Abstract
The most common cystic fibrosis–causing mutation in the cystic fibrosis transmembrane conductance regulator (CFTR) is deletion of phenylalanine at residue 508 (ΔF508). The ΔF508 mutation impairs folding of nucleotide binding domain 1 (NBD1) and interfacial interactions of NBD1 and the membrane spanning domains. Here, we report a domain-targeted screen to identify ΔF508-CFTR modulators that act on NBD1. A biochemical screen for ΔF508-NBD1 cell surface expression was done in Madin–Darby canine kidney cells expressing a chimeric reporter consisting of ΔF508-NBD1, the CD4 transmembrane domain, and an extracellular horseradish peroxidase (HRP) reporter. Using a luminescence readout of HRP activity, the screen was robust with a Z′ factor of 0.7. The screening of ~20,000 synthetic small molecules allowed the identification of compounds from four chemical classes that increased ΔF508-NBD1 cell surface expression by up to 4-fold; for comparison, a 12-fold increased cell surface expression was found for a wild-type NBD1 chimera. While the compounds were inactive as correctors of full-length ΔF508-CFTR, several carboxamide-benzothiophenes had potentiator activity with low micromolar EC50. Interestingly, the potentiators did not activate G551D or wild-type CFTR. Our results provide a proof of concept for a cell-based NBD1 domain screen to identify ΔF508-CFTR modulators that target the NBD1 domain.
Keywords: cystic fibrosis, CFTR, high-throughput screen, potentiator
Introduction
The cystic fibrosis transmembrane conductance regulator (CFTR) is a cAMP-regulated chloride channel expressed in the apical plasma membrane of airway, intestinal, pancreatic, and other epithelia. CFTR has cytoplasmic amino- and carboxy-termini and contains two membrane-spanning domains (MSD1 and MSD2), each of which is associated with a nucleotide binding domain (NBD1 and NBD2). The MSD1-NBD1 and MSD2-NBD2 domains are linked by a regulatory domain that can be phosphorylated.1,2 Deletion of phenylalanine 508 (ΔF508) in the NBD1 domain is the most common CFTR mutation causing cystic fibrosis.3 The ΔF508 mutation causes focal and global CFTR misfolding that results in endoplasmic reticulum retention, accelerated degradation, and impaired chloride channel function.4
The ΔF508 mutation destabilizes the NBD1 domain of CFTR both thermodynamically and kinetically.5–7 Suppressor mutations, such as R1S (G550E, R553Q, R555K, and F494N),8 stabilize ΔF508-NBD1 but only partially rescue, by 10%–40%, defective ΔF508-CFTR folding and plasma membrane expression.8 More robust rescue of up to 80% has been achieved by combinations of suppressor mutations, such as R1S and R1040W, which stabilize both ΔF508-NBD1 and the NBD1-MSD2 interface.8 These observations have suggested that CFTR modulator combinations that target the two major structural defects—defective ΔF508-NBD1 stability and the NBD-MSD interfacial defect—might rescue ΔF508-CFTR function to near wild-type levels.9 Whereas specific modulators have been identified that target NBD1-MSD interfacial defects, such as VX-809, there are no small molecules that selectively improve ΔF508-NBD1 stability. The robust ΔF508-CFTR correction achieved using VX-809, together with chemical chaperones, such as glycerol and myo-inositol, or second-site suppressor mutations that stabilize ΔF508-NBD1,9 offers further rationale to identify NBD1-targeted CFTR modulators.
The objective of this study was to develop a screen to identify ΔF508-NBD1-targeted modulators. A domain-selective screen was implemented in which the ΔF508-NBD1 domain of human CFTR was linked to the single MSD of CD4 and an extracellular horseradish peroxidase (HRP) tag (Fig. 1A). Small molecules targeting ΔF508-NBD1 might rescue ΔF508-CFTR misfolding and/or function by direct interaction or indirect proteostasis mechanisms.
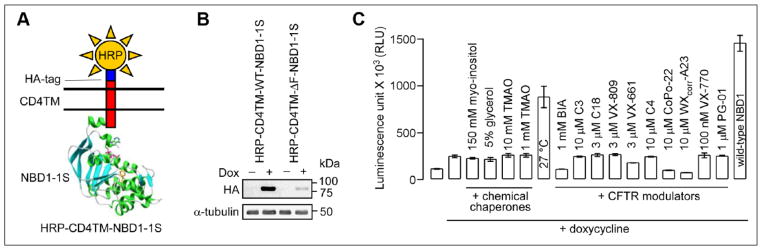
Figure 1.
Cell-based NBD1-targeted screen. (A) Schematic showing chimera construct consisting of the NBD1 domain of human CFTR linked to a single MSD of CD4 containing extracellular HA and HRP tags. (B) Immunoblot of MDCK cells expressing inducible HRP-CD4TM-WT-NBD1-1S (HRP-wild-type-NBD1) or HRP-CD4TM-ΔF508-NBD1-1S (HRP-ΔF508-NBD1) under the control of the TetON doxycycline (Dox)-regulated transactivator in the absence or presence of 500 ng/mL doxycycline for 2 days. Tubulin was the loading control. (C) Luminescence readout of cell surface HRP activity of MDCK cells expressing HRP-ΔF508-NBD1 after 24 h incubation with 500 ng/mL Dox at 37 °C with indicated compounds (SEM, n = 4) or at 27 °C. Data for MDCK cells expressing HRP-wild-type-NBD1 were shown as the positive control. Details of compounds in C are described in the text.
Materials and Methods
Chemicals and Compounds
VX-809, VX-661, VX-770, and CFTRinh-172 were purchased from Selleck Chemicals (Boston, MA). Correctors C3, C4, and C18 were obtained from the CFTR Compound Program, Cystic Fibrosis Foundation Therapeutics Inc., which is administered at Rosalind Franklin University of Medicine and Science; CoPo-22, PG-01, and WXcorr-A23 were obtained from an in-house repository of CFTR modulators. Bromoindole-acetic acid (BIA) was purchased from Santa Cruz Biotechnology (Dallas, TX). For screening, approximately 20,000 diverse drug-like synthetic compounds (>90% with molecular size 250–500 Da; ChemDiv Inc., San Diego, CA) were tested. One hundred and twenty commercially available carboxamide-benzothiophene analogs were purchased from ChemDiv for structure–activity studies.
Cell Lines
Doxycycline-inducible expression systems were generated by lentiviral transduction as described.10 CD4TM-NBD1 expression constructs were generated by insertion of PCR-amplified NBD1 cDNA containing the 1S mutation into XmaI/ApaI sites of pcDNA3-CD4T.11 For expression of extracellular HRP-tagged CD4TM-ΔF508-NBD1-1S chimeras, the extracellular CD4 domain of previously described CD4T-ΔF508-NBD1-1S8 was replaced in-frame with the catalytic domain of HRP. Madin–Darby canine kidney type II (MDCK) cells were transduced with lentivirus, and for each construct, >40 single clones were screened to identify clones with high expression and signal-to-background ratio. MDCK cells expressing HRP-CD4TM-ΔF508-NBD1-1S (HRP-ΔF508-NBD1) and HRP-CD4TM-WT-NBD1-1S (HRP-wild-type-NBD1) were grown at 37 °C and maintained in Dulbecco’s modified Eagle’s medium (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS) under puromycin (3 μg/mL) and G418 (0.2 mg/mL) selection. For low-temperature rescue experiments, cells were grown to 90%–100% confluence at 37 °C and then maintained at 27 °C for 18–24 h.
Fisher rat thyroid (FRT) epithelial cells stably expressing ΔF508, G551D, or wild-type CFTR were used as described.12 FRT cells were cultured in Coon’s modified Ham’s F-12 medium supplemented with 10% FBS, 2 mM L-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin. CFBE41o-TetON (CFBE) cells expressing ΔF508-CFTR-HRP were used as described.10,13 CFBE cells were grown in minimal essential medium supplemented with 10% FBS, 2 mM L-glutamine, and 10 mM HEPES.
Screening
For screening, MDCK cells were plated in black, 96-well microplates (Costar; Corning Inc., Tewksbury, MA) at 10,000 cells/well. MDCK cells (passages 2–5) were grown to confluency before plating. HRP-ΔF508-NBD1 expression was induced 24 h after plating with 0.5 μg/mL doxycycline for 2 days before screening. Screening was performed using a Beckman Coulter (Fullerton, CA) Biomek FX platform. MDCK cells expressing HRP-ΔF508-NBD1 were incubated with 100 μL of medium containing 25 μM test compound and 0.5 μg/mL doxycycline for 24 h at 37 °C. All compound plates contained negative controls (DMSO vehicle) and positive controls (MDCK cells expressing HRP-wild-type-NBD1). The cells were washed four times with phosphate-buffered saline (PBS), and HRP activity was assayed by the addition of 50 μL/well of HRP substrate (WesternBright Sirius Kit; Advansta Corp., Menlo Park, CA). After shaking for 5 min, chemiluminescence was measured using a Tecan Infinite M1000 plate reader (Tecan Groups Ltd., Mannedorf, Switzerland) equipped with an automated stacker. The Z′ factor was computed as 1 – [(3 × standard deviation of maximum signal control + 3 × standard deviation of minimum signal control)/absolute (mean of maximum signal control – mean of minimum signal control)]. A Z′ factor >0.5 is considered a robust assay.
Short-Circuit Current
Short-circuit current in cells expressing full-length ΔF508-CFTR was measured as described.12 For the corrector assay, FRT cells expressing ΔF508-CFTR were cultured on inserts (Snapwell; Corning) for 5–7 days and then incubated with test compounds (with or without 3 μM VX-809) for 18–24 h at 37 °C before measurements. For the potentiator assay, 3 μM VX-809 was added for 18–24 h at 37 °C before measurements. The basolateral solution contained 120 mM NaCl, 3 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 10 mM glucose, 25 mM NaHCO3, and 5 mM HEPES, pH 7.4. In the apical bathing solution, 60 mM NaCl was replaced by Na gluconate and CaCl2 was increased to 2 mM. Solutions were bubbled with 95% O2/5% CO2 and maintained at 37 °C. The basolateral membrane was permeabilized with 250 μg/mL amphotericin B. Hemichambers were connected to a DVC-1000 voltage clamp (World Precision Instruments Inc., Sarasota, FL) via Ag/AgCl electrodes and 1 M KCl agar bridges.
CFTR Plasma Membrane Expression
ΔF508-CFTR cell surface expression was measured as described.14 Briefly, ΔF508-CFTR-HRP expression in CFBE cells was induced after 24 h with 0.5 μg/mL doxycycline for 2 days before assay. Cells were incubated with test compound (20 μM) and 0.5 μg/mL doxycycline for 24 h at 37 °C. Plates contained negative controls (DMSO vehicle) and positive controls (3 μM VX-809). Cells were washed, HRP substrate was added, and luminescence (reported as relative luminescence units) was measured.
Differential Scanning Fluorimetry
Recombinant human ΔF508-NBD1 containing a single suppressor mutation (1S; F494N) was purified, and melting temperature measurement was done as described.8 Differential scanning fluorometry of ΔF508-NBD1 (6 μM) was done in 150 mM NaCl, 20 mM MgCl2, 10 mM HEPES, and 2.5 mM ATP, pH 7.5, using a Stratagene Mx3005p (Agilent Technologies, La Jolla, CA) quantitative PCR instrument in the presence of 2× Sypro Orange.
Immunoblotting
Immunoblotting was done using standard procedures.8 The HRP-CD4TM-NBD1-1S constructs were detected with mouse monoclonal anti-hemagglutinin (HA) antibody (Covance) with anti-tubulin antibody as the loading control.
Statistics
Statistical analysis was done using the Prism 5 GraphPad Software package (San Diego, CA). Data are presented as the mean ± standard error of the mean (SEM), and statistical significance was set at p < 0.05.
Results
Screen for NBD1-Targeted Modulators of ΔF508-CFTR
The assay used a chimeric transmembrane reporter consisting of NBD1 linked to the single transmembrane helix of CD4 and an extracellular reporter to provide a readout of cell surface presentation. As shown in Figure 1A, human wild-type and ΔF508 NBD1 domains (amino acids 389–679) carrying the single 1S (F494N) mutation that stabilizes NBD1 were linked at their C-termini to the CD4 transmembrane domain, and a HRP isoenzyme C moiety was linked at the endoplasmic reticulum lumen/extracellular end of the CD4 transmembrane domain to report cell surface presentation. In addition, a single HA tag was incorporated to facilitate detection by immunoblot. The MDCK cells used for HTS were engineered using a doxycycline transactivator-inducible expression system to control HRP-CD4TM-NBD1-1S levels as described.10 By immunoblot (Fig. 1B), HRP-CD4TM-WT-NBD1-1S (HRP-wild-type-NBD1) or HRP-CD4TM-ΔF508-NBD1-1 (HRP-ΔF508-NBD1) protein was not seen without doxycycline induction. With doxycycline, significantly greater HRP-wild-type-NBD1 expression was observed than HRP-ΔF508-NBD1, consistent with misfolding and degradation of the ΔF508-NBD1 reporter. The rationale of the screen is that compounds that interact with the ΔF508-NBD1 protein and correct its folding defect will permit accumulation at the plasma membrane.
MDCK cells expressing the HRP-ΔF508-NBD1 and HRP-wild-type-NBD1 chimeras were assayed for cell surface HRP activity (Fig. 1C). Minimal HRP activity was seen without doxycycline induction; doxycycline induction produced somewhat greater activity in cells expressing the ΔF508-NBD1 reporter, and much greater activity for cells expressing the wild-type NBD1 reporter. Chemical chaperones, including myo-inositol, glycerol, and trimethylamine oxide (TMAO), which increase the thermal stability of the ΔF508-NBD1 domain in vitro,10 did not increase the HRP signal. However, low temperature (27 °C) incubation strongly increased the HRP signal. BIA, a reported low-affinity ΔF508-NBD1 binder,15 did not increase the HRP signal. Also, a panel of known CFTR modulators was tested, including correctors VX-809, VX-661, C3, and C18, which target the NBD1-MSD interface,9 C4, which targets NBD2,16 CoPo-22, a CFTR modulator with dual corrector and potentiator function,12 and W1282Xcorr-A23, which corrects CFTR1281, the protein product generated by the W1282X-CFTR nonsense mutation.17 None of these correctors increased ΔF508-NBD1 cell surface expression; nor did previously described potentiators VX-770 and PG-01.
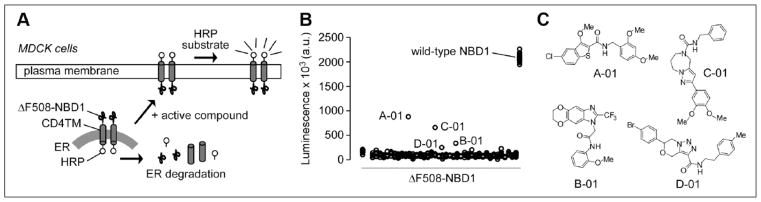
High-throughput screening was done in order to identify NBD1 modulators. Figure 2A diagrams the screening approach in which small molecules were tested for rescue of defective ΔF508-NBD1 folding/trafficking. Primary screening was done in 96-well plates in which MDCK cells expressing HRP-ΔF508-NBD1 were induced with doxycycline 48 h prior to screening, with test compounds incubated at 25 μM for 24 h prior to HRP assay. The screen was robust with a Z′ factor of 0.7. Figure 2B shows a scatterplot of the original HRP luminescence data for each of the compounds tested, along with the positive control (MDCK cells expressing HRP-wild-type-NBD1). Figure 2C shows the chemical structures of four active compounds (A-01, B-01, C-01, and D-01) that were verified on retesting. Scaffolds A-01, B-01, C-01, and D-01 include a carboxamide-benzothiophene, pyrazolo-diazepine, dioxino-benzimidazole, and triazolooxazine, respectively. These scaffolds are distinct from known ΔF508-CFTR correctors and potentiators.
Figure 2.
Screening results. (A) Schematic of primary screening assay using MDCK cells expressing HRP-ΔF508-NBD1. Small molecules that correct the folding defect and/or stabilize ΔF508-NBD1 will increase the ΔF508-NBD1 cell surface expression as read out by the increased HRP luminescence signal. (B) Summary of primary screen showing data for all compounds, with cells expressing HRP-wild-type-NBD1 as the positive control. (C) Chemical structures of active compounds identified from the screen.
Compound Action on Full-Length ΔF508-CFTR
Compound effects on the function of full-length ΔF508-CFTR were studied by short-circuit current in FRT cells expressing ΔF508-CFTR. The apical membrane chloride current was measured in the presence of a transepithelial chloride gradient and after basolateral membrane permeabilization, so that current provides a quantitative, linear measure of CFTR chloride conductance. Compounds B-01, C-01, and D-01 did not change chloride conductance, either alone or when added for 24 h together with 3 μM VX-809 (data not shown). Compound A-01, when added alone, did not change chloride conductance (short-circuit current 14 ± 4 μA/cm2 and 12 ± 5 μA/cm2 for 20 μM A-01 vs control, respectively). However, as shown in Figure 3A, A-01 together with 3 μM VX-809 increased the response to forskolin significantly greater than that of VX-809 alone (43 ± 5 μA/cm2 vs 29 ± 3 μA/cm2, respectively), though it did not increase maximal chloride conductance after addition of potentiator VX-770. The increase in the forskolin response to A-01 could represent potentiator and/or corrector actions.
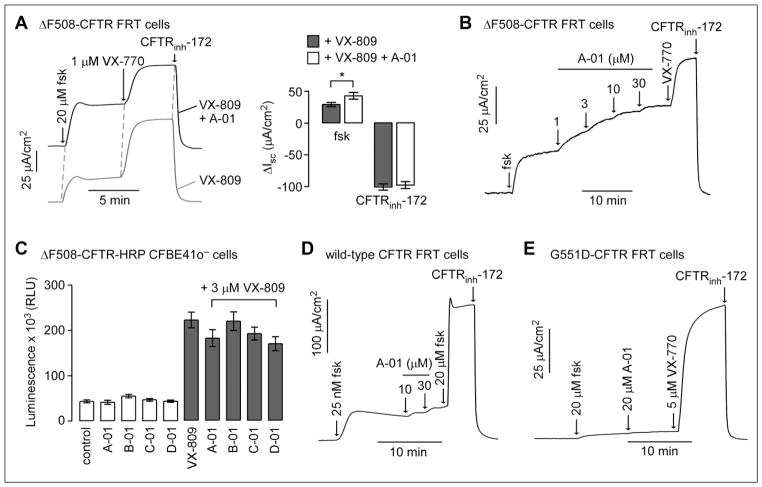
Figure 3.
Characterization of compounds identified from the screen. (A, left) Short-circuit current (Isc) measured in FRT cells expressing full-length ΔF508-CFTR showing responses to forskolin, VX-770, and CFTRinh-172 (10 μM). Cells were incubated at 37 °C for 24 h before measurement with (top) 20 μM A-01 plus 3 μM VX-809 or (bottom) 3 μM VX-809 only. (A, right) Summary of ΔIsc data (mean ± SEM, n = 3). (B) Isc in FRT cells expressing full-length ΔF508-CFTR showing responses to forskolin (20 μM) and indicated concentrations of A-01. Cells were incubated at 27 °C for 24 h before measurement. VX-770 (1 μM) and CFTRinh-172 (10 μM) were added as indicated. (C) Luminescence readout of cell surface ΔF508-CFTR in CFBE41o-cells expressing ΔF508-CFTR-HRP after 24 h of incubation with indicated compounds (20 μM) in the absence or presence of 3 μM VX-809 (SEM, n = 4). (D) Isc in FRT cells expressing wild-type CFTR showing responses to low forskolin (25 nM) and indicated concentrations of A-01. Maximal forskolin and CFTRinh-172 (10 μM) were added as indicated. (E) Isc in FRT cells expressing G551D-CFTR showing responses to indicated forskolin, A-01, VX-770, and CFTRinh-172 (10 μM).
To investigate possible potentiator activity, compounds were added acutely to cells in which ΔF508-CFTR cell surface expression was rescued by low-temperature (27 °C) incubation. Following the addition of forskolin, compound A-01 increased chloride current in a concentration-dependent manner with a low micromolar EC50 and maximal current (at 30 μM A-01) that was ~50% of that produced by VX-770. Similar studies done for compounds B-01, C-01, and D-01 did not show potentiator activity (data not shown). To investigate possible corrector activity, compounds at 20 μM were incubated with CFBE41ocells expressing ΔF508-CFTR with HRP engineered into the fourth extracellular loop (to report cell surface expression) for 24 h, and then cell surface HRP activity was assayed (Fig. 3C). None of the compound, when added alone, produced a signal increase. Though a substantial increase in HRP signal was produced by VX-809, no further signal increase was found when a test compound was included with VX-809. These results suggest that compound A-01 has ΔF508-CFTR potentiator but not corrector activity.
Compound A-01 was also tested for potentiator activity in FRT cells expressing wild-type CFTR (Fig. 3D). A low concentration of forskolin (25 nM) was added prior to the addition of A-01 to produce a low basal level of CFTR activation. Maximal forskolin (20 μM) was added after A-01 to fully activate wild-type CFTR. A-01 at a high concentration of 30 μM produced minimal (~8%) activation of wild-type CFTR. A-01 was also tested in FRT cells expressing G551D-CFTR, a gating mutant that produces little chloride current with maximal forskolin (Fig. 3E); A-01 did not activate G551D-CFTR at high concentration, with VX-770 shown as the positive control. The results suggest that A-01 is a selective ΔF508-CFTR potentiator.
Structure–Activity Analysis of Carboxamide-Benzothiophenes
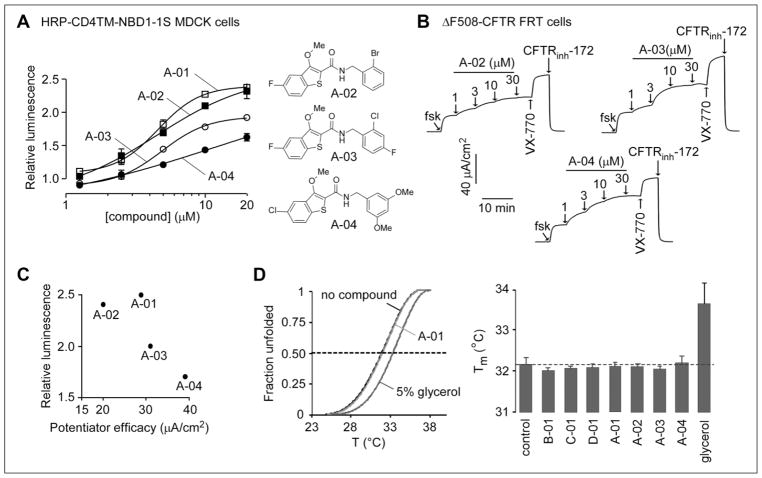
One hundred and twenty commercially available A-01 analogs were tested in MDCK cells expressing HRP-ΔF508-NBD1. Three additional carboxamide-benzothiophenes (A-02, A-03, and A-04) showed activity, with 2.4-, 2.0-, and 1.7-fold increased luminescence signal, respectively, over the control, DMSO-treated cells. Figure 4A (left) shows concentration dependence data. The structures of A-02, A-03, and A-04 were similar to that of A-01 (Fig. 4A right). Compounds were also assayed by short-circuit current for ΔF508-CFTR potentiator activity (Fig. 4B). All three analogs showed activity. Though all four carboxamide-benzothiophenes showed activity in the ΔF508-NBD1 assay (Fig. 4A) and the ΔF508-CFTR potentiator assay (Fig. 4B), the relative activities did not correlate (Fig. 4C).
Figure 4.
Structure–activity analysis of carboxamide-benzothiophene A-01 analogs. (A, left) Concentration dependence of compound action in MDCK cells expressing HRP-ΔF508-NBD1. Compounds were incubated with MDCK cells for 24 h at 37 °C prior to assay of HRP activity (mean ± SEM; n = 3). (A, right) Structures of active analogs. (B) Compound potentiator activity measured by short-circuit current in FRT cells expressing full-length ΔF508-CFTR showing responses to forskolin (20 μM) and indicated concentrations of compounds. Cells were incubated at 27 °C for 24 h before measurement. VX-770 (1 μM) and CFTRinh-172 (10 μM) were added as indicated. (C) Plot comparing biochemical (normalized relative luminescence, y axis) and functional (x axis) ΔF508-CFTR potentiator data for the four active carboxamide-benzothiophenes. (D) Representative melting curves (left) and melting temperature (right, n = 2) of human ΔF508-NBD1-1S determined by differential scanning fluorometry. The indicated compounds (30 μM) or the chemical chaperone glycerol (5%) was present during thermal unfolding.
Last, the compounds were tested for direct thermostabilization of ΔF508-NBD1-1S, based on melting temperature determination monitored by differential scanning fluorometry. As shown in Figure 4D left, compound A-01 did not alter NBD1-1S melting curves, whereas 5% glycerol (positive control) right-shifted the melting curves as reported before.9 None of the other compounds identified in this screen, including active analogs of A-01, altered NBD1-1S melting temperature, as shown in the data summary (Fig. 4D right). Table 1 summarizes the activity of selected compounds in the various assays.
Table 1.
Compound Activity in the Various Assays.
| Compound | MDCK ΔF508-NBD1 HRPa | CFBE ΔF508-CFTR HRPb | FRT ΔF508-CFTR Potentiationc | Recombinant ΔF508-NBD1 Thermostabilizationd |
|---|---|---|---|---|
| A-01 | Active | Inactive | Active | Inactive |
| A-02 | Active | Inactive | Active | Inactive |
| A-03 | Active | Inactive | Active | Inactive |
| A-04 | Active | Inactive | Active | Inactive |
| B-01 | Active | Inactive | Inactive | Inactive |
| C-01 | Active | Inactive | Inactive | Inactive |
| D-01 | Active | Inactive | Inactive | Inactive |
Surface presentation assay in MDCK cells expressing engineered ΔF508-NBD1 domain (HRP-ΔF508-NBD1).
Surface presentation assay in CFBE41o-cells expressing full-length ΔF508-CFTR with HRP in the fourth extracellular loop. Assays were performed with and without VX-809.
Short-circuit current assay in transfected FRT cells expressing full-length ΔF508-CFTR.
Melting temperature measurement assay using recombinant ΔF508-NBD1.
Discussion
The motivation for the screen developed here is the prediction that correction of defects in both ΔF508-NBD1 energetics and the NBD1-MSD interface may effectively rescue the defective folding and processing of full-length ΔF508-CFTR.8 While certain clinical and investigational correctors, including VX-809, C3, and C18, target the NBD1-MSD interfacial defects, no modulators identified to date selectively target ΔF508-NBD1. Herein, we implemented a cell-based biochemical screen to identify NBD1-targeted modulators using a chimera of the human ΔF508-NBD1 domain fused with the single transmembrane domain of CD4 containing an extracellular HRP tag. A panel of known CFTR modulators, including the correctors VX-809 and C4 (targeting the NBD2 domain), did not increase ΔF508-NBD1 cell surface expression as anticipated because these compounds act on other regions of ΔF508-CFTR. The compound BIA, which was shown to bind NBD1 protein in cocrystallization studies and to stabilize ΔF508-NBD1 energetics and enhance ΔF508-CFTR protein maturation in fibroblast cell models,15 was not active in the ΔF508-NBD1 assay.
The screen identified four compounds that increased ΔF508-NBD1 cell surface expression. Though the compounds did not show corrector activity in cells expressing full-length ΔF508-CFTR, one of the compounds was found to have potentiator activity. The compound, A-01, a carboxamide-benzothiophene, is chemically different from previously described ΔF508-CFTR modulators. Melting temperature shift studies showed that A-01 did not produce ΔF508-NBD1 domain thermostabilization. In prior studies, some correctors, including C6 and C12, produced ΔF508-NBD1 thermostabilization, as did chemical chaperones, including glycerol and TMAO.9 The in vitro domain thermostabilization assay might not correlate well with stabilizing efficacy in the context of full-length ΔF508-CFTR.
It was remarkable that A-01, a compound identified from the ΔF508-NBD1 cell surface presentation screen, had potentiator activity when tested on full-length ΔF508-CFTR. However, none of the active compounds identified in the domain screen of 20,000 small molecules functioned as correctors of full-length ΔF508-CFTR, though it is possible that a larger screen might produce correctors. We speculate that conformational changes induced by A-01 on the ΔF508-NBD1 domain in isolation are sufficient to partially rescue its defective trafficking, but not sufficient to rescue defective cellular processing of full-length ΔF508-CFTR, in which the NBD1 structural alterations are ineffective or its binding sites are not accessible. Modulators identified in the screen might also rescue ΔF508-NBD1 cell surface presentation indirectly as proteostasis regulators by modulating the cellular machinery responsible for its folding, degradation, and vesicular trafficking. However, such indirect mechanisms might not translate into compound efficacy as correctors of full-length ΔF508-CFTR. Previous studies have suggested that isolated NBD1 might not contain the necessary structural features to support high-affinity interactions.18
Another interesting finding was that A-01 acted as a potentiator of full-length ΔF508-CFTR but not of wild-type CFTR or G551D-CFTR. Generally, CFTR modulators identified for a specific mutant have demonstrated promiscuity toward other cystic fibrosis–causing CFTR mutants. Though VX-770 is a potentiator for G551D-CFTR,19 it has now been approved for >20 Class III gating mutants, certain splicing mutations,20 and in combination with VX-809, for the ΔF508-CFTR Class II mutation. VX-809 and VX-770, as well as several additional investigational correctors and potentiators, showed efficacy for the truncation product CFTR1281 generated by the W1282X-CFTR premature termination codon.17 The apparent selectivity of A-01 for ΔF508-CFTR could be due to its specific interaction with the ΔF508-NBD1 topological and electrostatic surfaces not found in wild-type CFTR or G551D-CFTR.
Screening to identify ΔF508-CFTR modulators has generally relied on heterologous systems expressing full-length ΔF508-CFTR constructs and functional assessment of activity using fluorescent potentiometric or halide sensors. 16,19,21,22 These studies identified C4 (corr-4a), the first corrector of ΔF508-CFTR,16 as well as VX-809.23 More recently, potentiometric sensors have also been used in human airway epithelial cell cultures to test CFTR modulators against ΔF508-CFTR and other CFTR mutations.24,25 A biochemical synergy screen of ΔF508-CFTR surface expression was developed using an engineered extracellular epitope14 to discover second-generation corrector combinations that target distinct ΔF508-CFTR folding defects. Novel ΔF508-CFTR therapeutics with two correctors and a potentiator are in clinical development.26 High-content screening approaches have recently been developed using dual-tagged ΔF508-CFTR with an amino-terminus mCherry and an extracellular epitope tag for ratiometric determination of surface presentation.27 Structure-based virtual screening has also been used to identify small molecules that inhibit keratin 8-ΔF508-CFTR interactions and mediate ΔF508-CFTR rescue in cell models.28 Assays of folding/solubility of CFTR NBD1 domains have been developed that rely on structural complementation of isolated domains from enzymes such as β-galactosidase,29,30 although to the best of our knowledge such assays have not been applied to the identification of CFTR modulators.
A few biological activities have been reported for the carboxamide-benzothiophene scaffold identified in this study. Carboxamide-benzothiophenes were shown to have antimalarial activity targeting the N-myristoyltransferase of Plasmodium falciparum and Plasmodium vivax.31 Carboxamide-benzothiophenes were also found to decrease the adherence of neutrophils to activated endothelial cells by inhibiting upregulation of the adhesion proteins E-selectin, ICAM-1, and VCAM-1.32 Compound A-01 has drug-like properties, including favorable molecular weight (391 Da), topological polar surface area (57 Å2), and cLogP (4.8).
In summary, the results here provide a proof of concept for NBD1 domain–targeted screening to identify ΔF508-NBD1 modulators. Additional screening, together with structural optimization, might produce more efficacious ΔF508-NBD1 modulators that act in synergy with ΔF508-CFTR modulators acting at other sites.
Acknowledgments
The authors than Robert Bridges, PhD, Rosalind Franklin University of Medicine and Science, for providing compounds through the CFTR Compound Program, CFFT.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by National Institutes of Health grants DK75302, DK72517, EB00415, and EY13574; the Cystic Fibrosis Foundation; the CIHR; and the Canadian Cystic Fibrosis Foundation. G.L.L. is a recipient of a Canada Research Chair.
Footnotes
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Liu F, Zhang Z, Csanady L, et al. Molecular Structure of the Human CFTR Ion Channel. Cell. 2017;169(1):85–95. doi: 10.1016/j.cell.2017.02.024. [DOI] [PubMed] [Google Scholar]
- 2.Riordan JR. CFTR Function and Prospects for Therapy. Annu Rev Biochem. 2008;77:701–726. doi: 10.1146/annurev.biochem.75.103004.142532. [DOI] [PubMed] [Google Scholar]
- 3.Ratjen F, Bell SC, Rowe SM, et al. Cystic Fibrosis. Nat Rev Dis Primers. 2015;1:15010. doi: 10.1038/nrdp.2015.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lukacs GL, Verkman AS. CFTR: Folding, Misfolding and Correcting the ΔF508 Conformational Defect. Trends Mol Med. 2012;18(2):81–91. doi: 10.1016/j.molmed.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qu BH, Thomas PJ. Alteration of the Cystic Fibrosis Transmembrane Conductance Regulator Folding Pathway. J Biol Chem. 1996;271:7261–7264. doi: 10.1074/jbc.271.13.7261. [DOI] [PubMed] [Google Scholar]
- 6.Thibodeau PH, Brautigam CA, Machius M, et al. Side Chain and Backbone Contributions of Phe508 to CFTR Folding. Nat Struct Mol Biol. 2005;12(1):10–16. doi: 10.1038/nsmb881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Du K, Sharma M, Lukacs GL. The DeltaF508 Cystic Fibrosis Mutation Impairs Domain-Domain Interactions and Arrests Post-Translational Folding of CFTR. Nat Struct Mol Biol. 2005;12(1):17–25. doi: 10.1038/nsmb882. [DOI] [PubMed] [Google Scholar]
- 8.Rabeh WM, Bossard F, Xu H, et al. Correction of Both NBD1 Energetics and Domain Interface Is Required to Restore ΔF508 CFTR Folding and Function. Cell. 2012;148:150–163. doi: 10.1016/j.cell.2011.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okiyoneda T, Veit G, Dekkers JF, et al. Mechanism-Based Corrector Combination Restores ΔF508-CFTR Folding and Function. Nat Chem Biol. 2013;9(7):444–454. doi: 10.1038/nchembio.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Veit G, Bossard F, Goepp J, et al. Proinflammatory Cytokine Secretion Is Suppressed by TMEM16A or CFTR Channel Activity in Human Cystic Fibrosis Bronchial Epithelia. Mol Biol Cell. 2012;23:4188–4202. doi: 10.1091/mbc.E12-06-0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Du K, Lukacs GL. Cooperative Assembly and Misfolding of CFTR Domains In Vivo. Mol Biol Cell. 2009;20(7):1903–1915. doi: 10.1091/mbc.E08-09-0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Phuan P-W, Yang B, Knapp JM, et al. Cyanoquinolines with Independent Corrector and Potentiator Activities Restore ΔPhe508-Cystic Fibrosis Transmembrane Conductance Regulator Chloride Channel Function in Cystic Fibrosis. Mol Pharmacol. 2011;80(4):683–693. doi: 10.1124/mol.111.073056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Veit G, Avramescu RG, Perdomo D, et al. Some Gating Potentiators, Including VX-770, Diminish ΔF508-CFTR Functional Expression. Sci Transl Med. 2014;6(246):246ra97. doi: 10.1126/scitranslmed.3008889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Phuan P-W, Veit G, Tan J, et al. Synergy-Based Small-Molecule Screen Using a Human Lung Epithelial Cell Line Yields ΔF508-CFTR Correctors That Augment VX-809 Maximal Efficacy. Mol Pharmacol. 2014;86(1):42–51. doi: 10.1124/mol.114.092478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He L, Aleksandrov AA, An J, et al. Restoration of NBD1 Thermal Stability Is Necessary and Sufficient to Correct ΔF508 CFTR Folding and Assembly. J Mol Biol. 2015;427(1):106–20. doi: 10.1016/j.jmb.2014.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pedemonte N, Lukacs GL, Du K, et al. Small-Molecule Correctors of Defective DeltaF508-CFTR Cellular Processing Identified by High-Throughput Screening. J Clin Invest. 2005;115(9):2564–2571. doi: 10.1172/JCI24898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haggie PM, Phuan P-W, Tan JA, et al. Correctors and Potentiators Rescue Function of the Truncated W1282X-Cystic Fibrosis Transmembrane Regulator (CFTR) Translation Product. J Biol Chem. 2017;292(3):771–785. doi: 10.1074/jbc.M116.764720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hall JD, Wang H, Byrnes LJ, et al. Binding Screen for Cystic Fibrosis Transmembrane Conductance Regulator Correctors Finds New Chemical Matter and Yields Insights into Cystic Fibrosis Therapeutic Strategy. Protein Sci. 2016;25(2):360–373. doi: 10.1002/pro.2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Goor F, Hadida S, Grootenhuis PD, et al. Rescue of CF Airway Epithelial Cell Function In Vitro by a CFTR Potentiator, VX-770. Proc Natl Acad Sci USA. 2009;106(44):18825–18830. doi: 10.1073/pnas.0904709106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vertex Pharmaceuticals. FDA Approves KALYDECO® (Ivacaftor) for More Than 600 People Ages 2 and Older with Cystic Fibrosis Who Have Certain Residual Function Mutations. Press release. 2017 http://www.businesswire.com/news/home/20170801005866/en/
- 21.Molinski SV, Ahmadi S, Hung M, et al. Facilitating Structure-Function Studies of CFTR Modulator Sites with Efficiencies in Mutagenesis and Functional Screening. J Biomol Screen. 2015;20(10):1204–1217. doi: 10.1177/1087057115605834. [DOI] [PubMed] [Google Scholar]
- 22.Maitra R, Sivashanmugam P, Warner K. A Rapid Membrane Potential Assay to Monitor CFTR Function and Inhibition. J Biomol Screen. 2013;18(9):1132–1137. doi: 10.1177/1087057113488420. [DOI] [PubMed] [Google Scholar]
- 23.Van Goor F, Hadida S, Grootenhuis PD, et al. Correction of the F508del-CFTR Protein Processing Defect In Vitro by the Investigational Drug VX-809. Proc Natl Acad Sci USA. 2011;108(46):18843–1888. doi: 10.1073/pnas.1105787108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahmadi S, Bozoky Z, Di Paola M, et al. Phenotypic Profiling of CFTR Modulators in Patient-Derived Respiratory Epithelia. NPJ Genom Med. 2017;2:12. doi: 10.1038/s41525-017-0015-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Molinski SV, Ahmadi S, Ip W, et al. Orkambi and Amplifier Co-Therapy Improves Function from a Rare CFTR Mutation in Gene-Edited Cells and Patient Tissue. EMBO Mol Med. 2017;9(9):1224–1243. doi: 10.15252/emmm.201607137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hanrahan JW, Matthes E, Carlile G, et al. Corrector Combination Therapies for F508del-CFTR. Curr Opin Pharmacol. 2017;34:105–111. doi: 10.1016/j.coph.2017.09.016. [DOI] [PubMed] [Google Scholar]
- 27.Botelho HM, Uliyakina I, Awatade NT, et al. Protein Traffic Disorders: An Effective High-Throughput Fluorescence Microscopy Pipeline for Drug Discovery. Sci Rep. 2015;5:9038. doi: 10.1038/srep09038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Odolczyk N, Fritsch J, Norez C, et al. Discovery of Novel Potent ΔF508-CFTR Correctors that Target the Nucleotide Binding Domain. EMBO Mol Med. 2013;5(10):1484–1501. doi: 10.1002/emmm.201302699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wigley WC, Stidham RD, Smith NM, et al. Protein Solubility and Folding Monitored In Vivo by Structural Complementation of a Genetic Marker Protein. Nat Biotechnol. 2001;19(2):131–136. doi: 10.1038/84389. [DOI] [PubMed] [Google Scholar]
- 30.Mendoza JL, Schmidt A, Li Q, et al. Requirements for Efficient Correction of ΔF508 CFTR Revealed by Analyses of Evolved Sequences. Cell. 2012;148(1–2):164–174. doi: 10.1016/j.cell.2011.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rackham MD, Brannigan JA, Moss DK, et al. Discovery of Novel and Ligand-Efficient Inhibitors of Plasmodium falciparum and Plasmodium vivax N-Myristoyltransferase. J Med Chem. 2013;56(1):371–375. doi: 10.1021/jm301474t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boschelli DH, Kramer JB, Khatana SS, et al. Inhibition of E-Selectin-, ICAM-1-, and VCAM-1-Mediated Cell Adhesion by Benzo[b]Thiophene-, Benzofuran-, Indole-, and Naphthalene-2-Carboxamides: Identification of PD 144795 as an Antiinflammatory Agent. J Med Chem. 1995;38(22):4597–4614. doi: 10.1021/jm00022a026. [DOI] [PubMed] [Google Scholar]