Abstract
Over the past decade, advances in the interdisciplinary field of network science have provided a framework for understanding the intrinsic structure and function of human brain networks. A particularly fruitful area of this work has focused on patterns of functional connectivity derived from noninvasive neuroimaging techniques such as functional magnetic resonance imaging. An important subset of these efforts has bridged the computational approaches of network science with the rich empirical data and biological hypotheses of neuroscience, and this research has begun to identify features of brain networks that explain individual differences in social, emotional, and cognitive functioning. The most common approach estimates connections assuming a single configuration of edges that is stable across the experimental session. In the literature, this is referred to as a static network approach, and researchers measure static brain networks while a subject is either at rest or performing a cognitively demanding task. Research on social and emotional functioning has primarily focused on linking static brain networks with individual differences, but recent advances have extended this work to examine temporal fluctuations in dynamic brain networks. Mounting evidence suggests that both the strength and flexibility of time-evolving brain networks influence individual differences in executive function, attention, working memory, and learning. In this review, we first examine the current evidence for brain networks involved in cognitive functioning. Then we review some preliminary evidence linking static network properties to individual differences in social and emotional functioning. We then discuss the applicability of emerging dynamic network methods for examining individual differences in social and emotional functioning. We close with an outline of important frontiers at the intersection between network science and neuroscience that will enhance our understanding of the neurobiological underpinnings of social behavior.
Key words: judgment and decision-making, cognitive abilities, social processes, network neuroscience, social neuroscience
Individuals often respond differently when put in the same exact situation. For example, if two individuals were put into a crowded social situation with strangers, they might behave very differently. One person might experience that situation as stressful and negatively arousing, and might cope by behaving quietly and exiting the situation as soon as possible, while the other person might experience the situation as energizing and exciting, and might float around the room interacting with as many people as possible. Recent advances at the intersection of network science and neuroscience have provided insight into how information is transferred across the brain (Sporns, 2013), and how brain regions might work together to navigate social situations (Schmälzle et al., 2017). In this review, we describe network approaches to characterizing complex interactions between brain regions, which can advance understanding of individual differences in social, emotional, and cognitive functioning.
Early work using neuroimaging techniques, including noninvasive functional magnetic resonance imaging (fMRI), attempted to map individual differences in social and emotional functioning to specific brain regions. These studies found robust associations between neural activation and individual differences in approach/avoidance tendencies (Gray et al., 2005), extraversion, neuroticism, and self-consciousness (Eisenberger, Lieberman, & Satpute, 2005), rejection sensitivity (Eisenberger & Lieberman, 2004), self-construal (Ma et al., 2012; Ray et al., 2010), social working memory (Meyer, Spunt, Berkman, Taylor, & Lieberman, 2012), and responses to persuasive health messages (Falk, Berkman, Whalen, & Lieberman, 2011), to name a few. Further, in some studies brain activation can predict individual variation in human behavior above and beyond self-report measures (Cooper, Tompson, O’Donnell, & Falk, 2015; Falk et al., 2015). This work represents an important first step in identifying neural correlates of individual differences in social, emotional, and cognitive functioning.
Brain regions, however, do not operate in isolation: temporal synchronization of neuronal firing across brain regions is a key way in which brain regions communicate and process information, and higher coordination between groups of neurons directly influences neuronal excitation (Fries, 2005, 2015). Thus, focusing on activation within single brain regions ignores potentially useful information about how these brain regions work together (Friston, 2011). To understand why these brain regions predict individual differences in social, emotional, and cognitive functioning, as well as improve our predictive models in applied domains, we must also understand the brain networks involved (Barrett & Satpute, 2013; Medaglia, Lynall, & Bassett, 2015; Sporns, 2014).
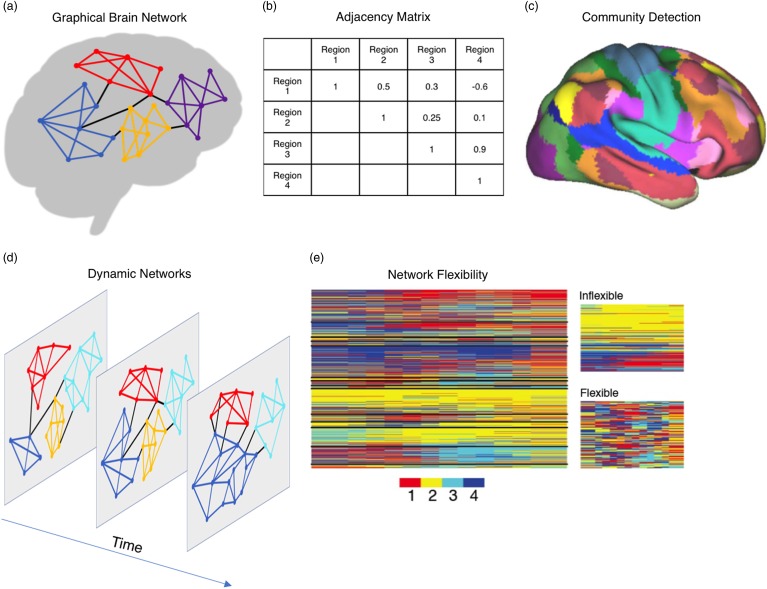
Brain networks exist at multiple time scales, including coordinated activity among regions that is consistent over time and tasks (static networks; Figure 1a) as well as time-evolving synchronization between regions that fluctuate and reconfigure in response to changing task demands (dynamic networks; see Figure 1c; Hutchison et al., 2013). The emerging field of network neuroscience (Bassett & Sporns, 2017) provides conceptual frameworks and computational tools to quantitatively measure and characterize the roles of brain regions in functional networks, to characterize the patterns of interconnections between regions of interest (ROIs) and the rest of the brain, and to link both these roles and patterns to social, emotional, and cognitive functioning.
Figure 1.
Approaches for analyzing brain networks. Brains can be represented as graphs consisting of nodes (regions) and connections between those nodes (connectivity; a), and connection strengths can be mathematically represented in adjacency matrices where each cell represents the strength of the connection between a pair of regions (b). Community detection algorithms take adjacency matrices and partition the brain into modules that contain greater (or stronger) within-community edges than expected in a statistical null model (c). Graphical approaches to studying brains can be extended across time (d). Dynamic networks capture how frequently brain regions (represented in rows) change their allegiance from one community to another (indexed by color), identifying what regions are inflexible (largely the same community affiliation across time steps) versus flexible (changing communities frequently across time steps; e).
1. Overview
In this review, we focus on three categories of individual differences: cognitive functioning, emotional functioning, and social functioning. Although much of the early work examining links between functional connectivity and individual differences in psychological processes focused on pairwise associations between two specific brain regions, we argue that network approaches to characterizing complex patterns of connectivity between brain regions can provide a more complete and richer understanding of how the brain facilitates effective cognitive, emotional, and social functioning.
Characterizing patterns of brain activity as networks is important for understanding how brains lead to psychological processes and behaviors for three key reasons. First, successful task performance often requires subnetworks of the brain to work together, whereas at other times, more competitive dynamics promote more effective performance (Fornito, Harrison, Zalesky, & Simons, 2012; Khambhati, Sizemore, Betzel, & Bassett, in press). As a result, the relationship between connectivity strength and task performance is contingent on the task being performed, the cooccurrence with task-irrelevant connections elsewhere in the brain, and the specificity of the recruited subnetworks. Second, an individual’s ability to flexibility reconfigure brain networks is an important mechanism that drives cognitive functioning (Bassett et al., 2011, 2013; Cole, Bassett, Power, Braver, & Petersen, 2014; Davison et al., 2015; Mattar, Cole, Thompson-Schill, & Bassett, 2015; Shine et al., 2016; Shine, Koyejo, & Poldrack, 2016). Third, network methods can often explain additional variance in behavioral outcomes beyond what is explained by activation in a single brain region. In some cases dynamic network methods can explain twice as much variance in cognitive functioning as static networks or pairwise connections (Jia, Hu, & Deshpande, 2014). Thus, both static and dynamic network methods have the potential to provide important insights into cognitive, emotional, and social functioning.
In this review, we first summarize common approaches for analyzing brain networks and existing evidence for intrinsic functional brain networks, which serve as a framework for understanding the links between brain networks and cognitive, emotional, and social functioning. Next, we describe existing evidence for both static and dynamic brain networks involved in cognitive functioning. Although research on social and emotional functioning has primarily focused on associations with static brain networks, applying dynamic network methods to examining individual differences in social and emotional functioning is an important next step for understanding the neurobiological underpinnings of social behavior. We discuss existing evidence for static brain networks involved in social and emotional functioning, as well as potential applications of dynamic network methods. Although there is an extensive literature linking individual differences in brain responses to clinical states and outcomes, these relationships are beyond the scope of this paper, and we refer readers interested in these topics to Vaidya and Gordon (2013) and Cao, Wang, and He (2015).
2. Approaches for analyzing brain connectivity
Approaches for analyzing static and dynamic brain networks build on earlier work that primarily focused on pairwise connections between brain regions. In this section, we first describe these pairwise approaches, as they form a basis for more advanced network approaches and provide insight into advantages as well as limitations of connectivity methods. We then summarize three common techniques for analyzing brain networks that could help personality researchers better understand how neurobiological processes contribute to individual differences in cognitive, emotional, and social functioning. Finally, we discuss practical considerations for how to implement network methods.
2.1. Pairwise connectivity
Early work examining functional connectivity and its links to psychological processes focused on pairwise connectivity between two brain regions. Pairwise approaches compute the average blood oxygen level-dependent (BOLD) time course from all voxels within a single ROI or seed, and then test the strength of the connectivity between the seed time course and the time course of the BOLD signal in other brain regions (Margulies et al., 2007). Connectivity strength is typically measured using a Pearson’s correlation coefficient (Biswal, Yetkin, Haughton, & Hyde, 1995) or wavelet coherence (Grinsted, Moore, & Jevrejeva, 2004; Müller et al., 2004). Psychophysiological interaction approaches use general linear models to further identify connectivity between two regions that is stronger in one task condition than another (Friston et al., 1997; McLaren, Ries, Xu, & Johnson, 2012), allowing researchers to focus on patterns of connectivity that are directly linked to what a person is doing during a task.
Pairwise approaches are useful for characterizing simplified patterns of connectivity, especially when there is an a priori hypothesis about how one brain region either regulates processing in another brain region or communicates information to it. For example, individual differences in emotion regulation are associated with different coupling between amygdala and prefrontal cortex, such that individuals who show greater decreases in amygdala activation as prefrontal cortex activation increases are better able to down-regulate negative emotions (Lee, Heller, van Reekum, Nelson, & Davidson, 2012).
However, by necessity pairwise connectivity measures ignore the thousands of other connections that are providing and receiving input from the two ROIs. Even relatively basic visual processes require input from complex, evolving, and expansive networks of brain regions (Parks & Madden, 2013). Pairwise approaches therefore offer an overly simplistic view of how brain regions work together to promote efficient information processing and cognitive, social, or emotional functioning (Mišić & Sporns, 2016). Therefore, network approaches for studying brain connectivity can advance understanding of how groups of brain regions work together to process information and ultimately facilitate effective cognitive, social, and emotional functioning (Barrett & Satpute, 2013).
2.2. Systems in intrinsic functional brain networks
Most studies of functional brain networks begin with brain activity that is measured either during a specific task or during what is called the “resting-state” (Raichle, 2015). Task-based fMRI is primarily used to identify functional brain networks that are recruited to perform a specific task. By contrast, resting-state fMRI is primarily used to measure intrinsic brain networks that are present in the absence of an experimentally driven task (Fox et al., 2005; Raichle, 2015; Raichle & Snyder, 2007). Intrinsic brain networks are preserved during sleep and are also present across a wide variety of task states (Cole et al., 2014). Thus, intrinsic brain networks provide an important framework for understanding the stable, fundamental organization of brain connectivity.
Resting-state functional connectivity reveals intrinsic, modular (Meunier, Achard, Morcom, & Bullmore, 2009) but flexible (Mattar, Betzel, & Bassett, 2016) subnetworks that appear to map onto cognitive systems. Some such systems (including default mode, sensory, and motor systems) are highly integrated internally and have relatively few connections to other systems, whereas some other such systems (including executive function systems) share numerous connections with other systems (Power et al., 2011). Moreover, brain systems identified using resting-state functional connectivity are relatively stable and present across a wide variety of task states (Cole et al., 2014). This consistent finding suggests that intrinsic subnetworks identified using resting-state fMRI may capture a stable set of brain states that are modified as necessary to implement task demands (Cole et al., 2014; Mattar et al., 2015). Often community changes are reflected in reduced within-system functional connectivity (Cole et al., 2014), and greater between-system communication as subnetworks work together to complete a task (Cole et al., 2013). Segregation of large-scale brain networks into subnetworks confers numerous advantages to the brain, including the ability to perform complex, highly specialized tasks while maintaining the ability to flexibly adapt to changing task demands (Wig, 2017).
On top of this relatively stable architecture across people, individual variability in connectivity is an important driver of individual differences in social, emotional, and cognitive functioning (Passaro et al., 2017; Vaidya & Gordon, 2013). Specifically, brain regions that show greater variability in connectivity across individuals are more likely to be associated with individual differences in personality traits, anxiety, risk-seeking tendencies, working memory, and perception (Mueller et al., 2013).
2.3. Approaches for analyzing brain networks
Most brain network analyses start with a set of a priori seeds (defined as nodes in the brain graph), and compute the pairwise connectivity (defined as edges in the brain graph) between each seed and every other seed. Using the measures of connectivity described above to quantify the strength of the connection between each brain region and every other brain region in the set, researchers can build an adjacency matrix where each cell in the matrix represents a pairwise connection (Figure 1b). Network science tools can then be applied to these adjacency matrices to describe and characterize the patterns of connectivity across the graph in order to identify topological features of more complex brain networks (Bullmore & Bassett, 2011; Bullmore & Sporns, 2009; Newman, 2010; Rubinov & Sporns, 2010). For the purposes of this review, we will highlight three useful tools that personality neuroscientists can use to examine brain networks.
The first approach examines functional segregation of subnetworks within large-scale brain networks. Segregation of large-scale brain networks into subnetworks facilitates performance by enabling the brain to perform multiple tasks simultaneously and adapt to changing task demands (Wig, 2017). Independent components analysis (ICA) takes the time course in each voxel in the brain and partitions the brain into a set of components where the voxels in each component share similar BOLD time courses (Allen, Erhardt, Wei, Eichele, & Calhoun, 2012; Beckmann, DeLuca, Devlin, & Smith, 2005; Calhoun, Liu, & Adali, 2009). Community detection algorithms (Porter, Onnela, & Mucha, 2009) take the connectivity between nodes in a graph (either voxels, brain regions, or seeds) and partition the brain to maximize within-community connectivity strength (Figure 1b; Sporns & Betzel, 2016; for review, see Garcia, Ashourvan, Muldoon, Vettel, & Bassett, 2017). The power of this first approach arises from the analysis of the resulting network’s topological features, such as the number of subnetworks, their regional configurations, and the proportion of their functional segregation versus integration (relative strength of within-subnetwork connections vs. between-subnetwork connections). Researchers interested in understanding personality have used resting-state fMRI to test for individual differences in the configuration of intrinsic brain networks and found that greater relative within-subnetwork connectivity (vs. between-subnetwork connectivity) is associated with increased neuroticism (Davis et al., 2013). This approach has also been used to study how reconfiguration of these networks during specific tasks might influence personality traits, and found that people who exhibit greater within-subnetwork connectivity while evaluating threat stimuli also score higher on trait neuroticism (Cremers et al., 2010).
The second approach examines network properties associated with information transfer and efficient information processing. For example, measures of path length and efficiency indicate how quickly or easily a piece of information can traverse from one location in the network to another location, under some suitable assumptions of information transmission dynamics (Cole, Yarkoni, Repovs, Anticevic, & Braver, 2012; Mišić, Sporns, & McIntosh, 2014). These measures can be computed globally for a large-scale network or computed locally for each subnetwork. Other measures of network topology include degree, density, rich club, diverse club, and core/periphery structure, and we direct interested readers to Rubinov and Sporns (2010). In short, network measures provide insight into how information may be strategically channeled through nodes with characteristic connectivity properties for specialized signal propagation in a network (Gu et al., 2015; Kim et al., 2018). For example, nodes in a diverse club have edges that are distributed across a large-scale network and are thought to make communication between subnetworks more efficient (Bertolero, Yeo, & D’Esposito, 2017). Taken together, these tools can provide rich insights into how various features of the network topology support effective cognitive, emotional, and social functioning.
The third approach examines temporal dynamics of brain networks, including how subnetworks reconfigure in response to changing task demands. Static network approaches described above can also be extended to study dynamic temporal fluctuations in functional networks. For example, multilayer community detection also partitions the brain into discrete communities, but is applied to data where the time course is separated into time windows (Garcia et al., 2017); this approach can be used to test how graph topology and network configuration might change over time (Figure 1d; Betzel & Bassett, 2016). Nonnegative matrix factorization is another useful algorithm that can be used to partition the brain into subgraphs that vary over time (Lee & Seung, 1999). This latter approach has the added benefit of defining subgraphs based on the extent to which the strength of connectivity between brain regions varies systematically over time, rather than just considering the average connectivity strength (Chai et al., 2017; Khambhati, Mattar, Wymbs, Grafton, & Bassett, 2018).
Dynamic network methods can also be useful for extending static measures of network efficiency (e.g., path length, centrality, etc.) to the temporal domain. For example, path length is a useful measure to examine the ease of information transfer throughout a static brain network (assuming that information is more easily transferred across shorter topological distances), while temporal path length or latency in a dynamic network can indicate the speed with which information can be transferred throughout dynamic brain networks, if the same assumptions hold (Sizemore & Bassett, in press). In addition, time-by-time graphs measure the similarity of the brain topology at each time point with every other time point and can provide useful information about how the brain traverses across cognitive states during a task (Khambhati, Sizemore, et al., in press). These measures have been linked to learning (Reddy et al., 2018) and development (Medaglia et al., 2018), and could also be applied to individual differences where network efficiency is a hypothesized mediator (e.g., intelligence, impulsivity, etc.; see below for examples).
2.4. Practical considerations
Although we argue that network neuroscience methods provide a fruitful set of tools for personality neuroscience, there are challenges and limitations that researchers interested in employing these techniques should consider. First, there is an ongoing debate about the mechanisms underlying measures of functional connectivity (Laumann et al., 2016; Lehmann, White, Henson, Cam-CAN, & Geerligs, 2017; Mateo, Knutsen, Tsai, Shih, & Kleinfeld, 2017; Winder, Echagarruga, Zhang, & Drew, 2017). At a minimum, measures of functional connectivity are extremely sensitive to artifacts, such that a significant proportion of the variance in edge strength is often accounted for by confounds including head motion and other physiological noise (Ciric et al., 2017; Laumann et al., 2016; Satterthwaite et al., 2017).
Furthermore, some researchers have argued that the majority of temporal variations in connectivity are due to physiological noise (Laumann et al., 2016) or spurious variations due to the choice of parameters (Lehmann et al., 2017). Importantly, much of this discussion centers around resting-state fMRI, and there is some evidence that the relationship between neuronal activity and BOLD-based measures of functional connectivity is stronger when completing functional tasks (Winder et al., 2017). The ability of these measures to predict theoretically relevant out-of-scanner tasks also adds confidence in their value (Chai et al., 2017).
Although the above debate has yet to resolve the issue of what percentage of functional connectivity measures is due to artifacts, researchers have developed tools for correcting for many of these confounds. Cleaning the data by regressing out head motion and physiological signals (i.e., global signal, white matter signal, and cerebrospinal fluid signal) and removing high-motion time points from the data can dramatically improve the quality of the connectivity data (Ciric et al., 2017). Thus, cleaning the BOLD signal is an important first step before computing pairwise connectivity metrics, which make up the network adjacency matrix.
In order to construct a brain network from cleaned neuroimaging data, researchers must define the nodes to include in their analyses. Most commonly, these network nodes are defined as contiguous clusters of voxels based on anatomical or functional features. For example, nodes can be defined based on peak voxels from a meta-analysis of a functional response of interest (e.g., using the Neurosynth database; Schmälzle et al., 2017), based on cortical architecture (Yeo et al., 2011), or based on a combination of these approaches (Glasser et al., 2016). One current limitation is that many popular atlases only include cortical regions or have limited precision in subcortical regions, making it difficult to study functions executed by subcortical structures (e.g., reward processing in subcortical regions such as ventral striatum).
Once the network adjacency matrix has been constructed, there are a number of publicly available toolboxes which can readily compute network metrics, including the Brain Connectivity Toolbox (Rubinov & Sporns, 2010). Researchers, however, might be interested in not just what the characteristic path length in a network is, for example, but also whether that path length is significantly longer or shorter than that expected in an appropriate random network null model. Permutation testing by randomly rewiring the network (randomly permuting the association of weights to edges, or randomly reassigning anatomical or subnetwork labels) is a common approach that can provide insight into whether network measures vary significantly from a null model as well as whether they vary between groups (Bassett et al., 2013; Betzel et al., 2017; Zalesky, Fornito, & Bullmore, 2010).
Finally, for the purposes of this review, we will focus on fMRI-based approaches to studying brain networks as they are by far the most common. However, these methods have also been applied to other neuroimaging modalities, including electroencephalography, intracranial electrocorticography, magnetoencephalography, positron emission topography, functional near infrared spectroscopy, and arterial spin labeled perfusion magnetic resonance imaging. We also limit our discussion to three primary psychological domains (cognitive, emotional, and social functioning) to illustrate the work that has been done to use network methods for advancing our understanding of psychology, as well as opportunities to further advance knowledge moving forward.
3. Cognitive functioning
Psychologists and neuroscientists have studied how variations in functional brain networks might explain individual variations in how people think and behave. Studies of functional brain networks have yielded useful insights into individual differences in cognitive functioning. Cognitive functioning frequently involves a combination of basic perceptual tasks and more complex cognitive tasks, and the ability to switch between different brain states leads to better overall performance (Khambhati, Medaglia, Karuza, Thompson-Schill, & Bassett, 2017; Sadaghiani, Poline, Kleinschmidt, & D’Esposito, 2015). To achieve better cognitive performance, the brain may involve multiple sets of functionally specialized regions that form domain-specific, core subnetworks (e.g., language, vision, audition, etc.) and more domain-general regions that flexibly switch between specialized core subnetworks depending on the task (Fedorenko & Thompson-Schill, 2014). Recent work suggests that greater functional separation (modularity) between brain subnetworks supports basic perceptual and cognitive tasks whereas stronger connections between subnetworks (increased integration) facilitates performance on more cognitively complex tasks (Kitzbichler, Henson, Smith, Nathan, & Bullmore, 2011; Shine et al., 2016).
3.1. Static brain networks and cognitive functioning
Much research on individual differences in cognitive functioning has focused on foundational cognitive processes, such as executive function, working memory, and perception. Across these processes, connectivity within the frontoparietal system and between the frontoparietal system and other systems appears to play an important role. Frontoparietal connectivity is positively correlated with working memory performance (Repovs, Csernansky, & Barch, 2011) and task-switching performance (Yin, Wang, Pan, Liu, & Chen, 2015; Zhang et al., 2009). In addition, working memory performance was influenced by network properties of the default mode system, such that greater network efficiency and within-system connectivity was associated with better working memory across individuals (Hampson, Driesen, Skudlarski, Gore, & Constable, 2006). Furthermore, in the case of working memory performance, stronger negative connectivity between default and frontoparietal systems was associated with better working memory performance (Hampson, Driesen, Roth, Gore, & Constable, 2010).
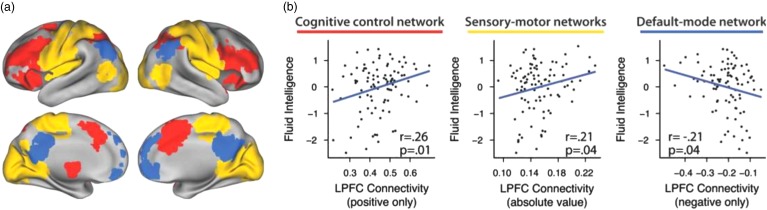
Recent studies have also examined how brain networks contribute to other cognitive traits, including intelligence and creativity. Research on brain networks and intelligence has leveraged graph theoretical approaches to quantify how efficient the brain is at transferring information across brain regions. Greater density of connectivity between the prefrontal cortex and the rest of the brain (Figure 2; Cole et al., 2012), shorter path lengths (Li et al., 2009), reduced interhemispheric connectivity (Santarnecchi, Tatti, Rossi, Serino, & Rossi, 2015), and stronger connections involving moderately weak, long-distance paths (Santarnecchi, Galli, Polizzotto, Rossi, & Rossi, 2014) predict increased IQ scores. Brain networks that are characterized by dense within-system connections with a few strong between-system connections optimize efficient information processing (Bullmore & Bassett, 2011; Muldoon, Bridgeford, & Bassett, 2016). Thus, individuals with brain networks that more efficiently process information have higher IQs than individuals with brain networks that less efficiently process information, providing insight into the neural architecture underlying intelligence.
Figure 2.
Brain subnetworks and cognitive functioning. Using meta-analyses and probabilistic cytoarchitecture, regions affiliated with three subnetworks were identified (cognitive control in red, sensory–motor in yellow, and default mode in blue; a). Measures of brain network efficiency predict fluid intelligence and cognitive control, yielding insights into how the brain processes complex cognitive tasks (b). Figure adapted with permission from Cole et al. (2012). LPFC=lateral prefrontal cortex.
Taken together, these results suggest a complicated relationship between default mode and frontoparietal systems that promotes improved cognitive function (Zanto & Gazzaley, 2013). In general, increased connectivity and efficiency within the frontoparietal system and greater connectivity between the frontoparietal system, subcortical, and sensory networks predicts better cognitive performance (Vaidya & Gordon, 2013; Cohen & D’Esposito, 2016). However, these results also suggest that the default mode system is not simply deactivated during cognitive tasks, and actually might interact with the frontoparietal system in important ways. One theory argues that the default mode, although demonstrating lower activation during tasks than during rest, is still facilitating or monitoring performance (Hampson et al., 2006) and might be important for integrating internally directed thought with processing of external stimuli. In fact, resting-state connectivity between the default mode and inferior frontal gyrus is associated with greater creativity (Beaty et al., 2014), suggesting that low-level spontaneous processes (mind-wandering, mental simulation, etc.) facilitate creativity, but also require some external attention resources to tap and harness those spontaneous processes. These results provide important insights into the complex nature of even basic cognitive functions.
3.2. Dynamic brain networks and cognitive functioning
Although overall activation and configuration of brain networks is important, the degree to which brain networks can flexibly adjust to changing task demands is also important for cognitive performance, and can predict individual differences in cognitive functioning. As evidenced above, successful task performance sometimes requires subnetworks of the brain to work together, whereas at other times more competitive dynamics promote more effective performance. The above work focuses on differences between tasks, but many cognitive tasks require multiple cognitive processes, and thus the optimal configuration of brain networks and cooperation/competition between those networks can also vary within a task.
Dynamic network approaches may be particularly useful in addressing the more complicated push–pull relationships that promote efficient and effective cognitive performance (Calhoun, Miller, Pearlson, & Adalı, 2014; Khambhati, Sizemore, et al., in press; Kopell, Gritton, Whittington, & Kramer, 2014). Flexibility within frontal cortex and integration across different frontal subnetworks is associated with greater working memory performance (Braun et al., 2015). Moreover, reconfiguration within and between frontal subnetworks was also associated with more general cognitive flexibility (Braun et al., 2015). Furthermore, executive function areas increase in strength and flexibility from adolescence to young adulthood, and this increase predicts both group and individual differences in neurocognitive performance (Chai et al., 2017). Moreover, in general, the ability of the brain to flexibly reconfigure has been linked to learning in a variety of domains (Bassett et al., 2011; Bassett, Yang, Wymbs, & Grafton, 2015; Mattar, Thompson-Schill, & Bassett, 2017). This work suggests a more general role of network flexibility in facilitating task switching and cognitive control during cognitively demanding tasks.
Dynamic network methods can also provide insight into how interactions between subnetworks of the brain influence cognitive performance. Cooperation (positive connectivity) between executive function and cerebellar brain networks is positively associated with initiating new, low-demand cognitive tasks but negatively associated with performing complex, high-demand cognitive tasks (Khambhati, Medaglia, et al., 2017). On a basic perception task, reduced within-system connectivity and increased between-system connectivity in visual cortex and the default mode was associated with poorer performance (Sadaghiani et al., 2015). These findings suggest that some brain subnetworks are important for specific task demands, whereas other brain subnetworks are more generalized and support performance across varying task demands.
4. Social and emotional functioning
As in the cognitive domain, psychologists and neuroscientists have also studied how variations in functional brain networks might explain individual variations in how people think and behave in social and emotional domains. Paralleling the literature on cognitive functioning, social, and emotional functions likewise involve a combination of basic perceptual tasks and more complex and integrative tasks, but less work has investigated how the ability to switch between different brain states may or may not lead to better overall performance. For example, early work on neural correlates of social cognition identified brain regions in the default mode system (medial prefrontal cortex, posterior cingulate cortex, and temporoparietal junction) as being important for processing social information and predicting individual differences in social and emotional functioning (Adolfi et al., 2017; Lieberman, 2007; Van Overwalle, 2009), and cognitive control regions in frontotemporal cortex as important for regulating affective responses in the salience system (Buhle et al., 2014). Despite these interesting findings, most of the research on neural correlates of social and emotional functioning has historically focused on univariate or pairwise analyses, and would benefit from incorporating graph theoretical and dynamic network approaches. Just as network approaches to characterizing complex patterns of connectivity between brain regions have provided a more complete and richer understanding of how brain regions work together in the context of cognitive function, we suggest that similar gains may be realized in applying these tools to understanding individual differences in emotional and social functioning. As described in more detail below, these approaches can advance knowledge about how people navigate their social world.
4.1. Static brain networks and emotional functioning
The majority of research using fMRI to study individual differences in emotional functioning has applied seed-based approaches to identify region-to-region connections that are associated with a particular personality trait. These seed-based studies show that amygdala, striatum, and other limbic regions involved in emotion processing are associated with individual differences in emotional functioning. For example, neuroticism is associated with reduced functional connectivity between amygdala and anterior cingulate cortex during the viewing of negative emotional stimuli (Cremers et al., 2010; Gentili et al., 2017), as well as during a classical conditioning reward task (Schweckendiek, Stark, & Klucken, 2016). Although these studies give some interesting insight into the role of different brain regions and pairwise connections during tasks, they do not provide a complete mechanistic explanation; this gap by extension thus provides an avenue for graph theoretical and dynamic network approaches to augment our understanding.
Recently, researchers have begun using network approaches to study personality traits associated with emotional functioning. In this work, personality traits associated with individual differences in affective processing (e.g., anxiety, neuroticism, harm avoidance) appear to show differences in brain subnetworks that involve connections between salience hubs (insula and amygdala) and other cortical regions (Cremers et al., 2010; Davis et al., 2013; Gentili et al., 2017; Markett, Montag, Melchers, Weber, & Reuter, 2016; Schweckendiek, Stark, & Klucken, 2016). More efficient connections within affective subnetworks and greater integration between affective and cognitive subnetworks may help individuals control spontaneous affective responses to aversive or appetitive stimuli, with these connections changing over the course of development (Silvers et al., 2017).
Graph theoretical approaches have been used to identify network properties that are associated with emotional functioning. Greater network efficiency in the insular-opercular subnetwork during rest is associated with greater affective control (Markett et al., 2016). Shorter characteristic path length and reduced functional connectivity in the insular-opercular subnetwork during rest predicts decreased harm avoidance (Markett et al., 2016), and greater functional segregation of affective and cognitive brain regions during rest is associated with increased impulsivity (Davis et al., 2013). Davis et al. (2013) examined the modular structure of brain networks in high, medium, and low impulsivity individuals. They found that highly impulsive individuals showed greater density of within-system connections and a decreased strength of between-system connections during rest. Furthermore, regions associated with cognitive control were less connected to subcortical reward regions in highly impulsive individuals, suggesting that increased modularity of these brain networks might be implicated in a reduced ability to inhibit reward-related impulses.
In addition to focusing heavily on seed-based approaches, with a small but growing body of research employing graph theoretical approaches, research on brain networks and emotional functioning has focused almost exclusively on static brain networks. Moving forward, the inclusion of dynamic network methods can test novel hypotheses about how the brain influences individual differences in social behavior. The research reviewed in the section on dynamic networks and cognitive functioning shows that simply focusing on average connectivity across a scan can miss important information about how brain subnetworks are reconfiguring and interacting with one another both at rest (Hutchison et al., 2013) and in response to changing task demands (Telesford et al., 2016). Moreover, within-individual variation in dynamic brain networks tracks daily variations in mood (Betzel, Satterthwaite, Gold, & Bassett, 2017), suggesting that dynamic fluctuations in brain network connectivity might be linked to socioemotional outcomes.
Moreover, greater functional integration between affective and cognitive brain networks is linked to lower neuroticism (Davis et al., 2013). It is interesting to speculate about exactly how this link occurs. We note that network science defines connector nodes to be those that share connections with multiple subnetworks at once and that thereby facilitate communication between subnetworks (Bertolero, Yeo, & D’Esposito, 2015). We hypothesize that one route through which affective and cognitive networks might become integrated is by connector nodes that share connections with both cognitive and affective subnetworks; due to their location between the two subnetworks, connector hubs could therefore flexibly shift their allegiance between these subnetworks to help regulate negative emotional experiences.
4.2. Static brain networks and social functioning
Compared with cognitive and emotional functioning, fewer studies have examined brain networks and social functioning. The few studies that have been conducted tend to highlight within-system connectivity in the default mode, and between the default mode and regions involved in processing sensory input (e.g., visual areas). The default mode system are associated with internally directed and self-generated thought, sensory systems are associated with externally directed stimulus-evoked processing, and cortical hubs (both in default mode and frontoparietal systems) are thought to integrate information across internal and external modalities (Andrews-Hanna, Smallwood, & Spreng, 2014). Successfully navigating social interactions may require hubs in the default mode system that can efficiently integrate external information about the social environment with internal information about the self.
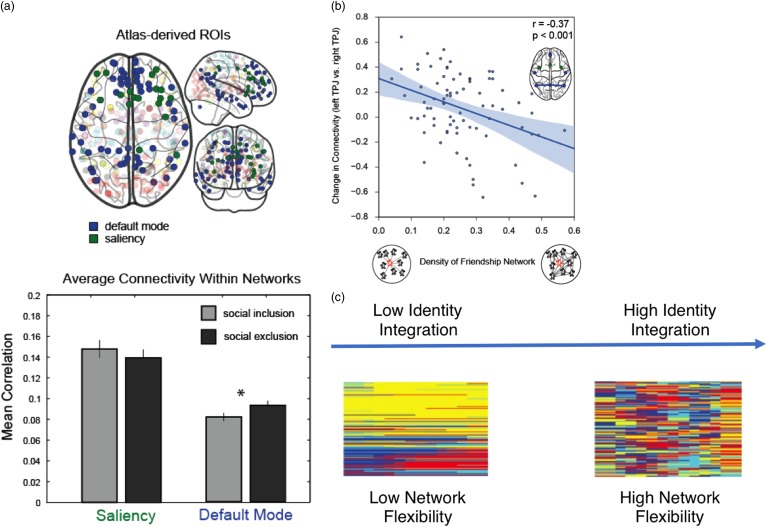
In one recent study, Schmälzle et al. (2017) examined functional connectivity during social exclusion and found differences in functional connectivity in default mode and mentalizing subnetworks during exclusion compared with inclusion (Figure 3a–b). Interestingly, they also found that this relationship was moderated by social network density, such that individuals with less dense friendship networks showed a stronger association between connectivity in the mentalizing network and rejection sensitivity (Schmälzle et al., 2017). It is possible that people with less dense social networks (vs. people with more dense social networks) rely on different strategies and mentalizing resources when interacting with others, which may shape how they respond to social exclusion. For example, the experience of frequently interacting with unconnected others might influence how people perceive and interpret others’ behavior, and whether they consider others’ perspectives during social interactions.
Figure 3.
Brain networks and social functioning. Recent work shows that network connectivity within parts of the default mode subnetwork (blue nodes) is greater following social exclusion (a), and that this effect is moderated by the density of an individual’s friendship network (b; adapted with permission from Schmälzle et al., 2017). We suggest that dynamic network methods can advance understanding of social functioning, including how people navigate multiple social identities. People who are better able to integrate multiple social identities may be able to do so, in part, because their brain flexibility adjusts to changing task demands and integrates information between subnetworks. In this case, people high in identity integration would have many brain regions that change communities frequently across time steps (c). ROIs=regions of interest; TPJ=temporoparietal junction.
This work is also consistent with an emerging literature that has investigated the association between static brain networks and personality traits. Personality traits linked to social processing, such as extraversion, are often associated with the default mode system (Adelstein et al., 2011; Vaidya & Gordon, 2013). Taken together, this work suggests that individual differences in social functioning might be facilitated by regions in the default mode, which can integrate external information (perhaps about the social environment) with internal information.
The above work, however, has only examined the association between static networks and social functioning. Dynamic network methods may provide additional insight into the mechanistic role that the default mode system is playing in facilitating social functioning. To the extent that default mode regions are operating as hubs that integrate external information about the social environment with internally directed thought, we might expect these regions to flexibly shift allegiance to different subnetworks depending on task demands (Mattar et al., 2015). Thus, a complementary hypothesis is that, in addition to the magnitude of connectivity, the flexibility with which cortical hubs in the default mode and frontoparietal subnetworks dynamically change the strength of their connections to different brain regions or other subnetworks might also be important for understanding individual differences in social and emotional functioning. For example, bicultural individuals who perceive their cultural identities as more overlapping and blended recruit dorsal medial prefrontal cortex more when thinking about close others (Huff, Yoon, Lee, Mandadi, & Gutchess, 2013). We hypothesize that flexibility and integration between cognitive control regions (executive function) and internally directed default mode regions might be important for successful integration of cultural identities.
5. Conclusion
In this review, we discuss recent advances in social and personality neuroscience, with a focus on the application of network science methods to understanding individual differences in social, emotional, and cognitive functioning. These efforts bridge the computational approaches of network science with the rich empirical data and biological hypotheses of neuroscience. Much of this work has focused on individual differences in cognitive functioning (creativity, intelligence, executive function), and we describe this research with the hope that it will demonstrate the potential utility of network approaches to understanding individual differences in social and emotional functioning. In particular, dynamic network methods can help unpack how brain networks fluctuate over time, and how those fluctuations might influence behavioral outcomes. These methods provide novel insights into the nature of individual differences in cognitive functioning and will serve as useful tools for social and personality research.
Financial Support
This work was supported by an award from the Army Research Laboratory (W911NF-10-2-0022) to support collaboration between D.S.B., E.B.F., and J.M.V. D.S.B. would also like to acknowledge support from the John D. and Catherine T. MacArthur Foundation, the Alfred P. Sloan Foundation, the Army Research Office through contract number W911NF-14-1-0679, the National Institute of Health (2-R01-DC-009209-11, 1R01HD086888-01, R01-MH107235, R01-MH107703, R01MH109520, 1R01NS099348, and R21-M MH-106799), the Office of Naval Research, and the National Science Foundation (BCS-1441502, BCS-1631550, and CNS-1626008). E.B.F. would also like to acknowledge support from NIH 1DP2DA03515601, DARPA YFA D14AP00048, and HopeLab. J.M.V. acknowledges support from mission funding to the US Army Research Laboratory. The content is solely the responsibility of the authors and does not necessarily represent the official views of any of the funding agencies.
Conflicts of Interest
The authors have nothing to disclose.
Inaugural Invited Paper
References
- Adelstein J. S., Shehzad Z., Mennes M., Deyoung C. G., Zuo X.-N., Kelly C., … Milham M. P. (2011). Personality is reflected in the brain’s intrinsic functional architecture. PloS One, 6, e27633 10.1371/journal.pone.0027633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolfi F., Couto B., Richter F., Decety J., Lopez J., Sigman M., … Ibáñez A. (2017). Convergence of interoception, emotion, and social cognition: A twofold fMRI meta-analysis and lesion approach. Cortex, 88, 124–142. 10.1016/j.cortex.2016.12.019 [DOI] [PubMed] [Google Scholar]
- Allen E. A., Erhardt E. B., Wei Y., Eichele T. Calhoun V. D. (2012). Capturing inter-subject variability with group independent component analysis of fMRI data: A simulation study. NeuroImage, 59, 4141–4159. 10.1016/j.neuroimage.2011.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna J. R., Smallwood J. Spreng R. N. (2014). The default network and self-generated thought: Component processes, dynamic control, and clinical relevance. Annals of the New York Academy of Sciences, 1316, 29–52. 10.1111/nyas.12360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett L. F. Satpute A. B. (2013). Large-scale brain networks in affective and social neuroscience: Towards an integrative functional architecture of the brain. Current Opinion in Neurobiology, 23, 361–372. 10.1016/j.conb.2012.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett D. S., Porter M. A., Wymbs N. F., Grafton S. T., Carlson J. M. Mucha P. J. (2013). Robust detection of dynamic community structure in networks. Chaos: An Interdisciplinary Journal of Nonlinear Science, 23, 13142 10.1063/1.4790830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett D. S. Sporns O. (2017). Network neuroscience. Nature Neuroscience, 20, 353–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett D. S., Wymbs N. F., Porter M. A., Mucha P. J., Carlson J. M. Grafton S. T. (2011). Dynamic reconfiguration of human brain networks during learning. Learning, 108, 19 10.1073/pnas.1018985108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett D. S., Yang M., Wymbs N. F. Grafton S. T. (2015). Learning-induced autonomy of sensorimotor systems. Nature Neuroscience, 18, 744–751. 10.1038/nn.3993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaty R. E., Benedek M., Wilkins R. W., Jauk E., Fink A., Silvia P. J., … Neubauer A. C. (2014). Creativity and the default network: A functional connectivity analysis of the creative brain at rest. Neuropsychologia, 64, 92–98. 10.1016/j.neuropsychologia.2014.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann C. F., DeLuca M., Devlin J. T. Smith S. M. (2005). Investigations into resting-state connectivity using independent component analysis. Philosophical Transactions of the Royal Society B: Biological Sciences, 360, 1001–1013. 10.1098/rstb.2005.1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolero M. A., Yeo B. T. T. D’Esposito M. (2015). The modular and integrative functional architecture of the human brain. Proceedings of the National Academy of Sciences of the United States of America, 112, E6798–E6807. 10.1073/pnas.1510619112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolero M. A., Yeo B. T. T. D’Esposito M. (2017). The diverse club. Nature Communications, 8 10.1038/s41467-017-01189-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betzel R. F. Bassett D. S. (2016). Multi-scale brain networks. NeuroImage, 160, 73–83. 10.1016/j.neuroimage.2016.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betzel R. F., Medaglia J. D., Papadopoulos L., Baum G. L., Gur R. C., Gur R. E., … Bassett D. S. (2017). The modular organization of human anatomical brain networks: Accounting for the cost of wiring. Network Neuroscience, 1, 42–68. 10.1162/NETN_a_00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betzel R. F., Satterthwaite T. D., Gold J. I. Bassett D. S. (2017). Positive affect, surprise, and fatigue are correlates of network flexibility. Scientific Reports, 7. 10.1038/s41598-017-00425-z. [DOI] [PMC free article] [PubMed]
- Biswal B. B., Yetkin F. Z., Haughton V. M. Hyde J. S. (1995). Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magnetic Resonance in Medicine, 34, 537–541. [DOI] [PubMed] [Google Scholar]
- Braun U., Schäfer A., Walter H., Erk S., Romanczuk-Seiferth N., Haddad L., … Bassett D. S. (2015). Dynamic reconfiguration of frontal brain networks during executive cognition in humans. Proceedings of the National Academy of Sciences, 112, 11678–11683. 10.1073/pnas.1422487112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks J. R., Garcia J. O., Kerick S. E., & Vettel J. M. (2016). Differential functionality of right and left parietal activity in controlling a motor vehicle. Frontiers in Systems Neuroscience, 10, 106 10.3389/fnsys.2016.00106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhle J. T., Silvers J. A., Wager T. D., Lopez R., Onyemekwu C., Kober H., … Ochsner K. N. (2014). Cognitive reappraisal of emotion: A meta-analysis of human neuroimaging studies. Cerebral Cortex, 24, 2981–2990. 10.1093/cercor/bht154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullmore E. Sporns O. (2009). Complex brain networks: Graph theoretical analysis of structural and functional systems. Nature Reviews Neuroscience, 10, 312 10.1038/nrn2575 [DOI] [PubMed] [Google Scholar]
- Bullmore E. T. Bassett D. S. (2011). Brain graphs: Graphical models of the human brain connectome. Annual Review of Clinical Psychology, 7, 113–140. 10.1146/annurev-clinpsy-040510-143934 [DOI] [PubMed] [Google Scholar]
- Calhoun V. D., Liu J. Adali T. (2009). A review of group ICA for fMRI data and ICA for joint inference of imaging, genetic, and ERP data. NeuroImage, 45, S163–S172. 10.1016/j.neuroimage.2008.10.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun V. D., Miller R., Pearlson G. Adalı T. (2014). The chronnectome: Time-varying connectivity networks as the next frontier in fMRI data discovery. Neuron, 84, 262–274. 10.1016/j.neuron.2014.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao M., Wang Z. He Y. (2015). Connectomics in psychiatric research: Advances and applications. Neuropsychiatric Disease and Treatment, 11, 2801–2810. 10.2147/NDT.S63470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai L. R., Khambhati A. N., Ciric R., Moore T., Gur R. C., Gur R. E., … Bassett D. S. (2017). Evolution of brain network dynamics in neurodevelopment. Network Neuroscience, 1, 14–30. 10.1162/NETN_a_00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciric R., Wolf D. H., Power J. D., Roalf D. R., Baum G. L., Ruparel K., … Satterthwaite T. D. (2017). Benchmarking of participant-level confound regression strategies for the control of motion artifact in studies of functional connectivity. NeuroImage, 154, 174–187. 10.1016/j.neuroimage.2017.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. R. D’Esposito M. (2016). The segregation and integration of distinct brain networks and their relationship to cognition. Journal of Neuroscience, 36, 12083–12094. 10.1523/JNEUROSCI.2965-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole M. W., Bassett D. S., Power J. D., Braver T. S. Petersen S. E. (2014). Intrinsic and task-evoked network architectures of the human brain. Neuron, 83, 238–251. 10.1016/j.neuron.2014.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole M. W., Reynolds J. R., Power J. D., Repovs G., Anticevic A. Braver T. S. (2013). Multi-task connectivity reveals flexible hubs for adaptive task control. Nature Neuroscience, 16, 1348–1355. 10.1038/nn.3470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole M. W., Yarkoni T., Repovs G., Anticevic A. Braver T. S. (2012). Global connectivity of prefrontal cortex predicts cognitive control and intelligence. Journal of Neuroscience, 32, 8988–8999. 10.1523/JNEUROSCI.0536-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper N., Tompson S., O’Donnell M. B., Falk E. B., Brook O, ’donnell M., & Falk E. B. (2015). Brain activity in self-and value-related regions in response to online antismoking messages predicts behavior change. Journal of Media Psychology, 27, 93–108. 10.1027/1864-1105/a000146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremers H. R., Demenescu L. R., Aleman A., Renken R., van Tol M.-J., van der Wee N. J. A., … Roelofs K. (2010). Neuroticism modulates amygdala—prefrontal connectivity in response to negative emotional facial expressions. NeuroImage, 49, 963–970. 10.1016/j.neuroimage.2009.08.023 [DOI] [PubMed] [Google Scholar]
- Davis F. C., Knodt A. R., Sporns O., Lahey B. B., Zald D. H., Brigidi B. D., & Hariri A. R. (2013). Impulsivity and the modular organization of resting-state neural networks. Cerebral Cortex, 23, 1444–1452. 10.1093/cercor/bhs126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison E. N., Schlesinger K. J., Bassett D. S., Lynall M.-E. E., Miller M. B., Grafton S. T., & Carlson J. M. (2015). Brain network adaptability across task states. PLoS Computational Biology, 11, e1004029 10.1371/journal.pcbi.1004029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger N. I. Lieberman M. D. (2004). Why rejection hurts: A common neural alarm system for physical and social pain. Trends in Cognitive Sciences, 8, 294–300. 10.1016/j.tics.2004.05.010 [DOI] [PubMed] [Google Scholar]
- Eisenberger N. I., Lieberman M. D. Satpute A. B. (2005). Personality from a controlled processing perspective: An fMRI study of neuroticism, extraversion, and self-consciousness. Cognitive, Affective, & Behavioral Neuroscience, 5, 169–181. 10.3758/CABN.5.2.169 [DOI] [PubMed] [Google Scholar]
- Falk E. B., Berkman E. T., Whalen D. Lieberman M. D. (2011). Neural activity during health messaging predicts reductions in smoking above and beyond self-report. Health Psychology, 30, 177–185. 10.1037/a0022259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk E. B., O’Donnell M. B., Tompson S., Gonzalez R., Dal Cin S. D., Strecher V., … An L. (2015). Functional brain imaging predicts public health campaign success. Social Cognitive and Affective Neuroscience, 11, 204–214. 10.1093/scan/nsv108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko E. Thompson-Schill S. L. (2014). Reworking the language network. Trends in Cognitive Sciences, 18, 120–126. 10.1016/j.tics.2013.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornito A., Harrison B. J., Zalesky A. Simons J. S. (2012). Competitive and cooperative dynamics of large-scale brain functional networks supporting recollection. Proceedings of the National Academy of Sciences, 109, 12788–12793. 10.1073/pnas.1204185109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M. D., Snyder A. Z., Vincent J. L., Corbetta M., Van Essen D. C. Raichle M. E. (2005). The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences of the United States of America, 102, 9673–9678. 10.1073/pnas.0504136102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries P. (2005). A mechanism for cognitive dynamics: Neuronal communication through neuronal coherence. Trends in Cognitive Sciences, 9, 474–480. 10.1016/j.tics.2005.08.011 [DOI] [PubMed] [Google Scholar]
- Fries P. (2015). Rhythms for cognition: Communication through coherence. Neuron, 88, 220–235. 10.1016/j.neuron.2015.09.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K. J. (2011). Functional and effective connectivity: A review. Brain Connectivity, 1, 13–36. 10.1089/brain.2011.0008 [DOI] [PubMed] [Google Scholar]
- Friston K. J., Buechel C., Fink G. R., Morris J., Rolls E. Dolan R. J. (1997). Psychophysiological and modulatory interactions in neuroimaging. NeuroImage, 6, 218–229. 10.1006/nimg.1997.0291 [DOI] [PubMed] [Google Scholar]
- Garcia J. O., Ashourvan A., Muldoon S., Vettel J. M. Bassett D. S. (2017). Applications of community detection techniques to brain graphs: Algorithmic considerations and implications for neural function. Proceedings of the IEEE. Advanced online publication. 10.1109/JPROC.2017.2786710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentili C., Cristea I. A., Ricciardi E., Vanello N., Popita C., David D., & Pietrini P. (2017). Not in one metric: Neuroticism modulates different resting state metrics within distinctive brain regions. Behavioural Brain Research, 327, 34–43. 10.1016/j.bbr.2017.03.031 [DOI] [PubMed] [Google Scholar]
- Glasser M. F., Coalson T. S., Robinson E. C., Hacker C. D., Harwell J., Yacoub E., … Van Essen D. C. (2016). A multi-modal parcellation of human cerebral cortex. Nature, 536, 171–178. 10.1038/nature18933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray J. R., Burgess G. C., Schaefer A., Yarkoni T., Larsen R. J. Braver T. S. (2005). Affective personality differences in neural processing efficiency confirmed using fMRI. Cognitive, Affective, and Behavioral Neuroscience, 5, 182–190. 10.3758/CABN.5.2.182 [DOI] [PubMed] [Google Scholar]
- Grinsted A, Moore J. C. Jevrejeva S. (2004). Application of the cross wavelet transform and wavelet coherence to geophysical time series. Nonlinear Processes in Geophysics, 11, 561–566. 10.5194/npg-11-561-2004 [DOI] [Google Scholar]
- Gu S., Pasqualetti F., Cieslak M., Telesford Q. K., Yu A. B., Kahn A. E., … Bassett D. S. (2015). Controllability of structural brain networks. Nature Communications, 6, 8414 10.1038/ncomms9414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M., Driesen N., Roth J. K., Gore J. C. Constable R. T. (2010). Functional connectivity between task-positive and task-negative brain areas and its relation to working memory performance. Magnetic Resonance Imaging, 28, 1051–1057. 10.1016/j.mri.2010.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M., Driesen N. R., Skudlarski P., Gore J. C. Constable R. T. (2006). Brain connectivity related to working memory performance. The Journal of Neuroscience, 26, 13338–13343. 10.1523/JNEUROSCI.3408-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huff S., Yoon C., Lee F., Mandadi A. Gutchess A. H. (2013). Self-referential processing and encoding in bicultural individuals. Culture and Brain, 1, 16–33. 10.1007/s40167-013-0005-1 [DOI] [Google Scholar]
- Hutchison R. M., Womelsdorf T., Allen E. A., Bandettini P. A., Calhoun V. D., Corbetta M., … Chang C. (2013). Dynamic functional connectivity: Promise, issues, and interpretations. NeuroImage, 80, 360–372. 10.1016/j.neuroimage.2013.05.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia H., Hu X. Deshpande G. (2014). Behavioral relevance of the dynamics of the functional brain connectome. Brain Connectivity, 4, 741–759. 10.1089/brain.2014.0300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khambhati A. N., Mattar M. G., Wymbs N. F., Grafton S. T. Bassett D. S. (2018). Beyond modularity: Fine-scale mechanisms and rules for brain network reconfiguration. NeuroImage, 166, 385–399. 10.1016/j.neuroimage.2017.11.015 [DOI] [PubMed] [Google Scholar]
- Khambhati A. N., Medaglia J. D., Karuza E. A., Thompson-Schill S. L. Bassett D. S. (2017). Subgraphs of functional brain networks identify dynamical constraints of cognitive control. Manuscript in preparation. 10.1101/147272 [DOI] [PMC free article] [PubMed]
- Khambhati A. N., Sizemore A. E., Betzel R. F. Bassett D. S. (in press). Modeling and interpreting mesoscale network dynamics. NeuroImage 10.1016/j.neuroimage.2017.06.029 [DOI] [PMC free article] [PubMed]
- Kim J., Soffer J. M., Kahn A. E., Vettel J. M., Pasqualetti F. Bassett D. S. (2018). Role of graph architecture in controlling dynamical networks with applications to neural systems. Nature Physics, 14, 91–98. 10.1038/nphys4268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitzbichler M. G., Henson R. N. A., Smith M. L., Nathan P. J. Bullmore E. T. (2011). Cognitive effort drives workspace configuration of human brain functional networks. Journal of Neuroscience, 31, 8259–8270. 10.1523/JNEUROSCI.0440-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopell N. J., Gritton H. J., Whittington M. A. Kramer M. A. (2014). Beyond the connectome: The dynome. Neuron, 83, 1319–1328. 10.1016/j.neuron.2014.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laumann T. O., Snyder A. Z., Mitra A., Gordon E. M., Gratton C., Adeyemo B., … Petersen S. E. (2016). On the stability of BOLD fMRI correlations. Cerebral Cortex, 27, 4719–4732. 10.1093/cercor/bhw265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D. D. Seung H. S. (1999). Learning the parts of objects by non-negative matrix factorization. Nature, 401, 788–791. 10.1038/44565 [DOI] [PubMed] [Google Scholar]
- Lee H., Heller A. S., van Reekum C. M., Nelson B. Davidson R. J. (2012). Amygdala-prefrontal coupling underlies individual differences in emotion regulation. NeuroImage, 62, 1575–1581. 10.1016/j.neuroimage.2012.05.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann B. C. L., White S. R., Henson R. N., Cam- CAN Geerligs L. (2017). Assessing dynamic functional connectivity in heterogeneous samples. NeuroImage, 157, 635–647. 10.1016/j.neuroimage.2017.05.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Liu Y., Li J., Qin W., Li K., Yu C., & Jiang T. (2009). Brain anatomical network and intelligence. PLoS Computational Biology, 5, e1000395. 10.1371/journal.pcbi.1000395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman M. D. (2007). Social cognitive neuroscience: A review of core processes. Annual Review of Psychology, 58, 259–289. 10.1146/annurev.psych.58.110405.085654 [DOI] [PubMed] [Google Scholar]
- Ma Y., Bang D., Wang C., Allen M., Frith C., Roepstorff A., & Han S. (2012). Sociocultural patterning of neural activity during self-reflection. Social Cognitive and Affective Neuroscience, 9, 73–80. 10.1093/scan/nss103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies D. S., Kelly A. M. C., Uddin L. Q., Biswal B. B., Castellanos F. X. Milham M. P. (2007). Mapping the functional connectivity of anterior cingulate cortex. NeuroImage, 37, 579–588. 10.1016/j.neuroimage.2007.05.019 [DOI] [PubMed] [Google Scholar]
- Markett S., Montag C., Melchers M., Weber B. Reuter M. (2016). Anxious personality and functional efficiency of the insular-opercular network: A graph-analytic approach to resting-state fMRI. Cognitive, Affective, & Behavioral Neuroscience, 16, 1039–1049. 10.3758/s13415-016-0451-2 [DOI] [PubMed] [Google Scholar]
- Mateo C., Knutsen P. M., Tsai P. S., Shih A. Y. Kleinfeld D. (2017). Entrainment of arteriole vasomotor fluctuations by neural activity is a basis of blood-oxygenation-level-dependent “resting-state” connectivity. Neuron, 96, 936–948.e3. 10.1016/j.neuron.2017.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattar M. G., Betzel R. F. Bassett D. S. (2016). The flexible brain. Brain, 139, 2110–2112. 10.1093/brain/aww151 [DOI] [PubMed] [Google Scholar]
- Mattar M. G., Cole M. W., Thompson-Schill S. L. Bassett D. S. (2015). A functional cartography of cognitive systems. PLoS Computational Biology, 11, e1004533 10.1371/journal.pcbi.1004533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattar M. G., Thompson-Schill S. L. Bassett D. S. (2017). The network architecture of value learning. Network Neuroscience, 0, 1–27. 10.1162/NETN_a_00021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren D. G., Ries M. L., Xu G. Johnson S. C. (2012). A generalized form of context-dependent psychophysiological interactions (gPPI): A comparison to standard approaches. NeuroImage, 61, 1277–1286. 10.1016/j.neuroimage.2012.03.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medaglia J. D., Lynall M.-E. Bassett D. S. (2015). Cognitive network neuroscience. Journal of Cognitive Neuroscience, 27, 1471–1491. 10.1162/jocn_a_00810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medaglia J. D., Satterthwaite T. D., Kelkar A., Ciric R., Moore T. M., Ruparel K., … Bassett D. S. (2018). Brain state expression and transitions are related to complex executive cognition in normative neurodevelopment. NeuroImage, 166, 293–306. 10.1016/j.neuroimage.2017.10.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier D., Achard S., Morcom A. Bullmore E. (2009). Age-related changes in modular organization of human brain functional networks. NeuroImage, 44, 715–723. 10.1016/j.neuroimage.2008.09.062 [DOI] [PubMed] [Google Scholar]
- Meyer M. L., Spunt R. P., Berkman E. T., Taylor S. E. Lieberman M. D. (2012). Evidence for social working memory from a parametric functional MRI study. Proceedings of the National Academy of Sciences of the United States of America, 109, 1883–1888. 10.1073/pnas.1121077109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mišić B. Sporns O. (2016). From regions to connections and networks: New bridges between brain and behavior. Current Opinion in Neurobiology, 40, 1–7. 10.1016/j.conb.2016.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mišić B., Sporns O. McIntosh A. R. (2014). Communication efficiency and congestion of signal traffic in large-scale brain networks. PLoS Computational Biology, 10, e1003427 10.1371/journal.pcbi.1003427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller S., Wang D., Fox M. D., Yeo B. T. T., Sepulcre J., Sabuncu M. R., … Liu H. (2013). Individual variability in functional connectivity architecture of the human brain. Neuron, 77, 586–595. 10.1016/j.neuron.2012.12.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muldoon S. F., Bridgeford E. W. Bassett D. S. (2016). Small-world propensity and weighted brain networks. Scientific Reports, 6, 22057 10.1038/srep22057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller K., Lohmann G., Neumann J., Grigutsch M., Mildner T. Von Cramon D. Y. (2004). Investigating the wavelet coherence phase of the BOLD signal. Journal of Magnetic Resonance Imaging, 20, 145–152. 10.1002/jmri.20064 [DOI] [PubMed] [Google Scholar]
- Newman M. (2010). Networks: An introduction. Oxford: Oxford University Press. [Google Scholar]
- Parks E. L. Madden D. J. (2013). Brain connectivity and visual attention. Brain Connectivity, 3, 317–338. 10.1089/brain.2012.0139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passaro A. D., Vettel J. M., McDaniel J., Lawhern V., Franaszczuk P. J. Gordon S. M. (2017). A novel method linking neural connectivity to behavioral fluctuations: Behavior-regressed connectivity. Journal of Neuroscience Methods, 279, 60–71. 10.1016/j.jneumeth.2017.01.010 [DOI] [PubMed] [Google Scholar]
- Porter M. A., Onnela J.-P. Mucha P. J. (2009). Communities in networks. American Mathematical Society, 56, 1082–1097. 10.1016/j.physrep.2009.11.002 [DOI] [Google Scholar]
- Power J. D. D., Cohen A. L. L., Nelson S. M. M., Wig G. S. S., Barnes K. A. A., Church J. A. A., … Petersen S. E. E. (2011). Functional network organization of the human brain. Neuron, 72, 665–678. 10.1016/j.neuron.2011.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle M. E. (2015). The brain’s default mode network. Annual Review of Neuroscience, 38, 433–447. 10.1146/annurev-neuro-071013-014030 [DOI] [PubMed] [Google Scholar]
- Raichle M. E. Snyder A. Z. (2007). A default mode of brain function: A brief history of an evolving idea. NeuroImage, 37, 1083–1090. 10.1016/j.neuroimage.2007.02.041 [DOI] [PubMed] [Google Scholar]
- Ray R. D., Shelton A. L., Hollon N. G., Matsumoto D., Frankel C. B., Gross J. J., & Gabrieli J. D. E. (2010). Interdependent self-construal and neural representations of self and mother. Social Cognitive and Affective Neuroscience, 5, 318–323. 10.1093/scan/nsp039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy P. G., Mattar M. G., Murphy A. C., Wymbs N. F., Grafton S. T., Satterthwaite T. D., & Bassett D. S. (2018). Brain state flexibility accompanies motor-skill acquisition. NeuroImage, 171, 135–147. 10.1016/j.neuroimage.2017.12.093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repovs G., Csernansky J. G. Barch D. M. (2011). Brain network connectivity in individuals with schizophrenia and their siblings. Biological Psychiatry, 69, 967–973. 10.1016/j.biopsych.2010.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinov M. Sporns O. (2010). Complex network measures of brain connectivity: Uses and interpretations. NeuroImage, 52, 1059–1069. 10.1016/j.neuroimage.2009.10.003 [DOI] [PubMed] [Google Scholar]
- Sadaghiani S., Poline J.-B., Kleinschmidt A. D’Esposito M. (2015). Ongoing dynamics in large-scale functional connectivity predict perception. Proceedings of the National Academy of Sciences, 112, 8463–8468. 10.1073/pnas.1420687112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarnecchi E., Galli G., Polizzotto N. R., Rossi A. Rossi S. (2014). Efficiency of weak brain connections support general cognitive functioning. Human Brain Mapping, 35, 4566–4582. 10.1002/hbm.22495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarnecchi E., Tatti E., Rossi S., Serino V. Rossi A. (2015). Intelligence-related differences in the asymmetry of spontaneous cerebral activity. Human Brain Mapping, 36, 3586–3602. 10.1002/hbm.22864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite T. D., Ciric R., Roalf D. R., Davatzikos C., Bassett D. S. Wolf D. H. (2017). Motion artifact in studies of functional connectivity: Characteristics and mitigation strategies. Human Brain Mapping. 10.1002/hbm.23665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmälzle R., Brook O’Donnell M., Garcia J. O., Cascio C. N., Bayer J., Bassett D. S., … Falk E. B. (2017). Brain connectivity dynamics during social interaction reflect social network structure. Proceedings of the National Academy of Sciences, 114, 5153–5158. 10.1073/pnas.1616130114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweckendiek J., Stark R. Klucken T. (2016). Neuroticism and extraversion moderate neural responses and effective connectivity during appetitive conditioning. Human Brain Mapping, 37, 2992–3002. 10.1002/hbm.23221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine J. M., Bissett P. G., Bell P. T., Koyejo O., Balsters J. H., Gorgolewski K. J., … Poldrack R. A. (2016). The dynamics of functional brain networks: Integrated network states during cognitive task performance. Neuron, 92, 544–554. 10.1016/j.neuron.2016.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine J. M., Koyejo O. Poldrack R. A. (2016). Temporal metastates are associated with differential patterns of time-resolved connectivity, network topology, and attention. Proceedings of the National Academy of Sciences, 113, 9888–9891. 10.1073/pnas.1604898113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvers J. A., Insel C., Powers A., Franz P., Helion C., Martin R. E., … Ochsner K. N. (2017). VlPFC-vmPFC-amygdala interactions underlie age-related differences in cognitive regulation of emotion. Cerebral Cortex, 27, 3502–3514. 10.1093/cercor/bhw073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sizemore A. E. Bassett D. S. (in press). Dynamic graph metrics: Tutorial, toolbox, and tale. NeuroImage. 10.1016/j.neuroimage.2017.06.081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporns O. (2013). Network attributes for segregation and integration in the human brain. Current Opinion in Neurobiology, 23, 162–171. 10.1016/j.conb.2012.11.015 [DOI] [PubMed] [Google Scholar]
- Sporns O. (2014). Contributions and challenges for network models in cognitive neuroscience. Nature Neuroscience, 17, 652–660. 10.1038/nn.3690 [DOI] [PubMed] [Google Scholar]
- Sporns O. Betzel R. F. (2016). Modular brain networks. Annual Review of Psychology, 67, 613–640. 10.1146/annurev-psych-122414-033634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telesford Q. K., Lynall M.-E., Vettel J., Miller M. B., Grafton S. T. Bassett D. S. (2016). Detection of functional brain network reconfiguration during task-driven cognitive states. NeuroImage, 142, 198–210. 10.1016/j.neuroimage.2016.05.078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya C. J. Gordon E. M. (2013). Phenotypic variability in resting-state functional connectivity: Current status. Brain Connectivity, 3, 99–120. 10.1089/brain.2012.0110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Overwalle F. (2009). Social cognition and the brain: A meta-analysis. Human Brain Mapping, 30, 829–858. 10.1002/hbm.20547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wig G. S. (2017). Segregated systems of human brain networks. Trends in Cognitive Sciences, 21, 981–996. 10.1016/j.tics.2017.09.006 [DOI] [PubMed] [Google Scholar]
- Winder A. T., Echagarruga C., Zhang Q. Drew P. J. (2017). Weak correlations between hemodynamic signals and ongoing neural activity during the resting state. Nature Neuroscience, 20, 1761–1769. 10.1038/s41593-017-0007-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo B. T. T., Krienen F. M., Sepulcre J., Sabuncu M. R., Lashkari D., Hollinshead M., … Buckner R. L. (2011). The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Journal of Neurophysiology, 106, 1125–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin S., Wang T., Pan W., Liu Y. Chen A. (2015). Task-switching cost and intrinsic functional connectivity in the human brain: Toward understanding individual differences in cognitive flexibility. PLoS One, 10, e0145826 10.1371/journal.pone.0145826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalesky A., Fornito A. Bullmore E. T. (2010). Network-based statistic: Identifying differences in brain networks. NeuroImage, 53, 1197–1207. 10.1016/j.neuroimage.2010.06.041 [DOI] [PubMed] [Google Scholar]
- Zanto T. P. Gazzaley A. (2013). Fronto-parietal network: flexible hub of cognitive control. Trends in Cognitive Sciences, 17, 602–603. 10.1016/j.tics.2013.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Lu G., Zhong Y., Tan Q., Yang Z., Liao W., … Liu Y. (2009). Impaired attention network in temporal lobe epilepsy: A resting FMRI study. Neuroscience Letters, 458, 97–101. 10.1016/j.neulet.2009.04.040 [DOI] [PubMed] [Google Scholar]