Abstract
The glycoprotein (GP) of lymphocytic choriomeningitis virus (LCMV), the prototype arenavirus, is a promising envelope protein of lentiviral pseudotype vectors for gene therapy. The distribution of dystroglycan, a known receptor for LCMV, cannot explain the narrow tropism of LCMV-GP-pseudotypes. Here, we examined whether infection of LCMV-GP-pseudotypes was affected by the expression of four cell surface molecules - Axl and Tyro3 (from the TAM family) and DC-SIGN and LSECtin (from the C-type lectin family) - that are known receptors of Lassa virus, another arenavirus. All four molecules enhanced LCMV-GP-pseudotype infection of cells. These results help explain the tropism of LCMV-GP-pseudotypes and further our understanding of LCMV infection in animals. (108 words)
Keywords: arenavirus, lymphocytic choriomeningitis virus, pseudotype, receptor
In gene therapy, lentiviral vectors have the advantage of stable gene induction in both dividing and non-dividing cells without viral protein expression. Glycoprotein G of vesicular stomatitis virus (VSV-G) is the most used envelope for lentiviral vectors due to its wide tropism and high transduction efficiency, characteristics that likely stem from the VSV receptor being a ubiquitous phospholipid present in most cell membranes [5]. However, VSV-G protein has cytotoxicity and establishment of cell lines constitutively expressing VSV-G has not been successful [2]. Therefore, the generation of stable vector-producing cells is an important step for the well-defined and safe production of vector particles [2] and for allowing target cells to be efficiently exposed to vectors by engrafting vector-producing cells [9]. In vivo toxicity caused by the injection of VSV-G-pseudotyped vectors has also been reported [14, 23]. The envelope protein, glycoprotein (GP), of lymphocytic choriomeningitis virus (LCMV) has several advantages over VSV-G as a viral vector envelope protein. LCMV-GP has low or no toxicity both in vitro and in vivo [14, 23], and cell lines stably expressing LCMV-GP have been established [2, 19]. In addition, LCMV-GP-pseudotypes show narrow infection tropism; they prefer to infect malignant glioma rather than normal neurons [2, 19, 31]. Such narrow tropisms avoid undesirable effects caused by the infection of unintended targets [25]. At present, there is no molecular explanation for how LCMV-GP affects viral tropism.
LCMV is a member of the Old World arenavirus group in the family Arenaviridae. Its natural hosts are Mus domesticus and Mus musculus. Through the saliva or urine of these hosts, LCMV is transmitted to humans and causes aseptic meningitis [4]. Because experimental infection with LCMV yields a variety of results in mice, depending on the viral strain, host age, and inoculation route, the murine LCMV model has been well used for research, advancing the fields of viral immunopathology and immunobiology [4]. Binding to and entry into cells by LCMV is conferred by the interaction of LCMV-GP with its cellular receptor(s). Dystroglycan (DG) is a transmembrane protein, composed of a peripheral α-subunit and a membrane-spanning β-subunit, that links extracellular matrices and intracellular cytoskeletons. α-DG serves as a receptor for the Old World arenaviruses, including LCMV and Lassa virus and for the clade C New World arenaviruses [7, 30]. Its O-mannosylation is important for its receptor function [11]. Recent studies on LCMV strongly suggest the existence of additional, as yet unidentified viral receptor(s) [11, 16, 29].
Recently, we analyzed the cell entry mechanisms of Lassa virus and identified four human molecules as viral receptors that mediate DG-independent infection [28]. These molecules are Axl and Tyro3 from the TAM family and dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin (DC-SIGN) and liver and lymph node sinusoidal endothelial calcium-dependent lectin (LSECtin) from the C-type lectin family. Because LCMV and Lassa virus share common characteristics in receptor usage [15, 30], here, we examined the effects of these four molecules on infection by LCMV-GP-pseudotypes.
Jurkat T cells expressing the four molecules, feline CD2 lacking cytoplasmic tail (fCD2∆CT, a negative control), and acetylglucosaminyltransferase-like protein (LARGE, a positive control, see below) were prepared as described previously [28]. Expression of Axl, Tyro3, and DC-SIGN in Jurkat cells was examined by flow cytometry with monoclonal antibodies against each molecule, resulting in the observation of approximately 95%, 80%, and 95% positivities for Axl-, Tyro3-, and DC-SIGN-expressing cells, respectively. Expression of LSECtin was assessed by examining the reactivity of LSECtin-expressing Jurkat cells with recombinant Ebola virus GP1 protein using flow cytometry [8], and over 90% positivity was observed. We observed no positive signals from naïve Jurkat cells to monoclonal antibodies against Axl, Tyro3, and DC-SIGN or to Ebola virus GP1 (data not shown). The strains of LCMV from which GPs were used were cl13, ARM53b, WE54, and WE2.2. For cl13 and WE54 GP expression, cDNAs encoding each (sequence accession numbers DQ361065 and AJ318512) were cloned into the expression plasmid pCAGGS/MCS [13, 22]. To express ARM53b GP, PCR-based mutagenesis was performed with the cl13 GP cDNA to cause an amino acid change at position 260 from lysine to phenylalanine [24]; the mutated cDNA was then cloned into pCAGGS/MCS. To express WE2.2 GP, mutagenesis was performed with the WE54 GP cDNA to change the amino acid at position 153 from serine to phenylalanine [32]. Human immunodeficiency virus-based lentiviral vectors carrying the LCMV-GP and Venus gene [21] as the envelope protein and reporter gene, respectively, were prepared as described previously [27]. Titration of vectors was performed by inoculation of Jurkat cells with serially diluted virus stocks and counting reporter-positive cells under a fluorescence microscope 48 hr later.
The GPs of the LCMV strains used in this study have different affinities for O-mannosylated DG; cl13 and WE54 have high affinities, whereas ARM53b and WE2.2 have low and no affinity, respectively [16, 29]. In Jurkat cells, DG is not O-mannosylated, but ectopic LARGE expression causes hyperglycosylation of DG [28]. As shown in Fig. 1, LARGE expression in Jurkat cells resulted in higher titers of GP-cl13- and GP-WE54-, but not of GP-ARM53b- and GP-WE2.2-, pseudotypes, indicating that our assay with LCMV-GP-pseudotypes and Jurkat cells reflected the characteristics of LCMV-GPs. The higher titer of the GP-ARM53b-pseutotype in control cells, when compared with those of other GP-pseudotypes (Fig. 1), may indicate the existence of as yet unknown receptor(s) specific for ARM53b GP in this cell line or the efficient production of pseudotype particles with this GP. When Axl, Tyro3, DC-SIGN, or LSECtin was expressed instead of LARGE, the titers of the pseudotypes with any of the LCMV-GPs were higher than those without these molecules (Fig. 2). These results suggest that DG-independent infection by LCMV [11, 16, 29] is caused by Axl, Tyro3, DC-SIGN, or LSECtin expression, and that infection caused by the expression of these four molecules does not correlate directly with LCMV virulence, because LCMV virulence has been shown to be correlated with DG-affinity [1, 18, 26, 29, 32] and infection of any LCMV-GP-pseudotypes with various DG-affinities was enhanced by the expression of the four molecules (Fig. 2). As with Lassa virus replication [28], LCMV replication in the liver may be supported by Axl and LSECtin expression [1, 18]; DG is not functional as a receptor for LCMV in this organ due to its lack of O-mannosylation [15]. At present, we cannot exclude the possibility that Gas6/protein S ligands for the TAM family in fetal calf serum enhance infection of LCMV-GP-pseudotypes by bridging molecules of the TAM family to the virion envelope component phosphatidylserine [20]. The use of mutant molecules of the TAM family that lack affinity to Gas6/protein S may help clarify this point.
Fig. 1.
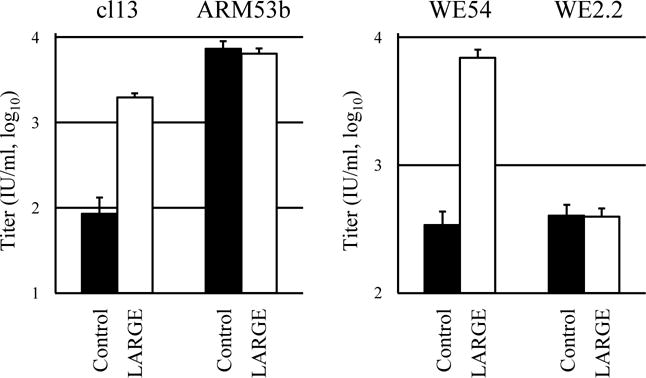
Titers of lentiviral pseudotypes carrying LCMV-GP as an envelope protein in Jurkat cells expressing the indicated molecules. GPs from the cl13, ARM53b, WE54, and WE2.2 strains of LCMV were used. Molecules expressed were control (fCD2ΔCT) and LARGE. Serially diluted pseudotypes were inoculated onto cells and, 48 hr later, reporter (Venus)-expressing cells were counted under a fluorescence microscope. Data are means ± standard deviations (n = 3). IU, infectious units.
Fig. 2.
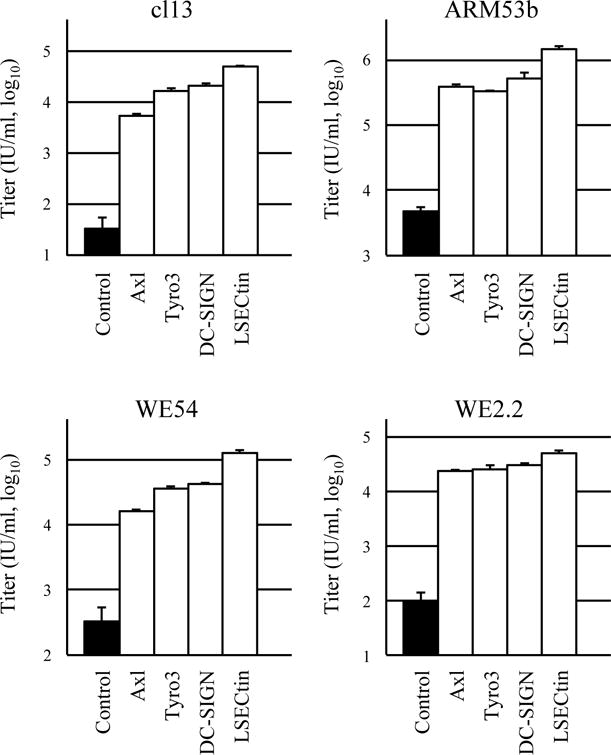
Titers of lentiviral pseudotypes carrying LCMV-GP as an envelope protein in Jurkat cells expressing control (fCD2ΔCT), Axl, Tyro3, DC-SIGN, and LSECtin. Titers were determined by using the method described in the Fig. 1 legend. Data are means ± standard deviations (n = 3).
In glioma, proteolytic cleavage occurs within the ectodomain of β-DG, resulting in a reduced level of cell surface α-DG [6], and, therefore, the DG of glioma should be inefficient or nonfunctional as a receptor for LCMV. In contrast, Axl is frequently expressed at a high level in glioma [33] and, in fact, Axl overexpression in glioma is predictive of a poor prognosis [10]. The fact that LCMV-GP-pseudotypes show tropism to glioma but not to normal neurons [2, 19, 31] may be attributed to the increased expression level of Axl on tumor cells, because Axl expression strikingly enhanced cellular susceptibility to infection by the pseudotypes (Fig. 2). Because reduced α-DG and high Axl/Tyro3 expression levels have also been reported in many malignant tumors including breast, pancreatic, prostate, and colon carcinoma cells [3, 12, 17], LCMV-GP-pseudotypes may be useful in gene therapy against these tumors as well as glioma. We hope that our findings will help improve targeted gene therapy with viral vectors and further our understanding of the pathogenesis of LCMV infection.
Acknowledgments
We thank Dr. Lukas Flatz (University of Geneva, Geneva, Switzerland) for providing cDNAs encoding the LCMV-GP cl13 and WE54 strains. This work was supported in part by Grants-in-Aid for Exploratory Research, by a Contract Research Fund for the Program of Founding Research Centers for Emerging and Reemerging Infectious Diseases, by ERATO (Japan Science and Technology Agency), by the Special Coordination Funds for Promoting Science and Technology from the Ministry of Education, Culture, Sports, Science, and Technology of Japan, and by National Institute of Allergy and Infectious Diseases Public Health Service research grants.
References
- 1.Bergthaler A, Merkler D, Horvath E, Bestmann L, Pinschewer DD. Contributions of the lymphocytic choriomeningitis virus glycoprotein and polymerase to strain-specific differences in murine liver pathogenicity. J Gen Virol. 2007;88:592–603. doi: 10.1099/vir.0.82428-0. [DOI] [PubMed] [Google Scholar]
- 2.Beyer WR, Westphal M, Ostertag W, von Laer D. Oncoretrovirus and lentivirus vectors pseudotyped with lymphocytic choriomeningitis virus glycoprotein: generation, concentration, and broad host range. J Virol. 2002;76:1488–1495. doi: 10.1128/JVI.76.3.1488-1495.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brennan PA, Jing J, Ethunandan M, Górecki D. Dystroglycan complex in cancer. Eur J Surg Oncol. 2004;30:589–592. doi: 10.1016/j.ejso.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 4.Buchmeier MJ, de la Torre JC, Peters CJ. Arenaviridae: The viruses and their replication. In: Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE, editors. Fields Virology. 5th. Lippincott, Williams & Wilkins; Philadelphia: 2007. pp. 1791–1827. [Google Scholar]
- 5.Burns JC, Friedmann T, Driever W, Burrascano M, Yee JK. Vesicular stomatitis virus G glycoprotein pseudotyped retroviral vectors: Concentration to very high titer and efficient gene transfer into mammalian and nonmammalian cells. Proc Natl Acad Sci U S A. 1993;90:8033–8037. doi: 10.1073/pnas.90.17.8033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calogero A, Pavoni E, Gramaglia T, D’Amati G, Ragona G, Brancaccio A, Petrucci TC. Altered expression of α-dystroglycan subunit in human gliomas. Cancer Biol Ther. 2006;5:441–448. doi: 10.4161/cbt.5.4.2546. [DOI] [PubMed] [Google Scholar]
- 7.Cao W, Henry MD, Borrow P, Yamada H, Elder JH, Ravkov EV, Nichol ST, Compans RW, Campbell KP, Oldstone MBA. Identification of α-dystroglycan as a receptor for lymphocytic choriomeningitis virus and Lassa fever virus. Science. 1998;282:2079–2081. doi: 10.1126/science.282.5396.2079. [DOI] [PubMed] [Google Scholar]
- 8.Gramberg T, Soilleux E, Fisch T, Lalor PF, Hofmann H, Wheeldon S, Cotterill A, Wegele A, Winkler T, Adams DH, Pöhlmann S. Interactions of LSECtin and DC-SIGN/DC-SIGNR with viral ligands: Differential pH dependence, internalization and virion binding. Virology. 2008;373:189–201. doi: 10.1016/j.virol.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hutterer M, Gunsilius E, Stockhammer G. Molecular therapies for malignant glioma. Wien Med Wochenschr. 2006;156:351–363. doi: 10.1007/s10354-006-0308-3. [DOI] [PubMed] [Google Scholar]
- 10.Hutterer M, Knyazev P, Abate A, Reschke M, Maier H, Stefanova N, Knyazeva T, Barbieri V, Reindl M, Muigg A, Kostron H, Stockhammer G, Ullrich A. Axl and growth arrest-specific gene 6 are frequently overexpressed in human gliomas and predict poor prognosis in patients with glioblastoma multiforme. Clin Cancer Res. 2008;14:130–138. doi: 10.1158/1078-0432.CCR-07-0862. [DOI] [PubMed] [Google Scholar]
- 11.Imperiali M, Spörri R, Hewitt J, Oxenius A. Post-translational modification of α-dystroglycan is not critical for lymphocytic choriomeningitis virus receptor function in vivo. J Gen Virol. 2008;89:2713–2722. doi: 10.1099/vir.0.2008/004721-0. [DOI] [PubMed] [Google Scholar]
- 12.Jiang X, Rieder S, Giese NA, Friess H, Michalski CW, Kleeff J. Reduced α-dystroglycan expression correlates with shortened patient survival in pancreatic cancer. J Surg Res. 2011;171:120–126. doi: 10.1016/j.jss.2009.11.730. [DOI] [PubMed] [Google Scholar]
- 13.Kobasa D, Rodgers ME, Wells K, Kawaoka Y. Neuraminidase hemadsorption activity, conserved in avian influenza A viruses, does not influence viral replication in ducks. J Virol. 1997;71:6706–6713. doi: 10.1128/jvi.71.9.6706-6713.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kobinger GP, Deng S, Louboutin JP, Vatamaniuk M, Matschinsky F, Markmann JF, Raper SE, Wilson JM. Transduction of human islets with pseudotyped lentiviral vectors. Hum Gene Ther. 2004;15:211–219. doi: 10.1089/104303404772680010. [DOI] [PubMed] [Google Scholar]
- 15.Kunz S, Rojek JM, Kanagawa M, Spiropoulou CF, Barresi R, Campbell KP, Oldstone MBA. Posttranslational modification of α-dystroglycan, the cellular receptor for arenaviruses, by the glycosyltransferase LARGE is critical for virus binding. J Virol. 2005;79:14282–14296. doi: 10.1128/JVI.79.22.14282-14296.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kunz S, Sevilla N, Rojek JM, Oldstone MBA. Use of alternative receptors different than α-dystroglycan by selected isolates of lymphocytic choriomeningitis virus. Virology. 2004;325:432–445. doi: 10.1016/j.virol.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 17.Linger RMA, Keating AK, Earp HS, Graham DK. TAM receptor tyrosine kinases: biologic functions, signaling, and potential therapeutic targeting in human cancer. Adv Cancer Res. 2008;100:35–83. doi: 10.1016/S0065-230X(08)00002-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lukashevich IS, Rodas JD, Tikhonov II, Zapata JC, Yang Y, Djavani M, Salvato MS. LCMV-mediated hepatitis in rhesus macaques: WE but not ARM strain activates hepatocytes and induces liver regeneration. Arch Virol. 2004;149:2319–2336. doi: 10.1007/s00705-004-0385-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miletic H, Fischer YH, Neumann H, Hans V, Stenzel W, Giroglou T, Hermann M, Deckert M, Von Laer D. Selective transduction of malignant glioma by lentiviral vectors pseudotyped with lymphocytic choriomeningitis virus glycoproteins. Hum Gene Ther. 2004;15:1091–1100. doi: 10.1089/hum.2004.15.1091. [DOI] [PubMed] [Google Scholar]
- 20.Morizono K, Xie Y, Olafsen T, Lee B, Dasgupta A, Wu AM, Chen ISY. The soluble serum protein Gas6 bridges virion envelope phosphatidylserine to the TAM receptor tyrosine kinase Axl to mediate viral entry. Cell Host & Microbe. 2011;9:286–298. doi: 10.1016/j.chom.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nagai T, Ibata K, Park ES, Kubota M, Mikoshiba K, Miyawaki A. A variant of yellow fluorescent protein with fast and efficient maturation for cell-biological applications. Nat Biotechnol. 2002;20:87–90. doi: 10.1038/nbt0102-87. [DOI] [PubMed] [Google Scholar]
- 22.Niwa H, Yamamura K, Miyazaki J. Efficient selection for high-expression transfectants with a new eukaryotic vector. Gene. 1991;108:193–200. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- 23.Park F. Correction of bleeding diathesis without liver toxicity using arenaviral-pseudotyped HIV-1-based vectors in hemophilia A mice. Hum Gene Ther. 2003;14:1489–1494. doi: 10.1089/104303403769211691. [DOI] [PubMed] [Google Scholar]
- 24.Salvato M, Borrow P, Shimomaye E, Oldstone MBA. Molecular basis of viral persistence: a single amino acid change in the glycoprotein of lymphocytic choriomeningitis virus is associated with suppression of the antiviral cytotoxic T-lymphocyte response and establishment of persistence. J Virol. 1991;65:1863–1869. doi: 10.1128/jvi.65.4.1863-1869.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schauber CA, Tuerk MJ, Pacheco CD, Escarpe PA, Veres G. Lentiviral vectors pseudotyped with baculovirus gp64 efficiently transduce mouse cells in vivo and show tropism restriction against hematopoietic cell types in vitro. Gene Ther. 2004;11:266–275. doi: 10.1038/sj.gt.3302170. [DOI] [PubMed] [Google Scholar]
- 26.Sevilla N, Kunz S, Holz A, Lewicki H, Homann D, Yamada H, Campbell KP, de La Torre JC, Oldstone MBA. Immunosuppression and resultant viral persistence by specific viral targeting of dendritic cells. J Exp Med. 2000;192:1249–1260. doi: 10.1084/jem.192.9.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shimojima M, Ikeda Y, Kawaoka Y. The mechanism of Axl-mediated Ebola virus infection. J Infect Dis. 2007;196:S259–S263. doi: 10.1086/520594. [DOI] [PubMed] [Google Scholar]
- 28.Shimojima M, Ströher U, Ebihara H, Feldmann H, Kawaoka Y. Identification of cell surface molecules involved in dystroglycan-independent lassa virus cell entry. J Virol. 2012;86:2067–2078. doi: 10.1128/JVI.06451-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smelt SC, Borrow P, Kunz S, Cao W, Tishon A, Lewicki H, Campbell KP, Oldstone MBA. Differences in affinity of binding of lymphocytic choriomeningitis virus strains to the cellular receptor α-dystroglycan correlate with viral tropism and disease kinetics. J Virol. 2001;75:448–457. doi: 10.1128/JVI.75.1.448-457.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spiropoulou CF, Kunz S, Rollin PE, Campbell KP, Oldstone MB. New World arenavirus clade C, but not clade A and B viruses, utilizes alpha-dystroglycan as its major receptor. J Virol. 2002;76:5140–5146. doi: 10.1128/JVI.76.10.5140-5146.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steffens S, Tebbets J, Kramm CM, Lindemann D, Flake A, Sena-Esteves M. Transduction of human glial and neuronal tumor cells with different lentivirus vector pseudotypes. J Neurooncol. 2004;70:281–288. doi: 10.1007/s11060-004-6046-8. [DOI] [PubMed] [Google Scholar]
- 32.Teng MN, Borrow P, Oldstone MB, de la Torre JC. A single amino acid change in the glycoprotein of lymphocytic choriomeningitis virus is associated with the ability to cause growth hormone deficiency syndrome. J Virol. 1996;70:8438–8443. doi: 10.1128/jvi.70.12.8438-8443.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vajkoczy P, Knyazev P, Kunkel A, Capelle HH, Behrndt S, von Tengg-Kobligk H, Kiessling F, Eichelsbacher U, Essig M, Read TA, Erber R, Ullrich A. Dominant-negative inhibition of the Axl receptor tyrosine kinase suppresses brain tumor cell growth and invasion and prolongs survival. Proc Natl Acad Sci U S A. 2006;103:5799–5804. doi: 10.1073/pnas.0510923103. [DOI] [PMC free article] [PubMed] [Google Scholar]


