Abstract
Recent outbreaks of highly pathogenic avian influenza A virus infections (including those of the H5N1 subtype) in poultry and in humans (through contact with infected birds) have raised concerns that a new influenza pandemic will soon occur. Effective vaccines against H5N1 virus are, therefore, urgently needed. Reverse genetics-based inactivated vaccines have been prepared according to WHO recommendations and licensed in several countries following their assessment in clinical trials. However, the effectiveness of these vaccines in a pandemic is not guaranteed. We must, therefore, continue to develop alternative pandemic vaccine strategies. Here, we review the current strategies for the development of H5N1 influenza vaccines, as well as some future directions for vaccine development.
1. Introduction
Only type A influenza virus exhibits pandemic potential due to antigenic variation. Currently, 16 hemagglutinin (HA) and 9 neuraminidase (NA) subtypes have been identified among type A viruses (Wright et al. 2007). Three influenza pandemics emerged during the 20th century, the most devastating of which was the Spanish influenza, which was caused by an H1N1 virus and was responsible for the deaths of at least 40 million people in 1918–1919 (Johnson and Mueller 2002). Sequence information from resurrected lung tissue samples suggest that the 1918 virus was derived from “an unusual avian precursor” (Reid et al. 2004). The HA of the 1918 virus retained the residues in the host-receptor-binding site that are characteristic of an avian precursor HA, but could bind human cell-surface receptors containing α2,6-linked sialic acid (Gamblin et al. 2004; Stevens et al. 2004). Reverse genetics studies, including the reconstitution of the 1918 virus itself and experimental infection of macaques with the reconstituted virus, suggest that, unlike other human influenza, the 1918 virus induced dysregulation of the antiviral response, causing acute respiratory distress and a fatal outcome in the non-human primate model (Kobasa et al. 2007). The other two, less serious pandemics of the 20th century occurred in 1957 (Asian influenza [H2N2]), and 1968 (Hong Kong influenza [H3N2]) (Wright et al. 2007). The 1957 virus consisted of HA (H2), NA (N2), and PB1 gene segments from an avian virus, with the other gene segments derived from a previously circulating human virus. The 1968 virus had avian HA (H3) and PB1 segments in a background of human viral genes. The acquisition of novel surface antigens allowed these viruses to circumvent the human immune response, resulting in these pandemics. The HAs of these two pandemic strains also bind preferentially to human-type receptors, although they originated from avian viruses (Matrosovich et al. 2000). Thus, for a virus to become a pandemic strain, it appears to require a novel HA subtype, to which humans are immunologically naïve, that efficiently binds to human-type receptors. It must also possess internal proteins to promote efficient growth in human upper respiratory cells and thereby facilitate its human-to-human transmission (Horimoto and Kawaoka 2005).
Vaccination is considered one of the most effective preventive measures for the control of influenza pandemics. Recent direct transmissions of avian viruses to humans suggest that avian viruses of HA subtypes other than H1 and H3 have pandemic potential, emphasizing the need for vaccines against these viruses. In particular, the widespread circulation of H5N1 viruses has focused current research on the development of H5N1 vaccines.
2. Developing H5N1 vaccines for humans
Although antivirals against H5N1 influenza viruses such as NA inhibitors (oseltamivir and zanamivir) may be effective for pandemic control, the possible emergence of drug-resistant viruses highlights the need for vaccination (Le et al. 2005; Gupta et al. 2006). Vaccination is considered the most effective preventive measure to combat an influenza pandemic. Currently, inactivated vaccines are typically used for influenza prophylaxis. They are usually prepared from virus that is grown in chicken embryonated eggs, purified from the allantoic fluids of the inoculated eggs, and inactivated with formaldehyde or β-propiolactone for “whole virus” vaccine formulation. Alternatively, the purified virus is treated with detergent for “split” or “subunit” vaccine formulation. These inactivated vaccines are then inoculated intramuscularly or subcutaneously into individuals. However, the high pathogenicity of the currently circulating H5N1 viruses presents difficulties for this type of vaccine preparation. Highly pathogenic avian influenza (HPAI) H5N1 viruses cannot be used as seed viruses for inactivated vaccine production, because their virulence threatens the lives of vaccine producers and it is difficult to obtain high-quality allantoic fluid with acceptable virus titers from embryonated eggs.
2.1 The conventional approach
Seed viruses for inactivated vaccines must be antigenically similar to the circulating viruses and grow efficiently in eggs. Faced with a pandemic thread posed by the Hong Kong H5N1 outbreak in 1997, the low pathogenic avian influenza (LPAI) virus A/duck/Singapore/F119-3/97 (H5N3) was selected as a vaccine seed virus. Vaccine prepared with this virus was assessed in a randomized phase I clinical trial (Nicholson et al 2001; Stephenson et al 2004), the results of which showed that although antibody responses indicative of protection were achieved by administration of the vaccine with the oil-in-water MF59™ adjuvant, this strain was not suitable for large-scale vaccine production due to its inefficient growth in eggs. However, this adjuvanted vaccine candidate induced cross-reactive neutralizing antibody responses in humans to heterologous H5N1 viruses, including 2004 isolates (Stephenson et al. 2005), demonstrating its potential for use until an antigenically matched vaccine becomes available. Since no such antigenically matched natural avirulent isolates have been found for recent H5N1 viruses, an alternative approach is needed to produce safe vaccine seed viruses to protect humans from this virus infection.
2.2 The practical approach
New vaccines are being developed that exploit reverse genetics technology (Neumann et al. 1999; Fodor et al. 1999) and the knowledge that the pathogenicity of avian influenza viruses is primarily determined by HA cleavability (Kawaoka and Webster 1988; Horimoto and Kawaoka 1994). Conversion of the HA cleavage site sequence of HPAI viruses to that of avian influenza with low pathogenicity (LPAI) viruses attenuates virulence but does not affect antigenicity. Several researchers have used reverse genetics to produce candidate H5N1 vaccine strains, whose HA and NA were derived from a human H5N1 virus and the remainder of their genes from a virus (termed a backbone virus) that grows well in eggs (Takada et al. 1999; Subbarao et al. 2003; Webby et al. 2004; Wood and Robertson 2004; Lipatov et al. 2005; Horimoto et al. 2006; Govorkova et al. 2006). For these vaccine strains, the HA cleavage site sequence was modified from virulent- to avirulent-type sequences. The WHO has recommended the use of A/Puerto Rico/8/34 (H1N1; PR8) as a backbone virus. PR8, originally a human isolate, has been passaged extensively in eggs and proven to be attenuated for humans. Indeed, the PR8 backbone has been used to produce annual vaccines against human H1N1 and H3N2 virus infections. Several high-growth reassortant viruses (PR8/H5N1 6:2 reassortant virus) have been developed by reverse genetics, including the NIBRG-14 reference strain (produced by the National Institute for Biological Standards and Control, UK). Following extensive clinical testing, these viruses have now been licensed as vaccine seed viruses for inactivated vaccine in several countries (Treanor et al. 2006; Bresson et al. 2006). H5N1 inactivated vaccines require adjuvants in addition to the “whole virus” formulation for adequate immunogenicity in humans, unlike seasonal vaccines. To compensate for the low immunogenicity of H5N1 vaccines in humans, antigenic matching between vaccine seeds and circulating strains should be considered. A panel of vaccine seed viruses with antigenic variations that reflect the genetic diversity of H5N1 viruses is required.
2.3 A promising approach
2.3.1 A high-growth seed for an egg-based vaccine
To increase the total number of vaccine doses from the limited production capacity of current vaccine manufacturers, seed viruses with high growth properties in eggs are required. Given that the NIBRG-14 seed for H5N1 inactivated vaccine grows less efficiently in eggs than the seeds use for seasonal vaccines, the selection of other seeds with higher growth potential is a germane strategy for H5N1 vaccine production and stockpiling. The PR8(UW) strain maintained in our laboratory is a superior donor virus for H5N1 vaccine production than is the PR8(Cambridge) used to produce the NIBRG-14 seed virus, with respect to in ovo growth (Horimoto et al. 2007). PR8 strains differ in their growth properties depending on their passage histories; PR8(UW) may be more highly adapted in eggs than PR8(Cambridge). The high growth property in eggs of PR8(UW) was determined via several mutations in polymerases and NP.
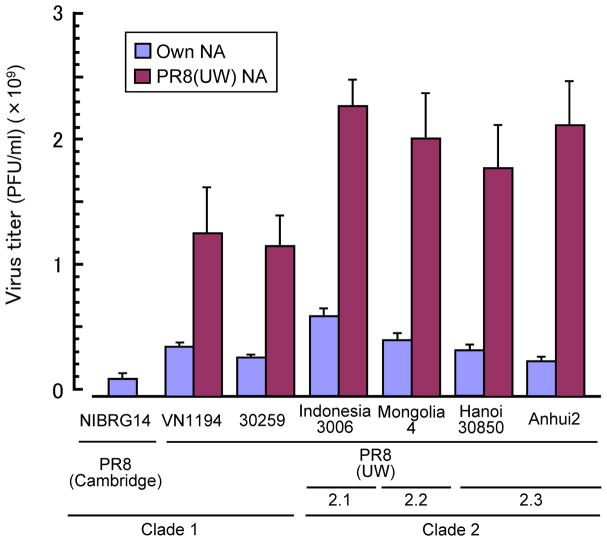
Inclusion of an alterative NA protein in PR8(UW) further enhances its growth in eggs. The HA-NA functional balance affects the growth in eggs of influenza viruses (Castrucci and Kawaoka 1993) and of seed strains for influenza seasonal vaccines (Lu et al. 2005). We found that HA-NA functional balance also determines the in ovo growth of H5N1 vaccine seed viruses; 7:1 reassortant viruses containing only modified HA from H5N1 viruses (and PR8 NA) grow significantly better than standard 6:2 reassortant viruses (Horimoto et al. 2007) (Figure 1). One might argue that reassortants that lack NA from an H5N1 isolate would induce a less protective immune response than recombinant viruses with H5N1 NA, because of antigenic differences in these proteins (even though the NA of PR8 is of the N1 subtype). However, since HA is the major protective antigen in inactivated vaccines, the enhanced growth potential conferred by the PR8 NA should offset the limited antigenic mismatch in this minor protective antigen. We propose that, in addition to the 6:2 reassortant viruses recommended by the WHO, 7:1 reassortant viruses (containing only a modified H5 derived from circulating strains) in the background of the PR8(UW) strain should be considered as vaccine seeds for H5N1 inactivated vaccine production. This approach would increase the available doses of pre-pandemic or pandemic H5N1 vaccines in a timely, cost-efficient manner.
Figure 1.
Growth enhancement of vaccine seed viruses in chicken embryonated eggs. PR8/H5N1 6:2 reassortants were prepared using H5N1 viruses of different clades with PR8(UW) donor virus; A/Vietnam/1194/04 (clade 1), A/Vietnam/30259 (clade 1), A/Indonesia/3006/05 (clade 2.1), A/whooper swan/Mongolia/4/05 (clade 2.2), A/Vietnam/30850/05 (clade 2.3), and A/Anhui/2/05 (clade 2.3). All reassortants replicated significantly better in eggs than did the reference seed NIBRG-14 with PR8(Cambridge). PR8/H5 7:1 reassortants containing PR8(UW) NA and HA from H5N1 viruses of different clades replicated significantly better than the corresponding PR8/H5N1 6:2 reassortants. Virus titers of the allantoic fluids were determined 48 hours post-inoculation after incubation at 33°C.
2.3.2 Cell culture-based vaccines
Given that chicken embryonated eggs, which are currently used for inactivated vaccine production, would be in a short supply during a pandemic, the development of cell culture-based H5 vaccines is an attractive alternative approach. In fact, inactivated influenza vaccines produced with Madin-Darby canine kidney (MDCK) and African green monkey Vero cells have been licensed in The Netherlands (Medema et al. 2006). Important considerations for this approach include the selection of background viruses that grow well in these cell cultures and monitoring for antigenic changes during propagation of the virus in cell culture. The safety of the vaccine products for human use is also important with respect to tumorigenicity.
We found that our PR8(UW) strain also supports better growth in MDCK cells than does the PR8(Cambridge) strain, due to its enhanced polymerase activity in this cell line. Interestingly, the NS gene of PR8(Cambridge) possesses higher interferon-antagonized activity in MDCK cells compared with that of PR8(UW). Accordingly, we believe that a chimeric PR8 construct whose NS gene is derived from the Cambridge strain and its remaining five internal genes from the UW strain would be the optimal donor for H5N1 vaccine production in MDCK cells. In addition, we found that inclusion of an HK213 (A/Hong Kong/213/2003) NA, whose stalk region does not have the deletion observed in most other H5N1 virus NAs, enhances the viral titers of 6:2 reassortants, indicating that the HA-NA functional balance is a determinant for viral growth in MDCK cells, as it is in eggs (Murakami et al. unpublished).
2.3.3 Live attenuated vaccines
To overcome the potential low immunogenicity of H5 inactivated vaccines for humans, live H5N1 attenuated vaccines whose HA has been altered to a nonpathogenic form have been developed. These vaccines are based on the recently licensed product FluMist®, possess a cold-adapted backbone virus, and are nonpathogenic in mammalian and chicken models (Li et al. 1999; Suguitan et al. 2006). Live influenza vaccines elicit systemic and local mucosal immune responses that include stimulating secretory IgA (sIgA) in the respiratory tract, a portal for the virus. They also elicit cellular immunity, which may provide better protection than that afforded by inactivated vaccines (Beyer et al. 2002). Live attenuated vaccines may also offer wider protection by protecting against viruses that have undergone antigenic drift. However, live H5N1 vaccines will not be used until the H5N1 virus has become widespread among humans, so as not to introduce new influenza viral HA and NA genes into the human population. In addition, they may not be used for the major high-risk groups of infants and the elderly due to safety considerations, as is the case with FluMist®. The use of live influenza vaccines for a pandemic is also currently limited by production capacity.
2.3.4 Mucosal inactivated vaccines
The primary target tissue of influenza virus infection is the respiratory mucosa. Therefore, virus-specific sIgA antibody induced in this organ by the mucosal immune system would efficiently protect against virus infection. sIgA antibody also exhibits cross-protection to antigenically drifted viruses, since its response to subsequent infection is driven by nonantigen-specific bystander help (Sangster et al. 2003). In this context, the protective efficacy of the licensed parenteral H5N1 inactivated vaccines, which induce mainly serum IgG antibodies, may be less satisfactory in pandemic vaccines.
Mucosal inactivated vaccines can induce sIgA antibody in respiratory organs and are safer for the vaccinees compared with live vaccines. Therefore, they can be used for people of all ages, including high-risk patients. In clinical trials with seasonal vaccine both inactivated whole-virus particles and split vaccines are effective in preventing live virus infection when administrated intranasally; although stronger immunogenicity is seen with the whole-virus vaccine, probably due to the stimulation of innate immunity by single-strand RNA via toll-like receptor (TLR)-7 (Hasegawa et al. 2007).
To enhance the immunogenicity of nasal inactivated vaccines, mucosal adjuvants should be combined with these vaccines. Commercially available double-strand RNA, poly(I:C12U) (Ampligen®) is effective as an adjuvant for H5N1 nasal inactivated vaccines. In addition, NKT cell-specific glycolipid ligand (α-galactosylceramide), bacterial toxin-derived forms of cholera toxin B subunits (CTB) and Escherichia coli heat-labile enterotoxin (LT), as well as physiological complement component C3d all possess adjuvant effects with nasal vaccines, although neurological side effects were associated with toxin-derived LT in clinical use (Hasegawa et al. 2007).
The sublingual mucosal route is an attractive alternative to mucosal immunization routes for administering inactivated vaccines (Song et al. 2008). Studies in a mouse model revealed appreciably high levels of virus-specific IgG in serum and sIgA antibodies in mucosal secretions even in the absence of adjuvants. Co-administration of a toxin-derived mucosal adjuvant enhanced these immune responses.
2.4. An improved method for reverse genetics
Currently, pre-pandemic H5N1 vaccines are being stockpiled in many countries. These inactivated vaccines were produced from viruses propagated in chicken embryonated eggs following inoculation of the vaccine seed virus, generated by reverse genetics in an African green monkey Vero cell line that is approved for human vaccine production (Nicolson et al. 2005). However, the generation of the H5N1 vaccine seed viruses in Vero cells is not optimal due to the low plasmid transfection efficiency of these cells for reverse genetics. In a pandemic situation, vaccines whose antigenicities match the circulating strain(s) need to be rapidly produced. Therefore, a more robust reverse genetics system is desirable for pandemic vaccine preparedness. Although 12- or 8-plasmid reverse genetics systems may prove useful for the production of pandemic and interpandemic vaccines, the possibility exists for the transfection efficiency of sets of plasmids to be so low as to impede the rapid and robust generation of vaccine seed viruses. To overcome this possibility, we have reduced the number of plasmids required to generate virus by reverse genetics (Figure 2) (Neumann et al. 2005). In this system, one plasmid synthesizes the six gene segments for the internal proteins (PB2, PB1, PA, NP, M, and NS), and a second plasmid synthesize the HA and NA segments. Two viral protein-expressing plasmids (one expressing NP and the other expressing PB2, PB1, and PA) complete this system. Thus, only four plasmids are transfected into Vero cells, which results in the generation of virus with significantly higher efficiency than that achieved with traditional 12-plasmid systems. This 4-plasmid system could, therefore, be valuable in the future generation of pandemic vaccine seed viruses.
Figure 2.
Schematic diagram of a 4-plasmid-based reverse genetic approach to generate vaccine seed viruses. A Pol I plasmid that synthesizes modified HA and NA gene segments is prepared from the circulating wild-type strains. Another Pol I plasmid that synthesizes the other six gene segments is prepared from a backbone virus that grows well in eggs or in cell culture. Two plasmids, expressing polymerase proteins or NP, respectively, are also prepared. Thus, a total of 4 plasmids are used to generate the vaccine seed viruses, with higher efficiency than other current systems, which can contain more than 8 plasmids.
Besides Vero cells, a limited number of other cells are approved for human vaccine production, for example, MDCK cells and chicken embryonic fibroblasts (CEF). A modified reverse genetics system that uses the chicken RNA polymerase I (PolI) promoter also supports the generation of influenza virus in CEF (Massin et al. 2005), with an efficiency of virus generation comparable to that of the human PolI system in Vero cells. MDCK cells also support the efficient growth of influenza virus and are used as a substrate for the production of seasonal influenza vaccines (Brands et al. 1999, Govorkova et al. 1999, Halperin et al. 2002). In MDCK cells, however, reverse genetics with the human PolI promoter does not work well, due to the host specificity of the PolI promoter. Recently, another reverse genetics system with T7 RNA polymerase II was shown to support influenza virus generation in MDCK cells (de Wit et al. 2007), although the efficiency of virus generation was inconsistent. We and others also established an alternative reverse genetics system driven by canine PolI (Murakami et al. 2008, Wang and Duke 2007) and generated recommended H5N1 vaccine seed viruses in MDCK cells with high efficiency.
3. Concluding remarks
Research on H5N1 vaccine development has revealed that in clinical trials, the immune responses in humans to H5N1 inactivated vaccines are lower than those to annual vaccines, and therefore multiple doses of the vaccines with adjuvants would be required. Reverse genetics-based H5N1 inactivated vaccines with adjuvants have been licensed in several countries; however, whether these vaccines will be effective against antigenically different strains in humans is unknown. Thus, H5N1 vaccine libraries that reflect the different antigenicities of currently circulating strains are needed as we cannot predict which strain will cause a pandemic. Furthermore, the adverse effects of adjuvants might also become apparent upon large-scale vaccination.
In the event of a pandemic caused by an HPAI virus, chicken eggs will likely be in short supply. Under such conditions, a reassortant vaccine seed virus with higher growth properties in eggs than the current seed strains is needed to produce sufficient vaccine. For this reason, we propose the reselection of a background virus for the production of seed viruses. As an alternative approach, cell-culture-based vaccines are currently being developed. Such egg-free vaccines may also be useful for those who have egg allergies.
Another consideration is the development of vaccines against influenza viruses of other subtypes, such as H2N2, H9N2 and H7N7 viruses, which also possess pandemic potential (Hehme et al. 2002, de Wit et al. 2005, Stephenson et al. 2003). Essentially, the same strategies as those for H5N1 vaccines can be employed for the development of vaccines to these virus subtypes.
Lastly, there may be concerns regarding production capacity and global accessibility of vaccines, manufacturing costs, and the cooperation of international governments, which must be addressed. It is also essential that we continue to promote alterative approaches to the development of cross-reactive and long-lasting pandemic vaccines that are egg- and adjuvant-independent, although it will likely take years to achieve this objective.
Acknowledgments
We thank Susan Watson for editing the manuscript. We also thank those in our laboratories who contributed to the data cited in this review. Our original research was supported by National Institute of Allergy and Infectious Diseases Public Health Service research grants; by CREST (Japan Science and Technology Agency); by Grants-in-Aid for Specially Promoted Research and for Scientific Research (B); by a Contract Research Fund for Program of Founding Research Centers for Emerging and Reemerging Infectious Diseases; and by the Special Coordination Funds for Promoting Science and Technology from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
References
- Beyer WEP, Palache AM, de Jong JC, Osterhaus ADME. Cold-adapted live influenza vaccine versus inactivated vaccine: systemic vaccine reactions, local and systemic antibody response, and vaccine efficacy. A meta-analysis. Vaccine. 2002;20:1340–1353. doi: 10.1016/s0264-410x(01)00471-6. [DOI] [PubMed] [Google Scholar]
- Brands R, Visser J, Medema J, Palache AM, van Scharrenburg GJ. Influvac: a safe Madin Darby Canine Kidney (MDCK) cell culture-based influenza vaccine. Dev Biol Stand. 1999;98:93–100. discussion 111. [PubMed] [Google Scholar]
- Bresson JL, Perronne C, Launay O, Gerdil C, Saville M, Wood J, Höschler K, Zambon MC. Safety and immunogenicity of an inactivated split-virion influenza A/Vietnam/1194/2004 (H5N1) vaccine: phase I randomised trial. Lancet. 2006;367:1657–1664. doi: 10.1016/S0140-6736(06)68656-X. [DOI] [PubMed] [Google Scholar]
- Castrucci MR, Kawaoka Y. Biological importance of neuraminidase stalk length in influenza A virus. J Virol. 1993;67:759–764. doi: 10.1128/jvi.67.2.759-764.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit E, Munster VJ, Spronken MI, Bestebroer TM, Baas C, Beyer WE, Rimmelzwaan GF, Osterhaus AD, Fouchier RA. Protection of mice against lethal infection with highly pathogenic H7N7 influenza A virus by using a recombinant low-pathogenicity vaccine strain. J Virol. 2005;79:12401–12407. doi: 10.1128/JVI.79.19.12401-12407.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit E, Spronken MI, Vervaet G, Rimmelzwaan GF, Osterhaus AD, Fouchier RA. A reverse-genetics system for Influenza A virus using T7 RNA polymerase. J Gen Virol. 2007;88:1281–1287. doi: 10.1099/vir.0.82452-0. [DOI] [PubMed] [Google Scholar]
- Fodor E, Devenish L, Engelhardt OG, Palese P, Brownlee GG, Garcia-Sastre A. Rescue of influenza A virus from recombinant DNA. J Virol. 1999;73:9679–8296. doi: 10.1128/jvi.73.11.9679-9682.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamblin SJ, Haire LF, Russell RJ, Stevens DJ, Xiao B, Ha Y, Vasisht N, Steinhauer DA, Daniels RS, Elliot A, Wiley DC, Skehel JJ. The structure and receptor binding properties of the 1918 influenza hemagglutinin. Science. 2004;303:1838–1842. doi: 10.1126/science.1093155. [DOI] [PubMed] [Google Scholar]
- Govorkova EA, Webby RJ, Humberd J, Seiler JP, Webster RG. Immunization with reverse-genetics-produced H5N1 influenza vaccine protects ferrets against homologous and heterologous challenge. J Infect Dis. 2006;194:159–167. doi: 10.1086/505225. [DOI] [PubMed] [Google Scholar]
- Govorkova EA, Kodihalli S, Alymova IV, Fanget B, Webster RG. Growth and immunogenicity of influenza viruses cultivated in Vero or MDCK cells and in embryonated chicken eggs. Dev Biol Stand. 1999;98:39–51. discussion 73–74. [PubMed] [Google Scholar]
- Gupta RK, Nguyen-Van-Tam JS. Oseltamivir resistance in influenza A (H5N1) infection. N Eng J Med. 2006;354:1423–1424. doi: 10.1056/NEJMc060077. [DOI] [PubMed] [Google Scholar]
- Halperin SA, Smith B, Mabrouk T, Germain M, Trepanier P, Hassell T, Treanor J, Gauthier R, Mills EL. Safety and immunogenicity of a trivalent, inactivated, mammalian cell culture-derived influenza vaccine in healthy adults, seniors, and children. Vaccine. 2002;20:1240–1247. doi: 10.1016/s0264-410x(01)00428-5. [DOI] [PubMed] [Google Scholar]
- Hasegawa H, Ichinohe T, Tamura S, Kurata T. Development of a mucosal vaccine for influenza viruses: preparation for a potential influenza pandemic. Exp Rev Vaccines. 2007;6:193–201. doi: 10.1586/14760584.6.2.193. [DOI] [PubMed] [Google Scholar]
- Hehme N, Engelmann H, Künzel W, Neumeier E, Sänger R. Pandemic preparedness: lessons learnt from H2N2 and H9N2 candidate vaccines. Med Microbiol Immunol. 2002;191:203–208. doi: 10.1007/s00430-002-0147-9. [DOI] [PubMed] [Google Scholar]
- Horimoto T, Kawaoka Y. Influenza: lessons from past pandemics, warnings from current incidents. Nat Rev Microbiol. 2005;3:591–600. doi: 10.1038/nrmicro1208. [DOI] [PubMed] [Google Scholar]
- Horimoto T, Kawaoka Y. Reverse genetics provides direct evidence for a correlation of hemagglutinin cleavability and virulence of an avian influenza A virus. J Virol. 1994;68:3120–3128. doi: 10.1128/jvi.68.5.3120-3128.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horimoto T, Murakami S, Muramoto Y, Yamada S, Fujii K, Kiso M, Iwatsuki-Horimoto K, Kino Y, Kawaoka Y. Enhanced growth of seed viruses for H5N1 influenza vaccines. Virology. 2007;366:23–27. doi: 10.1016/j.virol.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horimoto T, Takada A, Fujii K, Goto H, Hatta M, Watanabe S, Iwatsuki-Horimoto K, Ito M, Tagawa-Sakai Y, Yamada S, Ito H, Ito T, Imai M, Itamura S, Odagiri T, Tashiro M, Lim W, Guan Y, Peiris M, Kawaoka Y. The development and characterization of H5 influenza virus vaccines derived from a 2003 human isolate. Vaccine. 2006;24:3669–3676. doi: 10.1016/j.vaccine.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Johnson NP, Mueller J. Updating the accounts: global mortality of the 1918–1920 “Spanish” influenza pandemic. Bull Hist Med. 2002;76:105–115. doi: 10.1353/bhm.2002.0022. [DOI] [PubMed] [Google Scholar]
- Kawaoka Y, Webster RG. Sequence requirement for cleavage activation of influenza virus hemagglutinin expressed in mammalian cells. Proc Natl Acad Sci USA. 1988;85:324–328. doi: 10.1073/pnas.85.2.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobasa D, Jones SM, Shinya K, Kash JC, Copps J, Ebihara H, Hatta Y, Kim JH, Halfmann P, Hatta M, Feldmann F, Alimonti JB, Fernando L, Li Y, Katze MG, Feldmann H, Kawaoka Y. Aberrant innate immune response in lethal infection of macaques with the 1918 influenza virus. Nature. 2007;445:319–323. doi: 10.1038/nature05495. [DOI] [PubMed] [Google Scholar]
- Le QM, Kiso M, Someya K, Sakai YT, Nguyen TH, Nguyen KH, Pham ND, Ngyen HH, Yamada S, Muramoto Y, Horimoto T, Takada A, Goto H, Suzuki T, Suzuki Y, Kawaoka Y. Avian flu: isolation of drug-resistant H5N1 virus. Nature. 2005;437:1108. doi: 10.1038/4371108a. [DOI] [PubMed] [Google Scholar]
- Li S, Liu C, Klimov A, Subbarao K, Perdue ML, Mo D, Ji Y, Woods L, Hietala S, Bryant M. Recombinant influenza A virus vaccines for the pathogenic human A/Hong Kong/97 (H5N1) viruses. J Inf Dis. 1999;179:1132–1138. doi: 10.1086/314713. [DOI] [PubMed] [Google Scholar]
- Lipatov AS, Webby RJ, Govorkova EA, Krauss S, Webster RG. Efficacy of H5 influenza vaccines produced by reverse genetics in a lethal mouse model. J Inf Dis. 2005;191:1210–1215. doi: 10.1086/428951. [DOI] [PubMed] [Google Scholar]
- Lu B, Zhou H, Ye D, Kemble G, Jin H. Improvement of influenza A/Fujian/411/02 (H3N2) virus growth in embryonated chicken eggs by balancing the hemagglutinin and neuraminidase activities, using reverse genetics. J Virol. 2005;79:6763–6771. doi: 10.1128/JVI.79.11.6763-6771.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massin P, Rodrigues P, Marasescu M, van der Werf S, Naffakh N. Cloning of the chicken RNA polymerase I promoter and use for reverse genetics of influenza A viruses in avian cells. J Virol. 2005;79:13811–13816. doi: 10.1128/JVI.79.21.13811-13816.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matrosovich M, Tuzikov A, Bovin N, Gambaryan A, Klimov A, Castrucci MR, Donatelli I, Kawaoka Y. Early alterations of the receptor-binding properties of H1, H2, and H3 avian influenza virus hemagglutinins after their introduction into mammals. J Virol. 2000;74:8502–8512. doi: 10.1128/jvi.74.18.8502-8512.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medema JK, Meijer J, Kersten AJ, Horton R. Safety assessment of Madin Darby canine kidney cells as vaccine substrate. Dev Biol (Basel) 2006;123:243–250. [PubMed] [Google Scholar]
- Murakami S, Horimoto T, Yamada S, Kakugawa S, Goto H, Kawaoka Y. Establishment of canine RNA polymerase I-driven reverse genetics for influenza A virus: its application for H5N1 vaccine production. J Virol. 2008;82:1605–1609. doi: 10.1128/JVI.01876-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann G, Fujii K, Kino Y, Kawaoka Y. An improved reverse genetics system for influenza A virus generation and its implications for vaccine production. Proc Natl Acad Sci USA. 2005;102:16825–16829. doi: 10.1073/pnas.0505587102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann G, Watanabe T, Ito H, Watanabe S, Goto H, Gao P, Hughes M, Perez DR, Donis R, Hoffmann E, Hobom G, Kawaoka Y. Generation of influenza A viruses entirely from cloned cDNAs. Proc Natl Acad Sci USA. 1999;96:9345–5093. doi: 10.1073/pnas.96.16.9345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolson C, Major D, Wood JM, Robertson JS. Generation of influenza vaccine viruses on Vero cells by reverse genetics: an H5N1 candidate vaccine strain produced under a quality system. Vaccine. 2005;23:2943–2952. doi: 10.1016/j.vaccine.2004.08.054. [DOI] [PubMed] [Google Scholar]
- Nicholson KG, Colegate AE, Podda A, Stephenson I, Wood J, Ypma E, Zambon MC. Safety and antigenicity of non-adjuvanted and MF59-adjuvanted influenza A/Duck/Singapore/97 (H5N3) vaccine: a randomised trial of two potential vaccines against H5N1 influenza. Lancet. 2001;357:1937–1943. doi: 10.1016/S0140-6736(00)05066-2. [DOI] [PubMed] [Google Scholar]
- Reid AH, Taubenberger JK, Fanning TG. Evidence of an absence: the genetic origins of the 1918 pandemic influenza virus. Nat Rev Microbiol. 2004;2:909–914. doi: 10.1038/nrmicro1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangster MY, Riberdy JM, Gonzalez M, Topham DJ, Baumgarth N, Doherty PC. An early CD4+ T cell-dependent immunoglobulin A response to influenza infection in the absence of key cognate T-B interactions. J Exp Med. 2003;198:1011–1021. doi: 10.1084/jem.20021745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J-H, Hguyen HH, Cuburu N, Horimoto T, Ko S-Y, Park S-H, Czerkinsky C, Kweon M-N. Sublingual vaccination with influenza virus protects mice against lethal viral infection. Proc Natl Acad Sci USA. 2008 doi: 10.1073/pnas.0708684105. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson I, Nicholson KG, Wood JM, Zambon MC, Katz JM. Confronting the avian influenza threat: vaccine development for a potential pandemic. Lancet Infect Dis. 2004;4:499–509. doi: 10.1016/S1473-3099(04)01105-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson I, Bugarini R, Nicholson KG, Podda A, Wood JM, Zambon MC, Katz JM. Cross-reactivity to highly pathogenic avian influenza H5N1 viruses after vaccination with nonadjuvanted and MF59-adjuvanted influenza A/duck/Singapore/97 (H5N3) vaccine: a potential priming strategy. J Inf Dis. 2005;191:1210–1215. doi: 10.1086/428948. [DOI] [PubMed] [Google Scholar]
- Stephenson I, Nicholson KG, Glück R, Mischler R, Newman RW, Palache AM, Verlander NQ, Warburton F, Wood JM, Zambon MC. Safety and antigenicity of whole virus and subunit influenza A/Hong Kong/1073/99 (H9N2) vaccine in healthy adults: phase I randomised trial. Lancet. 2003;362:1959–1966. doi: 10.1016/S0140-6736(03)15014-3. [DOI] [PubMed] [Google Scholar]
- Stevens J, Corper AL, Basler CF, Taubenberger JK, Palese P, Wilson IA. Structure of the uncleaved human H1 hemagglutinin from the extinct 1918 influenza virus. Science. 2004;303:1866–1870. doi: 10.1126/science.1093373. [DOI] [PubMed] [Google Scholar]
- Subbarao K, Chen H, Swayne D, Mingay L, Fodor E, Brownlee G, Xu X, Lu X, Katz J, Cox N, Matsuoka Y. Evaluation of a genetically modified reassortant H5N1 influenza A virus vaccine candidate generated by plasmid-based reverse genetics. Virology. 2003;305:192–200. doi: 10.1006/viro.2002.1742. [DOI] [PubMed] [Google Scholar]
- Suguitan AL, Jr, McAuliffe J, Mills KL, Jin H, Duke G, Lu B, Luke CJ, Murphy B, Swayne DE, Kemble G, Subbarao K. Live, attenuated influenza A H5N1 candidate vaccines provide broad cross-protection in mice and ferrets. PLoS Med. 2006;3:e360. doi: 10.1371/journal.pmed.0030360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada A, Kuboki N, Okazaki K, Ninomiya A, Tanaka H, Ozaki H, Itamura S, Nishimura H, Enami M, Tashiro M, Shortridge KF, Kida H. Avirulent avian influenza virus as a vaccine strain against a potential human pandemic. J Virol. 1999;73:8303–8307. doi: 10.1128/jvi.73.10.8303-8307.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treanor JJ, Campbell JD, Zangwill KM, Rowe T, Wolff M. Safety and Immunogenicity of an inactivated subvirion influenza A (H5N1) vaccine. N Eng J Med. 2006;354:1343–1351. doi: 10.1056/NEJMoa055778. [DOI] [PubMed] [Google Scholar]
- Wang Z, Duke GM. Cloning of the canine RNA polymerase I promoter and establishment of reverse genetics for influenza A and B in MDCK cells. Virology J. 2007;4:102. doi: 10.1186/1743-422X-4-102. xx. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webby RJ, Perez DR, Coleman JS, Guan Y, Knight JH, Govorkova EA, McClain-Moss LR, Peiris JS, Rehg JE, Tuomanen EI, Webster RG. Responsiveness to a pandemic alert: use of reverse genetics for rapid development of influenza vaccines. Lancet. 2004;363:1099–1103. doi: 10.1016/S0140-6736(04)15892-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JM, Robertson JS. From lethal virus to life-saving vaccine: the development of inactivated influenza vaccines for pandemic influenza. Nat Rev Microbiol. 2004;2:842–847. doi: 10.1038/nrmicro979. [DOI] [PubMed] [Google Scholar]
- Wright PF, Neumann G, Kawaoka Y. Orthomyxoviruses. In: Knipe, et al., editors. Fields Virology. 5. Lippincott Williams & Wilkins; 2007. pp. 1691–1740. [Google Scholar]