especially on its drier, western side (Winchester & Kapan 2003).
Madagascar
Two extensive surveys of mosquito faunas allow for a comparison of A. albopictus and A. aegypti over time in Madagascar (Fontenille & Rodhain 1989, Raharimala et al. 2012), a comparatively old island, at least part of which is derived geologically from the African continent. It is not currently clear whether A. aegypti is indigenous to Madagascar, but A. albopictus is believed to have been introduced before 1900 during human migrations from SE Asia (Raharimala et al. 2012). Surveillance for the two species in 2009 reported decreases in the range and abundances of A. aegypti, both at sites with and without A. albopictus, compared to similar surveys in the 1980s (Raharimala et al. 2012). Aedes aegypti were more likely to be found in drier forested areas, which suggests that the feral subspecies formosus (McClelland 1968) might be the morph represented on Madagascar. By contrast, A. albopictus on Madagascar are comparatively more anthropophilic and were incriminated as the main vector of chikungunya virus on the island during Southwest Indian Ocean (SWIO) outbreaks of this arbovirus in 2006–09 (Vazeille et al. 2007, Ratsitorahina et al. 2008).
Reunion
Decreases in A. aegypti abundance relative to A. albopictus have also been reported for Reunion Island, an overseas department of France of relatively recent (4.5mya) geological age, located east of Madagascar (Bagny et al. 2009a). On this island immatures of A. aegypti are recovered primarily from rock pools and bamboo stumps in isolated ravines, whereas A. albopictus is widespread in both natural and artificial container habitats in rural and urban areas (Bagny et al. 2009a) and is the more anthropophilic of the two species (Delatte et al. 2010). Although both insecticide use and interspecific competition have been postulated as explanations of reductions in A. aegypti on Reunion (Bagny et al. 2009a), habitat degradation may also contribute to its imputed decline.
Seychelles
Aedes albopictus is common throughout much of this nation of 116 islands about one thousand miles east of the coast of Kenya. Upon last reconnaissance, A. aegypti was restricted to the capital city of Victoria on Mahé Island (Lambrecht 1971), a reduction in range from the 1940s, when it was also recorded from the opposite side of this island (Mattingly & Brown 1955). Aedes aegypti on the Seychelles appear to be more domestic than counterparts on SWIO islands, so its restriction to the most urbanized portion of this country conforms to the behavior of the domestic subspecies, A. aegypti aegypti, elsewhere in its native and invasive ranges.
Mayotte
Mayotte, politically a protectorate of France, is geographically part of the Comoros archipelago, located off the coast of East Africa in the Mozambique Channel. The establishment of A. albopictus around 2001 was purportedly associated with declines in A. aegypti, which subsequent surveys showed to be more prevalent than A. albopictus in rural habitats. (Bagny et al. 2009b), a reversal of the more common displacements of these species on habitat gradients (see below). Morphologically and behaviorally, A. aegypti on Mayotte appear to correspond to the feral subspecies formosus (Mattingly 1967, Bagny et al. 2009b). A recently described mosquito species known only from Mayotte, Aedes pia, is closely related to A. aegypti (Le Goff et al. 2013), but is not believed to undermine the conclusions of Bagny et al. (2009b) because A. pia is relatively uncommon (Le Goff et al. 2013).
Well Documented Competitive Displacements
Continental USA
The eastward and northward expansion of A. albopictus from its original establishment in Houston, Texas has been documented from state and county surveillance records (Moore 1999). Nearly simultaneously, reductions in abundance and distribution of A. aegypti were reported from four states in the southeastern USA (Hobbs et al. 1991, Mekuria et al. 1995, Nasci et al. 1989, O’Meara et al. 1995). By 1993, the range of A. aegypti was reduced in the Southeast to southern Florida and redoubts in the centers of major southern port cities, such as Houston and New Orleans (Craig 1993, O’Meara et al. 1995). Recent surveillance in Florida indicates that the reduced range and abundance of A. aegypti recognized by 1993 persisted through 2014 (Lounibos et al. 2016), although subtle changes were detected, suggesting limited local recoveries of this species in relation to A. albopictus (Lounibos et al. 2016, Hopperstad & Reiskind 2016).
Bermuda
Aedes aegypti was eradicated from the subtropical island of Bermuda (U.K.) in the 1960s but rediscovered there in 1997 (Kaplan et al. 2010). Upon recognition of the establishment of A. albopictus in 2000, surveillance for both species by trapping and identifying eggs laid in containers showed a rapid decline in abundance of A. aegypti concomitant with the rise in numbers of A. albopictus (Kaplan et al. 2010). Based on Lotka-Volterra models of competition, Kaplan et al. (2010) concluded that interspecific competition among larvae, by itself, was inadequate to explain the rapid decline and disappearance of A. aegypti on Bermuda following invasion by A. albopictus. Kaplan et al.’s (2010) estimates of carrying capacities (K) and competition coefficients (α) suggested that the two species were nearly equal as larval competitors. They argued that exclusion of A. aegypti would only be expected when A. albopictus had approached its carrying capacity. In fact, disappearance occurred at densities well below that density. Kaplan et al. (2010) suggested some additional impact of A. albopictus on A. aegypti must also have contributed to the latter’s disappearance from the island.
Tropical Cities Where A. albopictus Has Been Displaced by A. aegypti
Phylogeographic and historical evidence supports that the invasion of A. aegypti into Asia did not occur until the 19th century, considerably later than the western expansion of this species into the Americas (Smith 1956, Tabachnick 1991). During its spread into cities of SE Asia, A. aegypti encountered native A. albopictus, which it displaced in Bangkok (Stanton 1920), Kuala Lumpur (Rudnick 1965), and Calcutta (Kolkata) (Gilotra et al. 1967); anecdotal information also supports urban displacements of A. albopictus in Manila and southern Taiwan (Gilotra et al. 1967). Distributions of these species in Calcutta in the 1960s (Gilotra et al. 1967) show patterns of habitat segregation similar to those described in the Americas decades later (e.g. Braks et al. 2003, Rey et al. 2006), where A. aegypti predominates in the absence of vegetation and A. albopictus in the presence of trees and shrubs (Table 2).
Table 2.
Examples of habitat segregation between A. aegypti and A. albopictus in rural-urban landscape gradients.
| Location | Urban Species | Test for Negative Intersp. Correlation | References |
|---|---|---|---|
| South Florida | A. aegypti | yes | Braks et al. 2003; Rey et al. 2006; Lounibos et al. 2016 |
| Rio de Janeiro | A. aegypti | yes | Braks et al. 2003 |
| Gabon | A. aegypti | not done | Paupy et al. 2010 |
| Mayotte | A. albopictus | not done | Bagny et al. 2009b |
| North Thailand | A. aegypti | yes | Tsuda et al 2006 |
| Vietnam | A. aegypti | yes | Higa et al. 2010 |
| Indonesia | A. aegypti | not done | Ishak et al. 1997 |
A more recent example of urban displacement occurred in the Colombian port of Leticia on the Amazon River, where invasive A. albopictus had been recognized in 1998 as established (Velez 1998). After A. aegypti re-invaded Leticia in 2009, it displaced A. albopictus to the periphery, outside of the borders of the concrete streets of this carefully planned riverine city (Carvajál 2013).
Species Distributions on Rural-Urban Gradients
Niche Compressions in Sympatry
Two separate studies have used aerial imagery to quantify patterns of habitat use by the two species in zones of sympatry in southern Florida. Sampling of these habitats with oviposition traps in three, non-contiguous counties confirmed that A. aegypti abundances were positively correlated to habitats associated with urbanization, such as buildings and pavement, while A. albopictus predominated in rural settings with vegetation (Rey et al. 2006). Similar patterns were confirmed from larval collections of the two species at tire shops and cemeteries elsewhere in southern Florida (Lounibos et al. 2016). These patterns are consistent with other reports of habitat segregation on urban-rural gradients in Rio de Janeiro (Braks et al. 2003), Gabon (Paupy et al. 2010), northern Thailand (Tsuda et al. 2006), Indonesia (Ishak et al. 1997), and Vietnam (Higa et al. 2010) (Table 2). However, a different pattern was observed on the French island of Mayotte, part of the Comoros archipelago, where larval collections of the two species in 2007 showed A. albopictus predominating in urban and suburban zones, especially in the dry season, and A. aegypti in rural habitats (Bagny et al. 2009b) (Table 2). A somewhat different pattern was observed by Leisnham & Juliano (2009), who found that within urban south Florida, A. aegypti attained greater relative abundance in urban residential neighborhoods, compared to cemeteries and industrial areas, where A. albopictus predominated. Locations of examples of habitat segregation and displacements between these species are shown in Figure 1.
Figure 1.
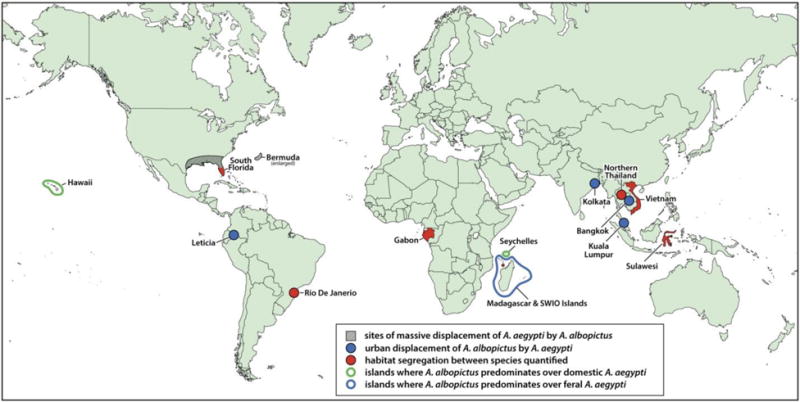
Geographic locations of different outcomes (see inset for interpretations) where A. aegypti and A. albopictus have met.
Niche Expansions in Allopatry
In Guangzhou, China, A. albopictus are more productive in urban, than in suburban or rural, habitats, and have shorter larval developmental times, higher adult emergence rates, and longer lifespans in the urban settings (Li et al. 2014). Thus, in the absence of A. aegypti, the fundamental niche of A. albopictus has expanded to, and is facilitated by, urban habitats (Li et al. 2014). In southern USA, prior to the arrival of A. albopictus, the fundamental niche of A. aegypti included rural habitats (Morlan & Tinker 1965), which, after the invasion of A. albopictus, are uncommonly occupied by this species (Braks et al. 2003, Rey et al. 2006).
AIAI and Invasions by Container Aedes
Juliano and Lounibos (2016) described three behavioral traits of domestic A. aegypti in Africa that support the hypothesis that this form of the species is pre-adapted for invasion success, as predicted by the Anthropogenically Induced Adaptation to Invade (AIAI) hypothesis of Hufbauer et al. (2012). The three adult female behaviors noted were: (1) house entry (endophily); (2) the preference for oviposition in human-made domestic containers; (3) preference for blood-feeding from humans (Juliano & Lounibos 2016). Although tendencies toward domestication in A. albopictus were not discussed, Juliano and Lounibos (2016) noted the behavior of this species to occupy disturbed, forest-urban ecotonal habitats in both its native and invasive ranges, a pattern also present in the feral subspecies A. aegypti formosus in East Africa (Lounibos 1981). This suggests that as edge species, the feral forms of both species may have been pre-adapted to domestication, and thus to human transport and invasion success in human dominated landscapes, supporting AIAI (Hufbauer et al. 2012). World-wide colonization and invasions by A. albopictus depended on acceptance by this species of tires as oviposition sites (Hawley et al. 1987), another behavioral trait facilitating use of human-dominated habitats and contributing to invasion success in a manner consistent with AIAI.
Climate and Transition Zones of Co-occurrence
Climate influences the distributions of A. aegypti and A. albopictus differently. Aedes albopictus can enter a protective state of egg diapause to endure harsher winter conditions of temperate zones (Urbanski et al. 2012). In southern China, the Tropic of Cancer represents the approximate latitudinal line, north of which A. aegypti is rare or absent on the mainland (Wu et al. 2010) and on Taiwan (Yang et al. 2014). In both Macao and Hong Kong, as well as southern mainland China, A. albopictus replaces A. aegypti as the primary dengue vector (Almeida et al. 2005, Li et al. 2014). Aedes aegypti eggs and adults are, in contrast, more tolerant of desiccation and higher temperatures than are A. albopictus (Sota & Mogi 1992, Juliano et al. 2002). Differential mortality in the egg stage of the two species is believed to contribute to their patchy distribution in cemeteries in southern Florida, where their co-existence is associated with warmer dry-season temperatures and lower humidities (Lounibos et al. 2010). The tolerance of dryness may contribute to occurrence of A. aegypti in the arid southwestern US (Reiter et al. 2003) and to a general pattern of A. aegypti association with drier conditions on islands like Madagascar and Hawaii (Fontenille & Rhodain 1989, Winchester & Kapan 2003), and the absence of invasive A. albopictus and dominance by A. aegypti in drier Northern Cameroon (Simard et al. 2005). Abundance of A. aegypti is strongly associated with seasonal dry periods in Florida (Juliano et al. 2002). In south Florida where the two species sometimes co-occur there is a consistent pattern of greater relative abundance of A. aegypti at the start of the wet season, suggesting relatively greater survivorship through the dry season, along with greater increase in relative abundance of A. albopictus as the wet season progresses (Juliano et al. 2002, Leisnham & Juliano 2009, Reiskind & Lounibos 2013). A similar seasonal pattern in areas of co-occurrence exists in Central African Republic (Kamgang et al. 2013) and in Thailand (Mogi et al. 1988). Camara et al. (2016) observed a similar pattern of greater success of A. aegypti in a field competition experiment in the dry spring (September – October) in Rio de Janeiro. Laboratory cage competition experiments demonstrated that experimental drying regimes imposed on populations were sufficient to eliminate the competitive advantage of A. albopictus over A. aegypti (Costanzo et al. 2005).
O’Neal & Juliano (2013) did field experiments testing specifically for effects of seasonal fluctuations in rainfall and differential mortality of eggs as a possible mechanism of coexistence in south Florida. They did not observe patterns of egg mortality consistent with this hypothesis (i.e., mortality of A. albopictus eggs was not greater in a dry-season experiment). They found, however, that interspecific competition was greatly reduced in the dry season, probably because of greater input of oak leaf detritus to containers, and greater input of oak flowers, which are a significantly more nutritious detrital resource for mosquito larvae (Lounibos et al. 1993). Thus, resource quantity and quality likely interact with seasonality, creating periods of reduced competitive interactions that may render coexistence more likely. See below for further review of the role of resource quality. Macroscopic climate variables such as temperature, precipitation, and seasonality thus encompass multiple, intercorrelated aspects of the environment – mortality due to desiccation and resource input, to name two - that influence these competitors in complex ways. This may mean that climate variables like rainfall or seasonality may be predictive of geographic ranges without a direct causal connection to population success. Whether climate variables in a new geographic area would be equally predictive of species’ success may depend on whether local species of trees respond to climate and seasonality in ways that are similar to those of the oak trees that dominate in Florida.
Mechanisms and Determinants of Co-existence and Exclusion
Although interspecific competition has been inferred or suggested to account for many observed changes in distribution and abundance where A. aegypti and A. albopictus co-occur, empirical tests of hypothesized mechanisms have been conducted only, to our knowledge, in the Western Hemisphere, where the two species co-exist as invasives. While many different mechanisms were proposed to account for competitive displacements observed in the USA (e.g., Craig 1993), over time only two have received sufficient empirical support to warrant inclusion for further consideration in this synthesis: reproductive (interference) competition and larval (resource) competition.
Asymmetric Mating Interference
A form of reproductive competition, often termed satyrization (Ribiero 1988), in which males of one species mate with a related species and produce inviable or less-fit hybrid offspring was proposed by Ribeiro (1988) as a biological control mechanism for insect pests or vectors. Nasci et al. (1989) provided some laboratory and field evidence that A. albopictus males copulating with A. aegypti females might account for competitive displacements of the latter species observed in Louisiana, USA, associated with the spread of A. albopictus in the late 1980s. However, the satyrization hypothesis received no support from early cage experiments that showed low rates of cross-insemination between these species (e.g., Harper & Paulson 1994).
The potential impact of satyrization generated renewed interest with the first report of interspecific matings in nature, in auto salvage yards in Florida, between A. albopictus and A. aegypti. This breakthrough was made possible by applying species-specific nucleotide probes to sperm dissected from spermathecae of wild-caught females (Tripet et al. 2011). Subsequent application of these methods to wild females captured at other sites of co-existence confirmed bidirectional interspecific matings at low (1–4%) frequencies on four continents (Bargielowski et al. 2015). Effects of cross-matings between these species are highly asymmetric, negatively affecting A. aegypti females much more than A. albopictus females, because male accessory gland products transferred during heterospecific matings permanently sterilize A. aegypti but not A. albopictus females (Tripet et al. 2011).
Testing multiple geographical populations of A. aegypti from the USA for susceptibility to cross-mating by A. albopictus, Bargielowski et al. (2013) reported that allopatric populations of A. aegypti were significantly more vulnerable than sympatric populations, and female origin, but not male origin, was a significant predictor in regression models that accounted for insemination success. The opposite cross, between A. albopictus females and A. aegypti males, occurred less frequently in the standardized cage environment and was influenced in the opposite direction by the allopatric-sympatric differences (Bargielowski et al. 2013). These authors concluded that reproductive character displacement had evolved in sympatric A. aegypti to mitigate the severe consequences of loss of reproduction in populations of this species in the presence of A. albopictus (Bargielowski et al. 2013). Character displacement attributable to reproductive competition was also demonstrated in male A. aegypti, allopatric populations of which were shown to be less effective than sympatric counterparts in mating conspecifics in the presence of A. albopictus females (Bargielowski et al. 2015b). Interestingly, the presence of conspecifics increased cross-matings in some cage exposures (Bargielowski et al. 2015b). Rapid selection for satyrization resistance was detectable in cage populations of allopatric A. aegypti females exposed to male A. albopictus, leading to reductions in cross-mating frequencies within 1–3 generations after exposure (Bargielowski & Lounibos 2014). Females derived from selected lines were significantly slower to mate with conspecific males, exposing a cost for the evolution of satyrization-resistance (Bargielowski & Lounibos 2014).
Aedes albopictus males from different geographical origins are not equally capable of satyrizing A. aegypti females. Males of A. albopictus derived from colonies of this species from three large cities in Brazil were relatively ineffective in inseminating A. aegypti from Brazil or the USA in a standard cage environment (Honório et al. 2017). As A. albopictus that invaded Brazil are of tropical origin, in contrast to the temperate-derived A. albopictus in the USA (Hawley et al. 1987, Birungi & Munstermann 2002), the capacity to satyrize may be under genetic control and account for some of the geographic differences in displacement outcomes between the two countries (Honório et al. 2017).
Resource competition among larvae
Most investigations of competition among larvae of these species are done as density manipulations (response surface, replacement series, or addition series). It is relatively uncommon for resource type or availability to be manipulated along with species densities. Even rarer are experiments that attempt to test models of resource competition (Tilman 1982; Grover 1998, Chase & Leibold 2003). Murrell & Juliano (2012) designed experiments to estimate the minimum amount of a standardized detritus source, a mix of leaf detritus and dead insects similar to what is seen in many field studies (e.g., Yee et al. 2007, Murrell et al. 2011), that would be necessary for stable populations of each species (Rindex). Their goal was an approximation of the R* values for resources that are at the heart of resource competition models (Tilman 1982). They found that A. albopictus had significantly lower Rindex than either A. aegypti or Culex pipiens, indicating it should exclude either of the other two species. Pairwise competition experiments using that resource environment were consistent with the prediction of exclusion of A. aegypti (Murrell & Juliano 2012). Relevant to the comparison of competitive abilities of A. albopictus and A. aegypti, Rindex for A. albopictus was not only significantly less than that of A. aegypti, but was also less than half the value of A. aegypti, suggesting that A. albopictus can persist as a population at detritus resource levels much lower than can A. aegypti and would thus be predicted to exclude A. aegypti in competition for the resource mix used in this experiment (Murrell & Juliano 2012). This remains the only experiment explicitly testing models of resource competition for any mosquitoes.
To evaluate the role of interspecific competition in changing field distributions, determining that natural densities of larvae are sufficient to produce significant interspecific competition is an important step toward establishing that interspecific competition is important under natural conditions. Field experiments on larvae at multiple sites in Florida showed that interspecific competition from A. albopictus had significant impacts on A. aegypti across a range of natural densities of larvae in cemeteries (Juliano et al. 2004). Similar experiments in suburban Brazil also showed that at natural densities, competitive effects of A. albopictus on A. aegypti were readily detectable in multiple seasons (Braks et al. 2004; Camara et al. 2016). Published accounts of field experiments on interspecific competition are rather rare (but see Kaplan et al. 2010). There have been several field experiments done at natural densities of larvae that show that intraspecific competition among larvae is also common. Two field experiments at natural densities of larvae of A. aegypti in residential areas in southern Mexico showed significant effects of intraspecific competition, and of delayed density dependence (i.e., effects of previous cohorts of larvae exploiting detritus resources in containers) acting on A. aegypti (Walsh et al. 2011, 2013). Similar studies done with A. albopictus in suburban residential areas of North Carolina demonstrated significant effects of delayed density dependence at natural densities of larvae (Walsh et al. 2012). Collectively these field studies indicate that both inter- and intra-specific competition among larvae of these Aedes are likely to occur relatively frequently in nature.
Field and laboratory experiments on competition among larval A. aegypti and A. albopictus have often found that A. albopictus is the superior competitor in many tested environments (reviewed by Juliano 2009, 2010). Meta-analysis of competition experiments (Juliano 2010) indicated significant heterogeneity of the outcome and degree of competitive asymmetry between these species that depended on the nature of the resources for larvae. When the resource base for larvae consisted of plant detritus of various kinds, A. albopictus had a clear competitive advantage, but when the resource base incorporated more rapidly decomposing substrates (e.g., dead insects) or resources that could be directly consumed (e.g., liver powder), the two species had approximately equal competitive effects and responses (Juliano 2010). Laboratory competition experiments show that some leaf types can also greatly reduce the competitive advantage of A. albopictus (Reiskind et al. 2012). Many of these laboratory results are consistent with field distributions in Florida, where A. aegypti populations persist and are robust in areas where nitrogen content of detritus is high, but are largely absent in areas where litter nitrogen content is lower (Murrell et al. 2011). Thus, it seems likely that one source of variation in the outcome of population encounters between A. aegypti and A. albopictus is environmental variation in the quality and composition (carbon, nitrogen, etc.) of the detrital resources encountered by larvae.
There is also evidence for interpopulation variation in competitive effects and responses of larvae of both species. Leisnham et al. (2009) tested nine North American populations of A. albopictus in competition with a standard population of A. aegypti. Although they found significant variation among A. albopictus populations in competitive effects on A. aegypti and in competitive responses to A. aegypti, this variation did not correlate with sites of coexistence or exclusion of A. aegypti following invasion by A. albopictus, or allopatry to A. aegypti. Thus, although variation among A. albopictus populations could contribute to local outcomes of competition when larvae meet, such variation does not seem to account for the broad pattern of coexistence or exclusion of A. aegypti in southern North America. Leisnham et al. (2010) similarly tested eight North American populations of A. aegypti in larval competition with a standard population of A. albopictus. They found that A. aegypti from allopatry to A. albopictus performed better in competition than did populations from sympatry, primarily because allopatric A. aegypti larvae had a greater competitive effect on A. albopictus. This result suggests that failure of A. albopictus to encroach further on the North American range of A. aegypti may in part be a result of superior competitive ability of larvae in some local populations of A. aegypti. If it is assumed that this difference in competitive ability between A. aegypti sympatric or allopatric to A. albopictus is a product of natural selection, it remains unknown what may have selected for that divergence. Improved performance in interspecific competition would seem more likely to be a product of selection in areas of sympatry, where encounters occur more frequently, but Leisnham et al. (2010) found, instead, better performance in interspecific competition in areas of allopatry to A. albopictus. The potential for evolution of competitive ability following contact of these species remains an important question for future research. No similar comparisons of intraspecfic variation in competitive abilities of these species has been conducted for other parts of their global invasive range.
Models of competition and mating interference
Although larval resource competition was formerly cited as the most common mechanism to account for competitive displacements among arthropod species (Reitz & Trimble 2002), there is a growing appreciation that reproductive interference may be an important cause of some displacements (Gröning & Hockrich 2008, Gao & Reitz 2017). It is fitting that several models analyze the joint effects of interspecific larval competition and reproductive interference on displacement and co-existence. Kishi & Nakazawa (2013) modeled how density dependent interactions affect population growth through both competition and reproductive interference and showed they may act synergistically to promote competitive exclusion. Their models do not explicitly include resource competition nor the stage structure inherent in the A. aegypti – A. albopictus system, wherein resource competition would occur among larvae, and affect survival, growth, and development and ultimately the production of adults, and reproductive interference among adults would affect fecundity and ultimately production of new larvae. Both Kishi & Nakazawa (2013) and the earlier models by Ribiero (1988) assume that interspecific competition (presumed among larvae) has a lesser impact than does intraspecific competition, rendering coexistence of competitors possible in the absence of reproductive interference. Owing to the potential for stronger, more direct, and more rapid impacts from reproductive interference, either co-existence or exclusion can prevail if advantages in the two competitive processes are in opposition (Kishi & Nakazawa 2013). Thus, the combination of interspecific competition among larvae and reproductive interference among adults could result in stable coexistence if there is a tradeoff in advantage, or competitive exclusion if one species has the advantage in both. These results are consistent with Ribeiro’s (1988) model, which like Kishi & Nakazawa’s (2013) is based on Lotka-Volterra dynamics, suggested that low levels of satyrization, enhanced by resource competition, could be used for control of pests and vectors. No one has yet modeled pure resource competition among larvae (i.e., the experimental system investigated by Murrell & Juliano 2012, described above) with one species having a strong advantage, and ability to exclude the other, in resource competition, and the added effect of reproductive interference. A stage-structured model seems likely to be necessary for this scenario.
Future Research Opportunities and Challenges
Testing Displacement Mechanisms
Despite the extensive sympatric ranges of these invasive Aedes species, experimental tests of putative displacement mechanisms have to date been conducted only for populations from the USA and Brazil (e.g., Juliano 1998, Braks et al. 2004, Camara et al. 2016, Honorio et al. 2017), leaving vast swaths of Asia and Africa unaccounted for to parameterize models that include interaction mechanisms. The opportunity to link the strength of a putative mechanism through experiments to the patterns of local displacement could extend models and increase understanding of competitive displacements in this system.
Population Genetics
The feral subspecies A. aegypti formosus is expected to behave differently than the domestic subspecies in encounters with A. albopictus, but populations of this species from Madagascar, Reunion and Mayotte have not been tested for genetic distinctiveness from A. aegypti aegypti that may account for different patterns of habitat segregation observed on these SWIO islands.
Interpopulation variation in larval competitive performance of A. aegypti and A. albopictus populations from the USA (Leisnham et al. 2009, Leisnham & Juliano 2010) has been quantified, but there has been no systematic investigation of evolution of competitive performance (of either species) when they meet. This contrasts markedly with investigations of evolution of resistance to reproductive interference, which has been shown in the lab (Bargielowski & Lounibos 2014) and detected in nature (Bargielowski et al. 2013, Lounibos et al. 2016).
Interpopulation variation in reproductive interference is also suggested by the failure of Brazilian populations of A. albopictus to satyrize in contrast to N. American populations (Honorio et al 2017). More information on the genetic bases of both larval resource competitive ability and adult satyrization would be valuable. Genetic profiles of satyrization-resistant A. aegypti have displayed consistent regions of the genome that appear to be targets of natural selection (Buford Reiskind et al. submitted), leading to the possibility of identifying the genetic basis for this example of reproductive character displacement. Evolution of satyrization resistance has been detected in natural populations (Bargielowski et al. 2013, Lounibos et al 2016) and selected lines of A. aegypti (Bargielowski & Lounibos 2014). Evolution of satyrization resistance or of resource competitive ability (or of both) may stabilize range boundaries of parapatric populations of A. albopictus and A. aegypti.
Recent analyses of mtDNA haplotype diversity have identified admixtures of unrelated genomes that have occurred as a consequence of unpredictable dispersion by invasive A. albopictus (Manni et al. 2017). Successive waves of colonization, allowing for genomic reassortment between founder populations, may also apply to worldwide dispersion of A. aegypti (e.g., Brown et al. 2014). Such genetic reassortment processes may not only change the invasive characteristics of both species but alter gene X environment interactions, which argues in support of population genetic approaches for understanding the outcomes of interspecific competition between these species.
Climate Change
The majority of models that predict abundance and distributional changes in A. albopictus and A. aegypti with climate change are based upon fundamental niches of the vector species, and do not incorporate interspecific interactions (e.g., Kearney et al. 2009, Rochlin et al. 2013, Proestos et al. 2015). For localities where these species might encounter one another, consideration of possible displacement effects and mechanisms would improve predicted future distributions under climate change. Although some climate change scenarios for these species only model effects of temperature changes (e.g., Thomas et al. 2011), precipitation patterns are known to affect A. albopictus population dynamics in cages (Alto & Juliano 2001) and the outcome of interactions of this species with A. aegypti (Costanzo et al. 2005). More, broadly, climate change may facilitate invasion by other mosquitoes, such as Aedes flavopictus, which has recently been observed displacing A. japonicus and A. albopictus with temperature increases in their native ranges near Nagasaki, Japan (Chaves 2016).
Conclusions
Worldwide, outcomes of interspecific encounters between A. albopictus and A. aegypti are extremely diverse, ranging from wide-scale competitive displacements to local habitat segregation to no evident effects. It is doubtful that the potential invasive geographic ranges of either species can be predicted accurately without using the multifaceted and context-dependent competitive interactions between these important vectors as predictors. Outcomes of interactions of these species appear to depend on which of the local genotypes of the two species interact, as well as on the local environments in which they interact.
We suggest that incorporating the effects of interspecific interactions in global distribution models of the two species (Benedict et al. 2007, Medley 2010, Kraemer et al. 2015, Ding et al. 2018) is likely to be both important for accurate prediction, and to be challenging, a point that was also made by Eisen and Moore (2013). We also suggest that incorporating species interactions into predictive models of invasive range will be generally important for most invasive species. Several authors working in other systems have found ways to add species interactions to environmental niche models (e.g., Leathwick & Austin 2001, Anderson et al. 2002) and these may provide a guide for researchers pursuing environmental niche models of these Aedes. Wisz et al. (2013) suggest multiple mathematical/statistical approaches that could be used to account for effects of species interactions in predictive environmental niche models. We think A. albopictus and A. aegypti could be an important and productive test case for some of these proposed approaches. First, the mechanisms by which they interact are well documented, limiting uncertainty about whether the interactions should be included in models. Second, as occupants of small water holding vessels in human-dominated habitats, the set of other species that might impact their distributions is more limited than those for organisms from more species-rich communities, which keeps this problem relatively simple (Wisz et al. 2013). Third, as important vectors of human disease, they are well studied and their distributions are the focus of considerable public health surveillance. Their roles as vectors also enhance the importance of developing predictive models of their invasive distributions.
Though we have illustrated the importance of the realized niche using interspecific competition as the interaction that is critical for distribution modeling and prediction for these two species, we expect a similar case could be made for predation among other invasive species, but that is beyond the scope of this synthesis. Development of local distribution maps, which are probably most relevant for epidemiological interpretations (e.g., Rochlin et al. 2013, Hahn et al. 2016), should be done with incorporation of the documented processes contributing to competitive displacements to improve spatial resolution. Where sterile insect techniques or other genetic approaches (e.g., Lees et al. 2015) are contemplated for control of one of these species, the potential for population replacement by the other competing vector species should be considered (e.g., Bonsall et al. 2010). Niche compressions following the establishment of either invasive species in habitats formerly occupied by the other are the most common evidence for interspecific competition between A. aegypti and A. albopictus. Although mechanisms whereby the two species achieve relative superiority in their realized niches are not completely understood, especially for A. aegypti in urban environments, the typical advantage of A. albopictus in larval competition and fluctuations in competitive advantages attributable to variations in resources and weather seem to account for the predominance of A. albopictus in vegetated habitats and the patchy co-existence of the two species in sympatry.
However, resource competition alone cannot account for the rapid and expansive displacements of A. aegypti observed in Bermuda (Kaplan et al. 2010) or the southeastern USA (Nasci et al. 1989, Bargielowski & Lounibos 2016). We consider asymmetric reproductive interference, or satyrization, to be the most probable additional contributing cause of these conspicuous, swift, and, at least for Florida (Lounibos et al. 2016), durable outcomes. The rarity of such massive displacements suggests that either they occur only under exceptional circumstances, such as at the margins of the range of A. aegypti, or that only some populations of A. albopictus are capable of satyrization (Honório et al, 2017). Although interspecific mating between these species, as estimated from heterospecific sperm detected in the spermathecae of wild-caught females, is relatively rare (1–4%), this interaction seems to be ecologically important. All sympatric field populations sampled on four continents had co-existed long enough to have evolved resistance to cross-mating (Bargielowski et al. 2013B, 2015) and A. aegypti from allopatry to A. albopictus are more susceptible to interspecific insemination by A. albopictus (Bargielowski et al. 2013, Lounibos et al. 2016). The frequency of satyrization has likely been underestimated because interspecific transfer of male accessory gland substances without successful sperm transfer to the spermathecae can produce the detrimental effects causing satyrization (Carrasquilla & Lounibos 2015).
Acknowledgments
The authors were supported during the writing of this review by US National Institute of Allergy & Infectious Disease grant R21AI095780 to LPL and US National Institute of Allergy & Infectious Disease grant R15AI124005 to SAJ. We thank VA Borowicz for useful comments on earlier drafts, and an anonymous referee, and D. Simberloff for additional valuable comments.
Footnotes
ORCID: 0000-0002-6178-4553
References
- Almeida APG, Batista SSSG, Sousa CAGCC. Bioecology and vectorial capacity of Aedes albopictus (Diptera: Culicidae) in Macao, China, in relation to dengue virus transmission. J Med Entomol. 2005;42:419–428. doi: 10.1093/jmedent/42.3.419. [DOI] [PubMed] [Google Scholar]
- Alto BW, Juliano SA. Precipitation and temperature effects on populations of Aedes albopictus (Diptera: Culicidae): implications for range expansion. J Med Entomol. 2001;38:646–656. doi: 10.1603/0022-2585-38.5.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R, Peterson AT, Gómez-Laverde M. Using niche-based GIS modeling to test geographic predictions of competitive exclusion and competitive release in South American pocket mice. Oikos. 2002;98:3–16. [Google Scholar]
- Bagny L, Delatte H, Quilici S, Fontenille D. Progressive decrease in Aedes aegypti distribution in Reunion Island since the 1900s. J Med Entomol. 2009a;46:1541–1545. doi: 10.1603/033.046.0644. [DOI] [PubMed] [Google Scholar]
- Bagny L, Delatte H, Elissa N, Quilici S, Fontenille D. Aedes (Diptera: Culicidae) vectors of arboviruses in Mayotte (Indian Ocean): distribution area and larval habitats. J Med Entomol. 2009b;46:198–207. doi: 10.1603/033.046.0204. [DOI] [PubMed] [Google Scholar]
- Bargielowski IE, Lounibos LP, Carrasquilla MC. Evolution of resistance to satyrization through reproductive character displacement in populations of invasive dengue vectors. Proc Natl Acad Sci. 2013;110:2888–2892. doi: 10.1073/pnas.1219599110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargielowski IE, Lounibos LP. Rapid evolution of reduced receptivity to interspecific mating in the dengue vector Aedes aegypti in response to satyrization by invasive Aedes albopictus. Evol Ecol. 2014;28:193–203. doi: 10.1007/s10682-013-9669-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargielowski IE, Lounibos LP. Satyrization and satyrization-resistance in competitive displacements of invasive mosquito species. Ins Sci. 2016;23:162–174. doi: 10.1111/1744-7917.12291. [DOI] [PubMed] [Google Scholar]
- Bargielowski IE, Lounibos LP, Shin D, Smartt CT, Carrasquilla MC, Henry A, Navarro JC, Paupy C, Dennett JA. Widespread evidence for interspecific mating between Aedes aegypti and Aedes albopictus (Diptera: Culicidae) in nature. Inf Gen Evol. 2015a;36:456–461. doi: 10.1016/j.meegid.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargielowski IE, Blosser E, Lounibos LP. The effects of interspecific courtship on the mating success of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) males. Ann Entomol Soc Amer. 2015b;108:513–518. doi: 10.1093/aesa/sav037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict MQ, Levine RS, Hawley WA, Lounibos LP. Spread of the tiger: global risk of invasion by the mosquito Aedes albopictus. Vect-Bor Zoon Dis. 2007;7:76–85. doi: 10.1089/vbz.2006.0562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birungi J, Munstermann LE. Genetic structure of Aedes albopictus (Diptera: Culicidae) populations based on mitochondrial ND5 sequences: evidence for an independent invasion into Brazil and the United States. Ann Entomol Soc Am. 2002;95:126–132. [Google Scholar]
- Bonsall MB, Yakob L, Alphey N, Alphey L. Transgenic control of vectors: the effects of interspecific interactions. Isr J Ecol Evol. 2010;56:353–370. [Google Scholar]
- Braks MAH, Honorio NA, Lourenco-de-Oliveira R, Juliano SA, Lounibos LP. Convergent habitat segregation of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) in southeastern Brazil and Florida, USA. J Med Entomol. 2003;40:785–794. doi: 10.1603/0022-2585-40.6.785. [DOI] [PubMed] [Google Scholar]
- Braks MAH, Honorio NA, Lourenco-de-Oliveira R, Lounibos LP, Juliano SA. Interspecific competition between two invasive species of container mosquitoes, Aedes aegypti and Aedes albopictus (Diptera: Culicidae), in Brazil. Ann Entomol Soc Am. 2004;97:130–139. [Google Scholar]
- Brown JE, Evans BR, Zheng W, et al. Human impacts have shaped historical and recent evolution in Aedes aegypti, the dengue and yellow fever mosquito. Evol. 2017;68:514–525. doi: 10.1111/evo.12281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burford Reiskind M, Labadie P, Bargielowski I, Lounibos P, Reiskind M. (submitted) Rapid evolution and the genomic consequences of selection against interspecific mating. Molec Ecol. doi: 10.1111/mec.14821. [DOI] [PubMed] [Google Scholar]
- Cadotte MW. Darwin to Elton: early ecology and the problem of invasive species. In: Cadotte MW, McMahon SM, Fukami T, editors. Conceptual ecology and invasion biology: reciprocal approaches. Springer; Dordrecht, Netherlands: 2006. pp. 15–33. [Google Scholar]
- Camara DCP, Codeço CT, Juliano SA, Lounibos LP, Riback TIS, Pereira GR, Honorio NA. Seasonal differences in density but similar competitive impact of Aedes albopictus (Skuse) on Aedes aegypti (L.) in Rio de Janeiro, Brazil. PLoS One. 2016;11(6):e0157120. doi: 10.1371/journal.pone.0157120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasquilla MC, Lounibos LP. Satyrization without evidence of successful insemination from interspecific mating between invasive mosquitoes. Biol Lett. 2015;11:20150527. doi: 10.1098/rsbl.2015.0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvajal Cortes JJ. Variação espacial e temporal dos vetores do Dengue Aedes (Stegomyia) albopictus (Skuse, 1894) e Aedes (Stegomyia) aegypti (Linnaeus, 1762) na área urbana do município de Letícia, (Amazonas-Colômbia) e sua associação com a transmissão do Dengue na tríplice fronteira Amazônica (Colômbia-Brasil-Peru) 2013 MSc Thesis. Inst. Osw. Cruz; Rio de Janeiro: 2013. [Google Scholar]
- Chase JM, Leibold MA. Ecological niches Linking classical and contemporary approaches. University of Chicago Press; Chicago: 2003. [Google Scholar]
- Chaves LF. Globally invasive, withdrawing at home: Aedes albopictus and Aedes japonicus facing the rise of Aedes flavopictus. Int J Biometeor. 2016 doi: 10.1007/s00484-016-1162-7. [DOI] [PubMed] [Google Scholar]
- Colautti R, Ricciardi A, Grigorovich I, MacIsaac H. Is invasion success explained by the enemy release hypothesis? Ecol Lett. 2004;7:721–733. [Google Scholar]
- Costanzo KS, Kesavaraju B, Juliano SA. Condition-specific competition in container mosquitoes: the role of non-competing life-history stages. Ecology. 2005;86:3289–3295. doi: 10.1890/05-0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig GB. The diaspora of the Asian tiger mosquito. In: McKnight B, editor. Biological pollution: the control and impact of invasive exotic species. Indiana Academy of Sciences; Indianapolis: 1993. pp. 101–120. [Google Scholar]
- Davis AJ, Jenkinson LS, Lawton JH, Shorrocks B, Wood S. Making mistakes when predicting shifts in species range in response to global warming. Nature. 1998;391:783–786. doi: 10.1038/35842. [DOI] [PubMed] [Google Scholar]
- Delatte H, Desvars A, Bou’etard A, et al. Blood-feeding behavior of Aedes albopictus, a vector of chikungunya on La Reunion. Vect-Bor Zoon Dis. 2010;10:249–58. doi: 10.1089/vbz.2009.0026. [DOI] [PubMed] [Google Scholar]
- Ding F, Fu J, Jiang L, Hao M. Mapping the spatial distribution of Aedes aegypti and Aedes albopictus. Acta Trop. 2018;178:155–162. doi: 10.1016/j.actatropica.2017.11.020. [DOI] [PubMed] [Google Scholar]
- Eisen L, Moore CG. Aedes (Stegomyia) aegypti in the continental United States: a vector at the cool margin of its geographic range. J Med Entomol. 2013;50:467–478. doi: 10.1603/me12245. [DOI] [PubMed] [Google Scholar]
- Elith J, Leathwick JR. Species distribution models: ecological explanation and prediction across space and time. Ann Rev Ecol Evol Syst. 2009;40:677–697. [Google Scholar]
- Elton CS. Animal ecology. The MacMillan Company; New York: 1927. [Google Scholar]
- Elton CS. The ecology of invasions of animals and plants. Methuen; London: 1958. [Google Scholar]
- Fontenille D, Rodhain F. Biology and distribution of Aedes albopictus and Aedes aegypti in Madagascar. J Am Mosq Cont Assoc. 1989;5:219–225. [PubMed] [Google Scholar]
- Gao Y, Reitz SR. Emerging themes in our understanding of species displacements. Ann Rev Entomol. 2017;62:165–83. doi: 10.1146/annurev-ento-031616-035425. [DOI] [PubMed] [Google Scholar]
- Gilotra SK, Rozeboom LE, Bhattacharya NC. Observations on possible competitive displacement between populations of Aedes aegypti Linnaeus and Aedes albopictus Skuse in Calcutta. Bull Wld Hlth Org. 1967;37:437–446. [PMC free article] [PubMed] [Google Scholar]
- Giller PS. Community structure and the niche. Chapman and Hall; London: 1984. [Google Scholar]
- Gröning J, Hochkirch A. Reproductive interference between animal species. Q Rev Biol. 2008;83:257–282. doi: 10.1086/590510. [DOI] [PubMed] [Google Scholar]
- Grover JP. Resource competition. Cambridge University Press; Cambridge: 1997. [Google Scholar]
- Grubaugh ND, Ladner JT, Kraemer MUG, et al. Genomic epidemiology reveals multiple introductions of Zika virus into the United States. Nature. 2017;546:401–409. doi: 10.1038/nature22400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn MB, Eisen RJ, Eisen L, et al. Reported distribution of Aedes (Stegomyia) aegypti and Aedes (Stegomyia) albopictus in the United States, 1995–2016 (Diptera: Culicidae) J Med Entomol. 2016 doi: 10.1093/jme/tjw072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper JP, Paulson SL. Reproductive isolation between Florida strains of Aedes aegypti and Aedes albopictus. J Am Mosq Cont Assoc. 1994;10:88–92. [PubMed] [Google Scholar]
- Hawley WA, Reiter P, Copeland RS, et al. Aedes albopictus in North America: probable introduction in used tires from Northern Asia. Science. 1987;236:1114–16. doi: 10.1126/science.3576225. [DOI] [PubMed] [Google Scholar]
- Higa Y, Thi Yen N, Kawada H, et al. Geographic distribution of Aedes aegypti and Aedes albopictus collected from used tires in Vietnam. J Am Mosq Cont Assoc. 2010;26:1–9. doi: 10.2987/09-5945.1. [DOI] [PubMed] [Google Scholar]
- Hobbs JH, Hughes EA, Eichold BH. Replacement of Aedes aegypti by Aedes albopictus in Mobile, Alabama. J Am Mosq Cont Assoc. 1991;7:488–89. [PubMed] [Google Scholar]
- Honório NA, Carrasquilla MC, Bargielowski I, et al. Male origin determines satyrization potential of Aedes aegypti by invasive Aedes albopictus. Biol Invas. 2017 doi: 10.1007/s10530-017-1565-3. [DOI] [Google Scholar]
- Hopperstad KA, Reiskind MH. Recent changes in the local distribution of Aedes aegypti L. (Diptera: Culicidae) in south Florida, USA. J Med Entomol. 2016;53:836–842. doi: 10.1093/jme/tjw050. [DOI] [PubMed] [Google Scholar]
- Hufbauer RA, Facon B, Ravigné V, et al. Anthropogenically induced adaptation to invade (AIAI): contemporary adaptation to human-altered habitats within the native range can promote invasions. Evol Appl. 2012;5:89–101. doi: 10.1111/j.1752-4571.2011.00211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson GE. Concluding remarks. Cold Spgs Har Symp Quan Biol. 1957;22:415–427. [Google Scholar]
- Hutchinson GE. An introduction to population ecology. Yale University Press; New Haven, CT: 1978. [Google Scholar]
- Ishak H, Miyagi I, Toma T, et al. Breeding habitats of Aedes aegypti (L.) and Aedes albopictus (Skuse) in village of Barru, South Sulawesi, Indonesia. SE Asia Trop Med Pub Hlth. 1997;28:844–850. [PubMed] [Google Scholar]
- Juliano SA. Species interactions among larval mosquitoes: Context dependence across habitat gradients. Ann Rev Entomol. 2009;54:37–56. doi: 10.1146/annurev.ento.54.110807.090611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano SA. Coexistence, exclusion, or neutrality? A meta-analysis of competition between Aedes albopictus and resident mosquitoes. Isr J Ecol Evol. 2010;56:325–351. doi: 10.1560/IJEE.55.3-4.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano SA, Lounibos LP. Invasions by mosquitoes: the roles of behaviour across the life cycle. In: Weis JS, Sol D, editors. Biological invasions and animal behaviour. Cambridge Univ Press; UK: 2016. pp. 245–265. [Google Scholar]
- Kamgang B, Ngoagouni C, Manirakiza A, Nakoune E, Paupy C, Kazanji M. Temporal patterns of abundance of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) and mitochondrial DNA analysis of Ae. albopictus in the Central African Republic. PLoS Negl Trop Dis. 2013;7(12):e2590. doi: 10.1371/journal.pntd.0002590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan L, Kendell D, Robertson D, Livdahl T, Khatchikian C. Aedes aegypti and Aedes albopictus in Bermuda: extinction, invasion and extinction. Biol Invas. 2010;12:3277–3288. [Google Scholar]
- Kearney M. Habitat, environment and niche: what are we modelling? Oikos. 2006;115:186–191. [Google Scholar]
- Kearney M, Porter W. Mechanistic niche modelling: combining physiological and spatial data to predict species’ ranges. Ecol Lett. 2009;12:334–350. doi: 10.1111/j.1461-0248.2008.01277.x. [DOI] [PubMed] [Google Scholar]
- Kearney M, Porter WP, Williams C, Ritchie S, Hoffmann AA. I ntegrating biophysical models and evolutionary theory to predict climatic impacts on species’ ranges: the dengue mosquito Aedes aegypti in Australia. Funct Ecol. 2009;23:528–538. [Google Scholar]
- Kishi S, Nakazawa T. Analysis of species coexistence co-mediated by resource competition and reproductive interference. Pop Ecol. 2013;55:305–313. [Google Scholar]
- Kraemer MUG, Sinka MA, Duka KA, et al. The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus. eLife. 2015;4:e08347. doi: 10.7554/eLife.08347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrecht FL. Notes on the ecology of Seychelles mosquitoes. Bull Entomol Res. 1971;60:513–532. [Google Scholar]
- Leathwick JR, Austin MP. Competitive interactions between tree species in New Zealand’s old-growth indigenous forests. Ecology. 2001;82:2560–2573. [Google Scholar]
- Lees RS, Gilles JR, Hendrichs J, Vreysen MJ, Bourtzis K. Back to the future: the sterile insect technique against mosquito disease vectors. Curr Opin Ins Sci. 2015;10:156–162. doi: 10.1016/j.cois.2015.05.011. [DOI] [PubMed] [Google Scholar]
- Le Goff G, Brengues C, Robert V. Stegomyia mosquitoes in Mayotte, taxonomic study and description of Stegomyia pia n. sp. Parasite. 2013 doi: 10.1051/parasite/2013030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leisnham PT, Juliano SA. Spatial and temporal patterns of coexistence between competing Aedes mosquitoes in urban Florida. Oecol. 2009;160:343–352. doi: 10.1007/s00442-009-1305-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leisnham PT, Juliano SA. Interpopulation differences in competitive effect and response of the mosquito Aedes aegypti and resistance to invasion of a superior competitor. Oecol. 2010;164:221–230. doi: 10.1007/s00442-010-1624-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leisnham PT, Lounibos LP, O’Meara GF, Juliano SA. Interpopulation divergence in competitive interactions of the mosquito Aedes albopictus. Ecology. 2009;90:2405–2413. doi: 10.1890/08-1569.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Kamara F, Zhou G, et al. Urbanization increases Aedes albopictus larval habitats and accelerates mosquito development and survivorship. PLoS Negl Trop Dis. 2014;8:e3301. doi: 10.1371/journal.pntd.0003301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lounibos LP. Habitat segregation in African treehole mosquitoes. Ecol Entomol. 1981;6:129–154. [Google Scholar]
- Lounibos LP. Invasions by insect vectors of disease. Ann Rev Entomol. 2002;47:233–266. doi: 10.1146/annurev.ento.47.091201.145206. [DOI] [PubMed] [Google Scholar]
- Lounibos LP, O’Meara GF, Juliano SA, Nishimura N, Escher RL, Reiskind MH, Cutwa M, Greene K. Differential survivorship of invasive mosquito species in south Florida cemeteries: do site-specific microclimates explain patterns of coexistence and exclusion? Ann Entomol Soc Am. 2010;103:757–770. doi: 10.1603/AN09142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lounibos LP, Bargielowski I, Carrasquilla MC, Nishimura N. Coexistence of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) in peninsular Florida two decades after competitive displacements. J Med Entomol. 2016 doi: 10.1093/jme/tjw122. [DOI] [PubMed] [Google Scholar]
- Lounibos LP, Nishimura N, Escher RL. Fitness of a treehole mosquito: influences of food type and predation. Oikos. 1993;66:114–118. [Google Scholar]
- MacArthur RH, Wilson EO. The theory of island biogeography. Princeton NJ: 1973. (Princeton Monographs in Population Biology). [Google Scholar]
- MacDougall AS, Gilbert B, Levine JM. Plant invasions and the niche. J Ecol. 2009;97:609–615. [Google Scholar]
- Manni M, Guglielmino CR, Scolari F, et al. Genetic evidence for a worldwide chaotic dispersion pattern of the arbovirus vector, Aedes albopictus. PLoS Negl Trop Dis. 2017;11(1):e0005332. doi: 10.1371/journal.pntd.0005332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattingly PF. Taxonomy of Aedes aegypti and related species. Bull Wld Hlth Org. 1967;36:552–554. [PMC free article] [PubMed] [Google Scholar]
- Mattingly PF, Brown ES. The mosquitos (Diptera, Culicidae) of the Seychelles. Bull Entomol Res. 1955;46:69–110. [Google Scholar]
- Medley KA. Niche shifts during the global invasion of the Asian tiger mosquito, Aedes albopictus Skuse (Culicidae), revealed by reciprocal distribution models. Glob Ecol Biog. 2010;19:122–133. [Google Scholar]
- Mekuria Y, Hyatt MG. Aedes albopictus in South Carolina. J Am Mosq Cont Assoc. 1995;11:468–70. [PubMed] [Google Scholar]
- Mitchell CE, Agrawal AA, Bever JD, Gilbert GS, Hufbauer RA, Klironomos JN, et al. Biotic interactions and plant invasions. Ecol Lett. 2006;9:726–740. doi: 10.1111/j.1461-0248.2006.00908.x. [DOI] [PubMed] [Google Scholar]
- Mogi M, Khamboonruang C, Choochote W, Suwanpanit P. Ovitrap surveys of dengue vector mosquitoes in Chiang Mai, northern Thailand—seasonal shifts in relative abundance of Aedes albopictus and Aedes aegypti. Med Vet Entomol. 1988;2:319–324. doi: 10.1111/j.1365-2915.1988.tb00203.x. [DOI] [PubMed] [Google Scholar]
- Monaghan AJ, Morin CW, Steinhoff DF, et al. On the seasonal occurrence and abundance of the Zika virus vector mosquito Aedes aegypti in the contiguous United States. PLoS Curr Outbr. 2016;16:8. doi: 10.1371/currents.outbreaks.50dfc7f46798675fc63e7d7da563da76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morlan HB, Tinker ME. Distribution of Aedes aegypti infestations in the United States. Am J Trop Med Hyg. 1965;14:892–899. doi: 10.4269/ajtmh.1965.14.892. [DOI] [PubMed] [Google Scholar]
- Murrell EG, Damal K, Lounibos LP, Juliano SA. Distributions of competing container mosquitoes depend on detritus types, nutrient ratios, and food availability. Ann Entomol Soc Am. 2011;104:688–698. doi: 10.1603/AN10158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrell EG, Juliano SA. Detritus type alters the outcome of interspecific competition between Aedes aegypti and Aedes albopictus (Diptera: Culicidae) J Med Entomol. 2008;45:375–383. doi: 10.1603/0022-2585(2008)45[375:dtatoo]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrell EG, Juliano SA. Competitive abilities in experimental microcosms are accurately predicted by a demographic index for R*. PLoS ONE. 2012;7(9):e43458. doi: 10.1371/journal.pone.0043458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasci RS, Hare CG, Willis FS. Interspecific mating between Louisiana strains of Aedes albopictus and Aedes aegypti in the field and the laboratory. J Am Mosq Cont Assoc. 1989;5:416–21. [PubMed] [Google Scholar]
- O’Meara GF, Evans LF, Gettman AD, Cuda JP. Spread of Aedes albopictus and decline of Ae. aegypti (Diptera:Culicidae) in Florida. J Med Entomol. 1995;32:554–562. doi: 10.1093/jmedent/32.4.554. [DOI] [PubMed] [Google Scholar]
- O’Neal PA, Juliano SA. Seasonal variation in competition and coexistence of Aedes mosquitoes: stabilizing effects of egg mortality or equalizing effects of resources? J Anim Ecol. 2013;82:256–265. doi: 10.1111/j.1365-2656.2012.02017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paupy C, Ollomo B, Kamgang B, et al. Comparative role of Aedes albopictus and Aedes aegypti in the emergence of dengue in Central Africa. Vec-Bor Zoon Dis. 2010;10:259–266. doi: 10.1089/vbz.2009.0005. [DOI] [PubMed] [Google Scholar]
- Pellissier L, Pradervand JN, Pottier J, Dubois A, Maiorano L, Guisan A. Climate-based empirical models show biased predictions of butterfly communities along environmental gradients. Ecography. 2012;35:1–9. [Google Scholar]
- Proestos Y, Christophides GK, Ergu¨ler K, Tanarhte M, Waldock J, Lelieveld J. Present and future projections of habitat suitability of the Asian tiger mosquito, a vector of viral pathogens, from global climate simulation. Phil Trans R Soc B. 2015;370:20130554. doi: 10.1098/rstb.2013.0554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raharimala FN, Ravaomanarivo LH, Ravelonandro P, et al. Biogeography of the major arbovirus mosquito vectors, Aedes aegypti and Aedes albopictus (Diptera: Culicidae), in Madagascar. Paras Vect. 2012;5:56. doi: 10.1186/1756-3305-5-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratsitorahina M, Harisoa J, Ratovonjato J, et al. Outbreak of dengue and Chikungunya fevers, Toamasina, Madagascar. Emerg Inf Dis. 2008;14:1135–1137. doi: 10.3201/eid1407.071521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiskind MH, Lounibos LP. Spatial and temporal patterns of abundance of Aedes aegypti L. (Stegomyia aegypti) and Aedes albopictus (Skuse) [Stegomyia albopictus (Skuse)] in southern Florida. Med Vet Entomol. 2013;27:421–429. doi: 10.1111/mve.12000. [DOI] [PubMed] [Google Scholar]
- Reiter P. Aedes albopictus and world trade in used tires, 1988–1995: the shape of things to come? J Am Mosq Cont Assoc. 1998;14:83–94. [PubMed] [Google Scholar]
- Reiter P, Lathrop S, Bunning M. Texas lifestyle limits transmission of dengue virus. Emer Inf Dis. 2003;9:86–89. doi: 10.3201/eid0901.020220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey JR, Nishimura N, Wagner B, Braks MA, O’Connell SM, Lounibos LP. Habitat segregation of mosquito arbovirus vectors in south Florida. J Med Entomol. 2006;43:1134–1141. doi: 10.1603/0022-2585(2006)43[1134:hsomav]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson DM, editor. Fifty years of invasion ecology: the legacy of Charles Elton. John Wiley & Sons; Oxford, UK: 2011. [Google Scholar]
- Ribeiro JMC. Can satyrs control pests and vectors? J Med Entomol. 1988;25:431–440. doi: 10.1093/jmedent/25.6.431. [DOI] [PubMed] [Google Scholar]
- Rochlin I, Ninivaggi DV, Hutchinson ML, Farajollahi A. Climate change and range expansion of the Asian tiger mosquito (Aedes albopictus) in northeastern USA: implications for public health practitioners. PLoS ONE. 2013;8(4):e60874. doi: 10.1371/journal.pone.0060874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudnick A. Studies of the ecology of dengue in Malaysia: a preliminary report. J Med Entomol. 1965;2:203–208. doi: 10.1093/jmedent/2.2.203. [DOI] [PubMed] [Google Scholar]
- Shea K, Chesson P. Community ecology theory as a framework for biological invasions. Trends Ecol Evol. 2002;17:170–176. [Google Scholar]
- Simard F, Nchoutpouen E, Toto C, Fontenille D. Geographic distribution and breeding site preference of Aedes albopictus and Aedes aegypti (Diptera: Culicidae) in Cameroon, Central Africa. J Med Entomol. 2005;42:726–731. doi: 10.1093/jmedent/42.5.726. [DOI] [PubMed] [Google Scholar]
- Smith CEG. The history of dengue in tropical Asia and its probable relationship to the mosquito Aedes aegypti. J Trop Med Hyg. 1956;59:243–251. [PubMed] [Google Scholar]
- Soberón J. Grinnellian and Eltonian niches and the geographic distributions of species. Ecol Lett. 2007;10:1115–1123. doi: 10.1111/j.1461-0248.2007.01107.x. [DOI] [PubMed] [Google Scholar]
- Sota T, Mogi M. Survival time and resistance to desiccation of diapause and non-diapause eggs of temperate Aedes (Stegomyia) mosquitoes. Entomol Exper Appl. 1992;63:155–161. [Google Scholar]
- Stanton AT. The mosquitos of far eastern ports with special reference to Stegomyia fasciata. F Bull Entomol Res. 1920;10:333–344. [Google Scholar]
- Tabachnick WJ. Evolutionary genetics and arthropod-borne disease. The yellow fever mosquito. Am Entomol. 1991;37:14–24. [Google Scholar]
- Thomas SM, Fischer D, Fleischmann S, Bittner T, Beierkuhnlein C. Risk assessment of dengue virus amplification in Europe based on spatial-temporal high resolution climate change projections. Erkunde. 2011;65:137–150. [Google Scholar]
- Tilman D. Resource competition and community structure. Princeton University Press; Princeton NJ, USA: 1982. [PubMed] [Google Scholar]
- Tripet F, Lounibos LP, Robbins D, Moran J, Nishimura N, Blosser EM. Competitive reduction by satyrization? Evidence for interspecific mating in nature and asymmetric reproductive competition between invasive mosquito vectors. Am J Trop Med Hyg. 2011;85:265–270. doi: 10.4269/ajtmh.2011.10-0677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda Y, Suwonkerd W, Chawprom S, et al. Different spatial distribution of Aedes aegypti and Aedes albopictus along an urban-rural gradient and the relating environmental factors examined in three villages in northern Thailand. J Am Mosq Cont Assoc. 2006;22:222–228. doi: 10.2987/8756-971X(2006)22[222:DSDOAA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Urbanski J, Mogi M, O’Donnell D, et al. Rapid adaptive evolution of photoperiodic response during Invasion and range expansion across a climatic gradient. Am Nat. 2012;179:490–500. doi: 10.1086/664709. [DOI] [PubMed] [Google Scholar]
- Vázquez DP. Exploring the relationship between niche breadth and invasion success. In: Cadotte MW, McMahon SM, Fukami T, editors. Conceptual ecology and invasion biology: reciprocal approaches. Springer; Dordrecht, Netherlands: 2006. pp. 307–322. [Google Scholar]
- Vazeille M, Moutailler S, Coudrier D, et al. Two chikungunya isolates from the outbreak of La Reunion (Indian Ocean) exhibit different patterns of infection in the mosquito Aedes albopictus. PLoS One. 2007;2:e1168. doi: 10.1371/journal.pone.0001168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vélez ID, Quiñones M, Suárez M, et al. Presencia de Aedes albopictus en Leticia, Amazonas, Colombia. Biomédica. 1998;18:192–198. [Google Scholar]
- Walsh RK, Aguilar CL, Facchinelli L, Valerio L, Ramsey JM, Scott TW, Lloyd AL, Gould F. Regulation of Aedes aegypti population dynamics in field systems: quantifying direct and delayed density dependence. Am J Trop Med Hyg. 2013;89:68–77. doi: 10.4269/ajtmh.12-0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh RK, Bradley C, Apperson CS, Gould F. An experimental field study of delayed density dependence in natural populations of Aedes albopictus. PLoS One. 2012;7:3–8. doi: 10.1371/journal.pone.0035959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh RK, Facchinelli L, Ramsey JM, Bond JG, Gould F. Assessing the impact of density dependence in field populations of Aedes aegypti. J Vect Ecol. 2011;36:300–307. doi: 10.1111/j.1948-7134.2011.00170.x. [DOI] [PubMed] [Google Scholar]
- Whittaker RH, Levin SA, Root RB. Niche, habitat, and ecotope. Am Nat. 1973;107:321–338. [Google Scholar]
- Winchester JC, Kapan DD. History of Aedes mosquitoes in Hawaii. J Am Mosq Cont Assoc. 2013;29:154–163. doi: 10.2987/12-6292R.1. [DOI] [PubMed] [Google Scholar]
- Wisz MS, Pottier J, Kissling WD, et al. The role of biotic interactions in shaping distributions and realised assemblages of species: implications for species distribution modelling. Biol Rev. 2013;88:15–30. doi: 10.1111/j.1469-185X.2012.00235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J-Y, Lun J-R, James AA, et al. Review: dengue fever in mainland China. Am J Trop Med Hyg. 2010;83:664–671. doi: 10.4269/ajtmh.2010.09-0755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CF, Hou JN, Chen TH, Chen WJ. Discriminable roles of Aedes aegypti and Aedes albopictus in establishment of dengue outbreaks in Taiwan. Acta Trop. 2014;130:17–23. doi: 10.1016/j.actatropica.2013.10.013. [DOI] [PubMed] [Google Scholar]
- Yee DA, Kaufman MG, Juliano SA. The significance of ratios of detritus types and microorganism productivity to competitive interactions between aquatic insect detritivores. J Anim Ecol. 2007;76:1105–1115. doi: 10.1111/j.1365-2656.2007.01297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


