Abstract
Purpose
To evaluate the association of baseline ellipsoid zone (EZ) parameters on optical coherence tomography (OCT) as calculated by a semi-automated computer algorithm with baseline visual acuity in eyes with retinal vein occlusion (RVO).
Design
Retrospective consecutive case series.
Subjects
Patients affected by RVO presenting from January 2011 to December 2014
Methods
Baseline demographics, clinical characteristics, and SD-OCT data at presentation were collected. Macular cube scans were exported into a retinal layer analysis software platform and outer retinal parameters were evaluated. Outer retinal/EZ parameters included EZ-retinal pigment epithelium (RPE) volume, central foveal EZ-RPE area, EZ-RPE central subfield thickness (CST), and EZ-RPE central foveal thickness (CFT). In addition, en face EZ mapping features were extracted including percent area with EZ attenuation (i.e., EZ-RPE thickness < 20 μm) and percent area with total EZ loss (i.e., EZ-RPE thickness = 0 μm).
Main Outcome Measure
Correlation of EZ parameters and baseline visual acuity (VA). Secondary outcome measures: Correlation of EZ parameters with other clinical characteristics and OCT measures of cube volume, cube average thickness, central subfield thickness.
Results
One hundred and twelve eyes were included in this analysis. Mean baseline VA was 56.53 ±17.68 ETDRS letters and was inversely associated with total EZ loss and EZ-RPE attenuation (r= − 0.33 and −0.38 respectively, p<0.001). VA was directly associated with all other EZ parameters (r=0.37 to 0.45, p<0.001). The presence of subretinal fluid was strongly linked to central parameters of central foveal EZ-RPE, EZ-RPE-CST, and EZ-RPE-CFT (Kruskal-Wallis test). Conventional OCT parameters (central subfield retinal thickness, cube volume and cube average thickness) did not have significant correlations with EZ measures (−0.3<R<0.3 and/or P>0.05).
Conclusion
Baseline EZ integrity is closely linked to presenting visual acuity in eyes with RVO and macular edema. EZ mapping provides an additional metric for evaluating RVO impact on retinal anatomy and potential function.
Keywords: Retinal vein occlusion, Ellipsoid Zone, Automated, Image processing, Optical Coherence Tomography, Macular edema
INTRODUCTION
Photoreceptors comprise the outermost cellular layer of the neuroretinal layer and are the main cells in visual transduction. Their loss has an undeniable effect on vision1. Optical coherence tomography (OCT) provides outstanding visualization of retinal anatomy including the outer retinal bands that represent the external limiting membrane, the ellipsoid zone (previously referred as the photoreceptor inner segment/outer segment (IS/OS) junction), and the interdigitation zone (also referred to as the cone outer segment tips). 2, 3 Ellipsoid zone (EZ) integrity on OCT has been linked with visual function in various diseases. 4–7
Retinal vein occlusions (RVOs) which include central RVOs (CRVO), branch RVOs (BRVO) and hemiretinal RVOs (HRVO) are a group of diseases caused by obstruction of venous flow. RVOs cause sudden painless visual loss accompanied by retinal hemorrhages, retinal edema, and venous engorgement, and vascular tortuosity. The cause of visual loss in RVOs are macular edema (ME), ischemia, and the presence of exudates and hemorrhages.8 As VEGF has an important role in pathogenesis of ME in RVOs9, an effective way of treatment of this type of ME secondary to RVO is the injection of anti-VEGF drugs. Presence of intra-retinal fluid, cystoid type of macular edema, among others are predictive factors for poor visual outcome after treatment with anti-VEGF agents in RVOs.10 Cystoid macular edema (CME) has been shown to be associated with photoreceptor damage, the degree of which correlates with visual outcome.11, 12 Among CRVOs, macular thickness and integrity of EZ have been found to correlate with visual acuity and prognosis.13 Initial visual acuity is a predictor of integrity of EZ after resolution of ME and of final visual acuity in CRVO.4
The extent of macular edema has been linked to poor visual outcomes in RVO and has been associated with photoreceptor loss and EZ loss.11, 12 Quantitative assessment of EZ integrity and outer retinal parameters has not been explored in RVOs. The ability to objectively evaluate EZ metrics would allow for a unique assessment of anatomic factors associated with functional parameters in RVOs. The EZ integrity provides a unique opportunity for outer retinal assessment and potentially overall integrity of the photoreceptor outer segment.14, 15 The purpose of this study was to evaluate quantitative EZ metrics in eyes presenting with RVO and secondary ME to better elucidate the impact of EZ status on baseline visual acuity and their association with other OCT parameters.
PATIENTS AND METHODS
This retrospective study was performed at Cole Eye Institute (Cleveland, Ohio) after approval from the Cleveland Clinic Investigational Review Board (IRB). All study related procedures were performed in accordance with good clinical practice and applicable FDA regulations. The IRB determined that informed consent was not necessary for this study because of its retrospective nature and absence of risks to subjects involved.
Patients with a diagnosis of RVO including CRVO, HRVO and BRVO (International Classification of Disease -9 codes: 362.35, 362.36, 362.37) presented between January 2011 and December 2014 were identified. Exclusion criteria were: (1) age less than 18 years; (2) absence of macular cube on SD-OCT; (3) presence of active macular diseases other than RVO; (4) CST of < 330 or > 600 microns; and (5) OCT data of limited quality or signal strength that limited analysis by the software. The previously listed CST was selected given the potential concern of significant shadowing resulting in artefactual EZ attenuation in eyes with severe edema, as well as the concern that inner retinal ischemia in eyes with minimal retinal thickness. Eyes were divided into quintiles to identify those eyes at extremes of retinal thickness. The central 3 quintiles were selected for analysis. This included eyes with CST between 330 and 600 microns. This range of CST was chosen to exclude both cases with retinal atrophy at presentation, and cases with severe macular edema, which by scattering and absorption of the incident light can attenuate OCT signal at the level of the EZ, causing artifactual low visibility of EZ. So all patients had some degree of mild to moderate ME.
Demographic and clinical characteristics of patients at presentation including age, gender, systemic co-morbidities (e.g., diabetes, hypertension), visual acuity, intraocular pressure (IOP), and type of RVO were collected and SD-OCT parameters, including central subfield thickness (CST), cube volume (CV) and cube average thickness (CAT) were documented. Snellen visual acuity, was converted to ETDRS letter scores using the formula: 85+50xlog (Snellen fraction).16
All eyes had undergone SD-OCT (Cirrus, Zeiss, Dublin, California), including a 6-mm 512x128 macular cube scan. SD-OCT data was exported and analyzed, with the EZ mapping and analysis platform, as previously described.14, 15 In brief, each macular cube scan was imported into the EZ mapping platform. Automated retinal layer segmentation, including internal limiting membrane, EZ, and RPE lines, was performed within the software platform. Following initial automated segmentation, line-by-line review of each scan was performed by a trained reader with manual correction of segmentation, as needed. After verification of optimal segmentation, multiple EZ parameters were exported for analysis including EZ-RPE volume, EZ-RPE central foveal area, EZ-RPE central foveal thickness (CFT), and the EZ-RPE CST. EZ-RPE volume represents the retinal volume contained within the macular cube bounded by the EZ and RPE lines. The EZ-RPE central foveal area is the retinal area bounded by the EZ and RPE on the foveal B-scan. The EZ-RPE central foveal thickness is the linear distance between the EZ and the RPE at the fovea. The EZ-RPE CST represented the mean thickness between the EZ-RPE lines within the central 1 mm area. En face assessment of EZ-RPE maps including evaluation of EZ attenuation percentage (i.e., EZ-RPE thickness < 20 microns) within the macular cube and percentage of total EZ loss (i.e., EZ-RPE thickness = 0) within the macular cube. The presence of the EZ for segmentation was determined based on whether it could be visibly discriminated at a given location on the B-scan regardless of the presence of other pathologic findings (e.g., intraretinal fluid, subretinal fluid). For descriptive purposes, EZ-RPE volume and the en face mapping metrics are referred to as global/panmacular parameters. The other are considered central parameters.
STATISTICAL METHODS
Categorical factors were summarized as frequencies and percentages, while continuous measures were summarized with medians and quartiles since many of the EZ parameters were not normally distributed. Associations with continuous measures were evaluated using Spearman correlations, while Wilcoxon rank sum and Kruskal-Wallis tests were used for associations with categorical factors. Although p-values are presented, as sample size is very influential of statistical significance in these comparisons, the focus of the analysis was on the magnitude of correlations. Correlations between −0.3 and 0.3 were considered to be weak. Analysis was performed using SAS software (version 9.4; Cary, NC). Additional analysis was performed to identify ranges of data where associations between CST and EZ measures were limited.
RESULTS
A total of 271 patients with RVO presented to Cole eye institute from January 2011 to December 2014. One hundred twelve eyes of 112 patients were included after applying the inclusion and exclusion criteria in this analysis. Demographic and baseline characteristics are presented in Table 1. Patients were from 45 to 93 years old (mean 69.17±13.05), 57 (50.9%) were female. There were 41 (36.6%) cases of CRVO, 62 (55.4%) cases of BRVO, and 9 (8%) cases of HRVO. Mean baseline VA was 56.53 ±17.68 ETDRS letters (equal to 20/57 in Snellen acuity) with a range of 4.79–85, (hand motion to 20/20 in Snellen acuity). Mean CST was 452.34±77.11 microns (range: 334–600), mean CV was 11.42±1.94 mm3, (range: 2.7–18), and mean CAT was 318.69±54.12 microns (range:75–499). EZ parameters are given in Table 2. Forty-three eyes (38.7%) had subretinal fluid SRF in OCT. There were no correlations between age, IOP, type of RVO, presence of SRF, CST, CV, and CAT with visual acuity in ETDRS letters (p>0.05 for all).
Table 1.
Baseline characteristics
| Age | 69.17±13.05 (45–93) |
|
| |
| Eye | |
| OD | 52(46.4%) |
| OS | 60 (53.6%) |
|
| |
| Sex | |
| Female | 57 (50.9%) |
| Male | 55(49.1%) |
|
| |
| Diabetes Mellitus | |
| Yes | 38(33.9%) |
|
| |
| IOP | 16.66±3.37 |
| Range | (9–26) |
|
| |
| Glaucoma | |
| Yes | 24 (21.6%) |
|
| |
| Lens status | |
| Phakic | 82(73.2%) |
| Pseudophakic | 30(26.8%) |
|
| |
| Type of RVO | |
| HRVO | 9 (8%) |
| BRVO | 62 (55.4%) |
| CRVO | 41 (36.6%) |
|
| |
| Subretinal Fluid | |
| Present | 43(38.7%) |
BRVO: branch retinal vein occlusion; CRVO: central retinal vein occlusion; HRVO: hemi-retinal vein occlusion; IOP: Intraocular pressure; OD: right eye; OS: left eye; RVO: retinal vein occlusion.
Table 2.
Ellipsoid zone parameters in Retinal Vein Occlusion.
| Minimum | Maximum | Mean ± SD | |
|---|---|---|---|
| Percentage of EZ attenuation* | 0.12 | 83.94 | 14.86 ±16.67 |
| Percentage of total EZ loss | 0.02 | 81.80 | 12.10 ±16.12 |
| EZ-RPE volume (mm3) | 0.22 | 1.36 | 1.06 ±0.21 |
| EZ-RPE central foveal area (mm2) | 0.00 | 0.29 | 0.16 ±0.05 |
| EZRPE_CFT (microns) | 0.00 | 47.97 | 26.57 ±8.15 |
| EZRPE_CST (microns) | 0.00 | 68.14 | 17.41 ±13.82 |
Thickness of less than 20 microns.
EZ: ellipsoid zone; EZ-RPE central foveal area: area of EZ-RPE in the central foveal B scan; EZ-RPE-CFT: thickness of EZ-RPE in central foveal B scan; EZ-RPE-CST: average thickness of EZ-RPE in central subfield area; EZ-RPE volume: volume of the EZ-RPE in the macular cube scan; RPE: retinal pigment epithelium; SD: standard deviation.
There was no association between EZ parameters and age, gender, lens status, or type of RVO. In the presence of SRF central EZ-RPE measures were significantly lower (p<0.01 for all central parameters). The presence or absence of SRF did not affect global EZ-RPE measures (Table 3).
Table 3.
EZ parameters in eyes with and without subretinal fluid.
| SRF absent (N=68) | SRF present (N=43) | p | |
|---|---|---|---|
| Percentage of EZ attenuation* | 11.90±16.13 | 12.63±16.37 | 0.383 |
| Percentage of total EZ loss | 14.46±16.28 | 15.80±17.52 | 0.582 |
| EZ-RPE volume (mm3) | 1.08±0.21 | 1.04±0.21 | 0.457 |
| EZ-RPE central foveal area (mm2) | 0.17±0.05 | 0.15±0.05 | 0.008 |
| EZ-RPE-CFT (microns) | 27.84±8.15 | 24.45±7.86 | 0.008 |
| EZ-RPE-CST (microns) | 21.2±13.29 | 11.47±12.81 | <0.001 |
Thickness of less than 20 microns.
EZ: ellipsoid zone; EZ-RPE central foveal area: area of EZ-RPE in the central foveal B scan; EZ-RPE-CFT: thickness of EZ-RPE in central foveal B scan; EZ-RPE-CST: average thickness of EZ-RPE in central subfield area; EZ-RPE volume: volume of the EZ-RPE in the macular cube scan; RPE: retinal pigment epithelium; SRF: subretinal fluid.
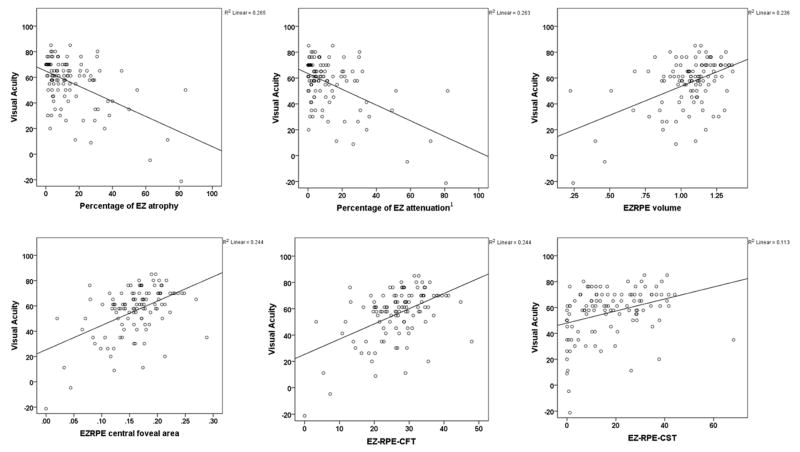
Visual acuity was strongly correlated to EZ parameters (Figure 2). Visual acuity was negatively associated with global measures of attenuation (r=−0.38, p<0.001) and atrophy (r=−0.33, p<0.001), which are measures of the percentage of attenuated EZ (EZ-RPE height of less than 20 μm) or total EZ loss in a cube scan, and positively associated with the total volume of EZ-RPE (r=0.38, p<0.001), EZ-RPE central foveal area (r=0.45, p<0.001), EZ-RPE CFT (r=0.45, p<0.001), and EZ-RPE CST (r=0.4, p<0.001). There were no strong and significant correlations among conventional SD-OCT parameters of CST, CV and CAT with EZ measures (−0.3> r <0.3, and/ or p>0.05).
Figure 2.
Scatter plots of correlations between different ellipsoid zone measures and visual acuity (in ETDRS letters). Abbreviations: EZ: ellipsoid zone; EZ-RPE central foveal area: area of EZ-RPE in the central foveal B scan; EZ-RPE-CFT: thickness of EZ-RPE in central foveal B scan; EZ-RPE-CST: average thickness of EZ-RPE in central subfield area; EZ-RPE volume: volume of the EZ-RPE in the macular cube scan; RPE: retinal pigment epithelium. 1. Attenuation is height of less than 20 microns.
DISCUSSION
In this study, all EZ measures including both global and central measures had significant correlation with visual acuity in eyes with RVO and ME. Percentages of EZ-RPE attenuation and loss had a negative correlation with visual acuity. EZ-RPE volume and the central measures of central foveal area, CFT and CST of EZ-RPE had a positive correlation with VA.
The ellipsoid zone, previously referred to as the third hyper reflective band, is a landmark in OCT commonly used for evaluation of photoreceptor health. Its integrity has correlation with visual function in various diseases including RVO.4–7 Most studies on EZ, are qualitative17–21, or quantitative with manual measurements on OCT B scans22, 23, or quantitative on en face C scans at the level of the EZ.24, 25
Evaluation of the EZ in the acute stages of RVO in the presence of edema is hindered by attenuation of the OCT signal in the outer retina,25, 26 which can affect output of algorithms for EZ measurement. Due to this effect, cases with severe edema (CST>600μm) were excluded in our study. In the presence of severe edema, quantification of EZ changes is very difficult or impossible manually, To overcome this problem, one report evaluated the integrity of EZ in uninvolved retina of BRVO eyes and found predictive value for the integrity of the band in uninvolved retina at 500 and 1000 μm from the fovea for visual acuity after reabsorption of edema.12 Others evaluated the EZ measures after resolution of macular edema4, 27 and observed that a preserved EZ after resolution of macular edema in eyes with CRVO was associated with better visual outcome as well as better initial vision and less edema at presentation.4
These studies highlight the importance of automated quantification of EZ parameters in the presence of macular edema where manual measurement of EZ is either not feasible or has low reliability. Although the effect of photoreceptor health on the visual outcome of RVOs is well-established, the inner retina also has an important function in visual outcome.4 Kadomoto et al found non-perfusion within the parafoveal area to be the most strongly correlated factor with vision, even more than the EZ defect on OCT, in eyes with resolved macular edema of BRVO.6 Reports have been published on the loss of photoreceptors in areas with deep capillary non perfusion in OCTA.25, 27, 28 Ischemia makes the inner retina more hyper reflective in OCT.29–31 Hyperreflectivity of inner retinal tissue can attenuate the OCT signal in outer retina, and may interfere with visibility of the EZ. The same applies to hemorrhages and exudates, which are hyper-reflective and attenuate the outer retinal signal32, 33 Kanakis et al25 used a method to remove shadowing from the OCT C scans and was able to relate areas with attenuation of the EZ with ischemic areas. Ischemia has been found to correlate with the presence of edema34, and severe edema is mostly seen in ischemic CRVO.35 Therefore, visibility of the EZ is dependent on a multitude of other interconnected factors in addition to photoreceptor loss, including optical and histologic effects of both edema and ischemia. Thus it is conceivable that EZ parameters may change with resolution of edema as observed in some studies.29 In the current study all baseline EZ parameters had significant correlations with baseline vision. The importance of this finding is not well defined, and more studies are needed to explore correlations with visual outcome. The association of EZ parameters with baseline vision which is similar to the results of previous studies, is confirmatory of reliability of the algorithm output in eyes with macular edema in the specified range. This paves the way for studies using automated quantitative measurement of EZ parameters.
Strengths to this study include the large sample size, automated quantification of EZ parameters with extensive human supervision, and use of the cube scan data of the SD-OCT with both B and C scans. A limitation to this study is inclusion of all types of RVO together and dividing the study into subtypes of RVO may result in more conclusive data. Cases with severe macular edema were excluded as well, due to the attenuation effect of edema on OCT signal at the level of EZ. A longitudinal study on these eyes with comparison of EZ parameters in the acute stage and after resolution of edema can help in determining the reliability of algorithm in cases with severe edema.
In this study, baseline VA was strongly correlated with all EZ parameters, which shows the applicability of quantitative EZ assessment in the presence of edema. Additional research is needed to better elucidate the longitudinal EZ dynamics and the impact of EZ alterations on overall outcomes.
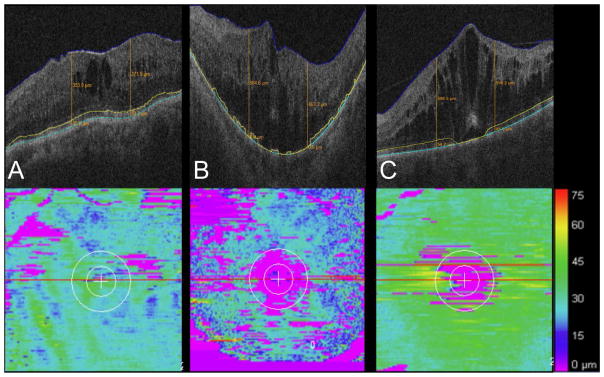
Figure 1. Ellipsoid zone mapping in retinal vein occlusion.
(A) B-scan (upper frame) with mild intraretinal fluid and segmentation lines. Mild-moderate ellipsoid zone (EZ) loss is noted on the EZ-retinal pigment epithelium (RPE) map. (B). B-scan (upper frame) with moderate-severe intraretinal fluid and segmentation lines. Moderate-severe EZ loss is noted on the EZ-RPE map. (C). B-scan (upper frame) with severe intraretinal fluid and segmentation lines. Mild-moderate EZ loss is noted on the EZ-RPE map.
Acknowledgments
Financial Support: NIH/NEI K23-EY022947-01A1 (JPE); Ohio Department of Development TECH-13-059 (JPE);
Footnotes
Meeting Presentation: Presented at the Association for Research in Vision and Ophthalmology (ARVO) conference 2017, Baltimore, MD.
Financial Disclosures: TB: None. RPS: grants and personal fees from Regeneron, grants and personal fees from Alcon, grants and personal fees from Genentech, personal fees from Shire , grants from Zeiss , personal fees from Biogen, outside the submitted work. FFC: None. KC: None; LB: None: JPE: research grants from Genentech, Regeneron, Alcon, Thrombogenics. Consulting fees from ThromboGenics, Alcon, Zeiss, Leica/Bioptigen, Genentech, Santen and Roche. All outside the submitted work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hejtmancik JF, Nickerson JM. Overview of the Visual System. Prog Mol Biol Transl Sci. 2015;134:1–4. doi: 10.1016/bs.pmbts.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 2.Staurenghi G, Sadda S, Chakravarthy U, Spaide RF. Proposed lexicon for anatomic landmarks in normal posterior segment spectral-domain optical coherence tomography: the IN*OCT consensus. Ophthalmology. 2014;121(8):1572–8. doi: 10.1016/j.ophtha.2014.02.023. [DOI] [PubMed] [Google Scholar]
- 3.Tao LW, Wu Z, Guymer RH, Luu CD. Ellipsoid zone on optical coherence tomography: a review. Clin Exp Ophthalmol. 2016;44(5):422–30. doi: 10.1111/ceo.12685. [DOI] [PubMed] [Google Scholar]
- 4.Ota M, Tsujikawa A, Kita M, Miyamoto K, Sakamoto A, Yamaike N, et al. Integrity of foveal photoreceptor layer in central retinal vein occlusion. Retina. 2008;28(10):1502–8. doi: 10.1097/IAE.0b013e3181840b3c. [DOI] [PubMed] [Google Scholar]
- 5.Roohipoor R, Mohammadi N, Ghassemi F, Karkhaneh R, Rezaei M, Nili-Ahmadabadi M, et al. Foveal Structure in Macula-off Rhegmatogenous Retinal Detachment after Scleral Buckling or Vitrectomy. J Ophthalmic Vis Res. 2015;10(2):172–7. doi: 10.4103/2008-322X.163780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kadomoto S, Muraoka Y, Ooto S, Miwa Y, Iida Y, Suzuma K, et al. EVALUATION OF MACULAR ISCHEMIA IN EYES WITH BRANCH RETINAL VEIN OCCLUSION: An Optical Coherence Tomography Angiography Study. Retina. 2017 doi: 10.1097/IAE.0000000000001541. [DOI] [PubMed] [Google Scholar]
- 7.Sayman Muslubas I, Karacorlu M, Arf S, Hocaoglu M, Ersoz MG. Features of the Macula and Central Visual Field and Fixation Pattern in Patients with Retinitis Pigmentosa. Retina. 2017 doi: 10.1097/IAE.0000000000001532. [DOI] [PubMed] [Google Scholar]
- 8.Jaulim A, Ahmed B, Khanam T, Chatziralli IP. Branch retinal vein occlusion: epidemiology, pathogenesis, risk factors, clinical features, diagnosis, and complications. An update of the literature Retina. 2013;33(5):901–10. doi: 10.1097/IAE.0b013e3182870c15. [DOI] [PubMed] [Google Scholar]
- 9.Campochiaro PA. Molecular pathogenesis of retinal and choroidal vascular diseases. Prog Retin Eye Res. 2015;49:67–81. doi: 10.1016/j.preteyeres.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chatziralli I, Theodossiadis G, Chatzirallis A, Parikakis E, Mitropoulos P, Theodossiadis P. RANIBIZUMAB FOR RETINAL VEIN OCCLUSION: Predictive Factors and Long-Term Outcomes in Real-Life Data. Retina. 2017 doi: 10.1097/IAE.0000000000001579. [DOI] [PubMed] [Google Scholar]
- 11.Hunter AA, Modjtahedi SP, Long K, Zawadzki R, Chin EK, Caspar JJ, et al. Improving visual outcomes by preserving outer retina morphology in eyes with resolved pseudophakic cystoid macular edema. J Cataract Refract Surg. 2014;40(4):626–31. doi: 10.1016/j.jcrs.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 12.Ota M, Tsujikawa A, Murakami T, Kita M, Miyamoto K, Sakamoto A, et al. Association between integrity of foveal photoreceptor layer and visual acuity in branch retinal vein occlusion. Br J Ophthalmol. 2007;91(12):1644–9. doi: 10.1136/bjo.2007.118497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodolfo M, Lisa T, Luca DA, Enrico B, Alfonso S, Marta DN, et al. Optical coherence tomography angiography microvascular findings in macular edema due to central and branch retinal vein occlusions. Sci Rep. 2017;7:40763. doi: 10.1038/srep40763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Itoh Y, Ehlers JP. Ellipsoid Zone Mapping and Outer Retinal Characterization after Intravitreal Ocriplasmin. Retina. 2016;36(12):2290–6. doi: 10.1097/IAE.0000000000001110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Itoh Y, Vasanji A, Ehlers JP. Volumetric ellipsoid zone mapping for enhanced visualisation of outer retinal integrity with optical coherence tomography. Br J Ophthalmol. 2016;100(3):295–9. doi: 10.1136/bjophthalmol-2015-307105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gregori NZ, Feuer W, Rosenfeld PJ. Novel method for analyzing snellen visual acuity measurements. Retina. 2010;30(7):1046–50. doi: 10.1097/IAE.0b013e3181d87e04. [DOI] [PubMed] [Google Scholar]
- 17.Sakamoto A, Nishijima K, Kita M, Oh H, Tsujikawa A, Yoshimura N. Association between foveal photoreceptor status and visual acuity after resolution of diabetic macular edema by pars plana vitrectomy. Graefes Arch Clin Exp Ophthalmol. 2009;247(10):1325–30. doi: 10.1007/s00417-009-1107-5. [DOI] [PubMed] [Google Scholar]
- 18.Shin HJ, Lee SH, Chung H, Kim HC. Association between photoreceptor integrity and visual outcome in diabetic macular edema. Graefes Arch Clin Exp Ophthalmol. 2012;250(1):61–70. doi: 10.1007/s00417-011-1774-x. [DOI] [PubMed] [Google Scholar]
- 19.Scarinci F, Jampol LM, Linsenmeier RA, Fawzi AA. Association of Diabetic Macular Nonperfusion With Outer Retinal Disruption on Optical Coherence Tomography. JAMA Ophthalmol. 2015;133(9):1036–44. doi: 10.1001/jamaophthalmol.2015.2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inoue M, Morita S, Watanabe Y, Kaneko T, Yamane S, Kobayashi S, et al. Inner segment/outer segment junction assessed by spectral-domain optical coherence tomography in patients with idiopathic epiretinal membrane. Am J Ophthalmol. 2010;150(6):834–9. doi: 10.1016/j.ajo.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 21.Inoue M, Morita S, Watanabe Y, Kaneko T, Yamane S, Kobayashi S, et al. Preoperative inner segment/outer segment junction in spectral-domain optical coherence tomography as a prognostic factor in epiretinal membrane surgery. Retina. 2011;31(7):1366–72. doi: 10.1097/IAE.0b013e318203c156. [DOI] [PubMed] [Google Scholar]
- 22.Maheshwary AS, Oster SF, Yuson RM, Cheng L, Mojana F, Freeman WR. The association between percent disruption of the photoreceptor inner segment-outer segment junction and visual acuity in diabetic macular edema. Am J Ophthalmol. 2010;150(1):63–7e1. doi: 10.1016/j.ajo.2010.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hariri AH, Velaga SB, Girach A, Ip MS, Le PV, Lam BL, et al. Measurement and Reproducibility of Preserved Ellipsoid Zone Area and Preserved RPE Area in a Cohort of Eyes with Choroideremia. Am J Ophthalmol. 2017 doi: 10.1016/j.ajo.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 24.Gattani VS, Vupparaboina KK, Patil A, Chhablani J, Richhariya A, Jana S. Semi-automated quantification of retinal IS/OS damage in en-face OCT image. Comput Biol Med. 2016;69:52–60. doi: 10.1016/j.compbiomed.2015.11.015. [DOI] [PubMed] [Google Scholar]
- 25.Kanakis MG, Giannouli K, Andreanos K, Papaconstantinou D, Koutsandrea C, Ladas I, et al. CAPILLARY NONPERFUSION AND PHOTORECEPTOR LOSS IN BRANCH RETINAL VEIN OCCLUSION: Spatial Correlation and Morphologic Characteristics. Retina. 2016 doi: 10.1097/IAE.0000000000001410. [DOI] [PubMed] [Google Scholar]
- 26.Spaide RF, Fujimoto JG, Waheed NK. Image Artifacts in Optical Coherence Tomography Angiography. Retina. 2015;35(11):2163–80. doi: 10.1097/IAE.0000000000000765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nesper PL, Scarinci F, Fawzi AA. Adaptive Optics Reveals Photoreceptor Abnormalities in Diabetic Macular Ischemia. PLoS One. 2017;12(1):e0169926. doi: 10.1371/journal.pone.0169926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scarinci F, Nesper PL, Fawzi AA. Deep Retinal Capillary Nonperfusion Is Associated With Photoreceptor Disruption in Diabetic Macular Ischemia. Am J Ophthalmol. 2016;168:129–38. doi: 10.1016/j.ajo.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coady PA, Cunningham ET, Jr, Vora RA, McDonald HR, Johnson RN, Jumper JM, et al. Spectral domain optical coherence tomography findings in eyes with acute ischaemic retinal whitening. Br J Ophthalmol. 2015;99(5):586–92. doi: 10.1136/bjophthalmol-2014-304900. [DOI] [PubMed] [Google Scholar]
- 30.Pichi F, Morara M, Veronese C, Lembo A, Nucci P, Ciardella AP. Perivenular whitening in central vein occlusion described by fundus autofluorescence and spectral domain optical coherence tomography. Retina. 2012;32(7):1438–9. doi: 10.1097/IAE.0b013e31825dd2a7. [DOI] [PubMed] [Google Scholar]
- 31.Sarda V, Nakashima K, Wolff B, Sahel JA, Paques M. Topography of patchy retinal whitening during acute perfused retinal vein occlusion by optical coherence tomography and adaptive optics fundus imaging. Eur J Ophthalmol. 2011;21(5):653–6. doi: 10.5301/EJO.2011.6374. [DOI] [PubMed] [Google Scholar]
- 32.Bolz M, Schmidt-Erfurth U, Deak G, Mylonas G, Kriechbaum K, Scholda C. Optical coherence tomographic hyperreflective foci: a morphologic sign of lipid extravasation in diabetic macular edema. Ophthalmology. 2009;116(5):914–20. doi: 10.1016/j.ophtha.2008.12.039. [DOI] [PubMed] [Google Scholar]
- 33.Munk MR, Dunavoelgyi R, Baratsits M, Matt G, Montuoro A, Buehl W, et al. Detection and Differentiation of Intraretinal Hemorrhage in Spectral Domain Optical Coherence Tomography. Curr Eye Res. 2015;40(10):1046–54. doi: 10.3109/02713683.2014.971931. [DOI] [PubMed] [Google Scholar]
- 34.Spaide RF. Volume-Rendered Optical Coherence Tomography of Retinal Vein Occlusion Pilot Study. Am J Ophthalmol. 2016;165:133–44. doi: 10.1016/j.ajo.2016.02.037. [DOI] [PubMed] [Google Scholar]
- 35.Martinet V, Guigui B, Glacet-Bernard A, Zourdani A, Coscas G, Soubrane G, et al. Macular edema in central retinal vein occlusion: correlation between optical coherence tomography, angiography and visual acuity. Int Ophthalmol. 2012;32(4):369–77. doi: 10.1007/s10792-012-9578-5. [DOI] [PubMed] [Google Scholar]