Abstract
Individuals vary substantially in their tendency to take risks. In the last two decades, a large number of neuroimaging studies in humans have explored the neural mechanisms of several cognitive processes that contribute to risk taking. Here I focus on functional and structural MRI studies that investigated uncertainty processing, one of the main features of risk behavior. Using decision making and learning paradigms, these studies implicated a network of brain areas, including posterior parietal cortex, anterior insula, anterior cingulate cortex and ventrolateral prefrontal cortex, in various aspects of uncertainty processing. Individual differences in behavior under uncertainty are reflected in the function and structure of some of these areas, and are integrated into value representations in ventromedial prefrontal cortex and ventral striatum, reinforcing the potential contribution of all of these brain structures to individual tendencies to take risks.
In August 1974 Philippe Petit, a young Frenchman, fixed a rope between the tops of the Twin Towers in New York, a quarter mile above the ground, and crossed it back and forth several times (Petit 2002). When Petit set out on this adventure, he took a risk. The outcome of his action was highly uncertain and could have been either highly positive - rewarding sensations of accomplishment, recognition and fame - or devastating. Fortunately for Petit, his endeavor was successful.
Some degree of risk-taking is generally deemed important for achieving progress in any creative activity, including, for example, in science (Rzhetsky, Foster, Foster and Evans 2015) or business (March and Shapira 1987). Extreme avoidance of risk is associated with trait anxiety (Maner and others 2007) and is a characteristic of anxiety-based disorders, such as obsessive compulsive disorder (Pushkarskaya and others 2015) and posttraumatic stress disorder (Ruderman and others 2016). Excessive risk-taking, however, is also maladaptive and is linked to other mental disorders, including schizophrenia and bipolar disorder (Reddy and others 2014). Increased risk-taking tendencies are also at the core of many harmful behaviors, such as substance abuse (Wagner 2001), reckless driving (Zuckerman and Kuhlman 2000) and unsafe sex (Donohew and others 2000).
What gives rise to risk behavior? Why do some people put their health, wealth and wellbeing at risk, while others avoid even the slightest uncertainty? Psychologists have been studying these questions for decades. In recent years they have been joined by neuroscientists, in an attempt to reveal the neural mechanisms underlying risk-taking behavior. A useful approach to studying these mechanisms is to examine the neural bases of several rudimentary mental processes that contribute to risk-taking behavior, including, but not limited to, sensitivity to rewards and punishments, self-control, and the processing of uncertainty.
Individual sensitivities to rewards and punishments likely play a role in risk-taking behaviors, which typically carry both positive and negative consequences. High sensitivity to rewards may lead a person to focus more on the rewarding aspects of a risky activity than on its possible adverse outcomes. Such an individual will be more drawn to that activity compared to a person who is less susceptible to reward. Conversely, sensitivity to punishment will attract the individual’s attention to the potential negative consequences of a risky activity, decreasing the likelihood that she will take part in that activity. An important aspect of these positive and negative outcomes of a risky action is that they typically occur at different points in time. For example, smoking a cigarette is immediately rewarding to a smoker, but its health hazards will only occur in the future. Conversely, a risky business decision may have an immediate cost, but pay off in the future. Thus, self-control, or the ability to forgo immediate rewards in order to avoid future large costs, may hinder some risky behaviors, while accepting immediate costs to achieve future large gains, may be associated with other types of risk taking.
But perhaps the hallmark of risky behaviors is that they involve uncertain outcomes. How an individual handles uncertainty will therefore strongly affect her tendency to engage in risky behaviors. A person who is tolerant of uncertainty will be more likely to take risks compared to one who finds uncertainty distressing. The processing of uncertainty is complex in itself and can be further decomposed into subprocesses. Most notably, economists distinguish between uncertainty with known outcome probabilities, termed risk1 (Box 1), and uncertainty with unknown outcome probabilities, referred to as ambiguity (Box 1), a distinction that is proving useful in studying risk-taking behavior. Individuals vary substantially in how they perceive outcome probabilities (subjective probability), how they tradeoff outcome magnitude against its probability (risk attitude) and how they treat ambiguity around outcome probabilities (ambiguity attitude). In this review I will survey the neuroanatomical substrates of the different cognitive processes that make risky behaviors in humans, focusing on uncertainty processing and individual differences in uncertainty attitudes. As we will see, prior research has made substantial stride in unraveling important uncertainty-related neural mechanisms, but many open questions remain for future research.
Box 1: Economic theory in the research of risky behavior.
In recent years neuroeconomic studies have turned to ideas and techniques from the field of economics for deconstructing risk-taking - and decision making in general - into their constituent components (Glimcher 2008; Glimcher and Fehr 2014). A basic concept in risky choice is that of expected value (EV), a concept that was first raised by Pascal (Pascal 1966). Pascal suggested that the desirability of any option that the decision maker considers is equal to the value of that option multiplied by the probability of obtaining that value:
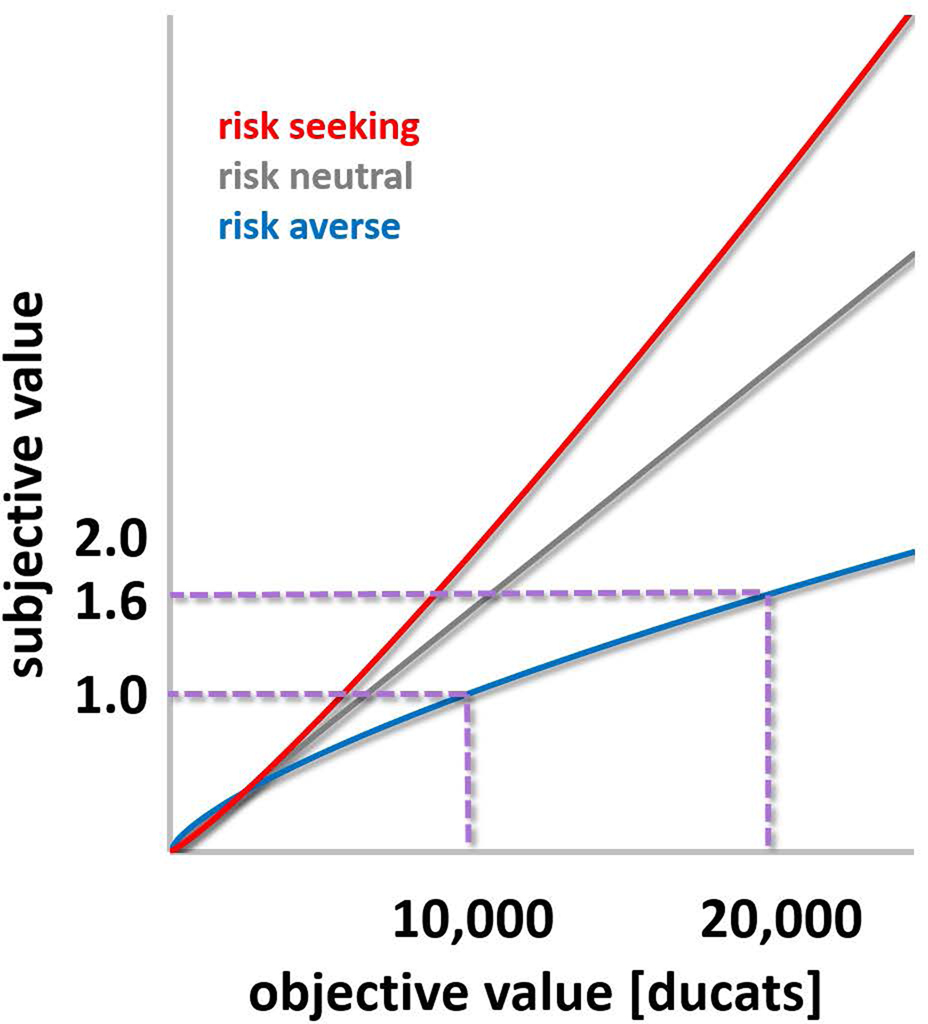
By that account, to make a choice between several options, the decision maker needs simply to compute the expected value of each option and choose the option of the highest value. This simple concept is, of course, too simple. Bernoulli (Bernoulli 1738/1954) has made this point using an example of a very poor fellow that obtains a lottery ticket with an equal probability to win either twenty thousand ducats or nothing. Should this man evaluate his chance of winning at ten thousand ducats (its expected value), asks Bernoulli? Or should he be willing to accept a smaller amount, say nine thousand ducats, in exchange for the lottery ticket? The intuitive answer, that this man should accept the nine thousand ducats, suggests that expected value is not a sufficient quantity for decision making. Rather, the expected utility or subjective value of an option should be considered. A subjective-value function that is concave in respect to the objective value (the amount of ducats) can account for the risk preference we expect the poor fellow to exhibit (Figure B1, blue curve). What happens in a concave value function is that subjective value increases more slowly than objective value. In the example in Figure B1, when the amount of money is doubled from 10,000 to 20,000 ducats, the subjective value only increases by 1.6. But the subjective value function can vary for different individuals, based on their wealth, as well as personal preferences and other characteristics. A power function of the following form:
could account for individual differences in risk preferences with a single parameter (α). Risk-averse behavior will be described with an α that is smaller than 1, while an α that is larger than one will capture risk-seeking behavior (Figure B1).
Following Bernoulli, in the 20th century, Samuelson (Samuelson 1938) has introduced the powerful idea of revealed preferences. What Samuelson realized was that by making some very simple assumptions, for example that if a person prefers an apple to an orange she will not also prefer an orange to an apple, one can make robust predictions about choice behavior. Based on a set of such simple assumptions, or “axioms”, Von Neumann and Morgenstern (Von Neumann and Morgenstern 1944) developed their Expected Utility (EU) theory. What they showed was, that if a decision maker obeys these simple axioms, her behavior appears “as if” she attempts to maximize some utility (or subjective value) function.
A common strategy for estimating risk attitudes is therefore to fit choice behavior with such a utility function, for example the power function above, and derive the a risk parameter (α in the case of a power function) that best describes each individual’s behavior. Note that in this approach, risk attitude results from the way the outcome magnitude is perceived, rather than from how outcome probability is perceived. A somewhat different, but related, approach to risk was developed in the finance literature, which conceptualizes risk as a cost due to the variance of potential outcomes (Markowitz 1991). For a risk averse individual, the higher the variance the higher the cost. While a debate about which approach is more useful still exists, both models make similar predictions under many conditions, and the studies described here have used both.
Regardless of whether we follow the economic or the financial approach, if people indeed obeyed the simple axioms that Von Neumann and Morgenstern posited, we could have stopped here, with little need for further research of decision making. Soon after Von Neumann and Morgenstern developed their theory, however, it became clear that the axioms are often violated. One of the earliest demonstrations of such violation was provided by Daniel Ellsberg (Ellsberg 1961). Ellsberg proposed the following thought experiment: a participant is presented with two urns, one with 50 blue chips and 50 red ones (the 50–50 urn) and another with 100 blue and red chips in an unknown or ambiguous proportion (the ambiguous urn). The participant is first asked to pick a color (red or blue) and then asked to state whether he would rather bet on drawing his chosen color from the 50–50 urn for a prize of $10 or from the ambiguous urn for a prize of $15. For the 50–50 urn, the probability of drawing either blue or red is 0.5; for the second urn the probabilities are not known. Since the subject chooses the winning color, however, the probability of winning by betting on the ambiguous urn is still 0.5. This is because even if, in the worst case, all of the chips were of a single unknown color it was the subject who randomly picked that color. The choice is thus between $10 at a probability of 0.5 (with the 50–50 urn) and $15, also at a probability of 0.5 (with the ambiguous urn). Therefore, regardless of the form of their particular utility functions, rational decision makers should prefer to bet on the ambiguous urn. Ellsberg has anticipated that many individuals would prefer to avoid the ambiguous urn, even at a substantial monetary cost, a prediction that has since received strong support from numerous empirical studies (Camerer and Weber 1992). It is important to remember, however, that although irrational in the lab, ambiguity aversion may, in many cases protect us from unnecessary risk taking. Thus, depending on the specific conditions, both too weak and too strong ambiguity aversion may be maladaptive. Importantly, since risk and ambiguity attitudes are not strongly correlated across individuals, both may independently contribute to risk-taking behavior.
Methodological issues of studying decision making under uncertainty
Risk-taking behavior inevitably involves uncertainty. Uncertainty, however, is not unique to risky behavior – it exists everywhere, in virtually any decision we make. Whether you choose a course from a menu, contemplate a retirement plan or entertain the idea of a bungee jump, the outcome of your choice is never certain. A large number of human neuroimaging studies investigated the neural basis of decision making under uncertainty. Many early studies employed learning tasks, in which participants learned to associate stimuli with uncertain rewards or punishments and to select the stimuli that lead to better outcomes. A widely used example of such task is the Iowa Gambling Task (IGT; Bechara, Damasio, Tranel and Damasio 1997). In the IGT participants are presented with four decks of cards, and on each trial draw a card from a deck of their choice. Each card is associated with either a gain or a loss. In two of the decks, most of the cards lead to large gains, but every now and then a card leads to an even larger loss, resulting in an overall loss in the long run. In the other two decks cards lead to lower gains, but even lower losses, resulting in a net gain. At the beginning of the task, participants have no information about outcome probabilities, which means that their initial decisions are made under complete ambiguity (Box 1). With time, ambiguity is reduced and healthy participants learn to limit their card choices to the “good” decks. Bechara and colleagues (Bechara, Damasio, Tranel and Damasio 1997), have shown that patients with brain lesion in ventromedial prefrontal cortex (vmPFC, Figure 1) are impaired on this task, supporting a role for vmPFC in risk-taking behavior and decision making in general. The complexity of the task, however, makes it difficult to delineate the specific role of vmPFC or the specific impairment in vmPFC-lesioned patients. In particular, learning from feedback is an important feature of the IGT, and reduced earnings on the task may result from a general learning impairment. Alternatively, deficient performance on the IGT may be due to overestimation of the positive value of potential gains, or underestimation of the negative value of potential losses. Therefore, while the IGT has been highly valuable in highlighting one of the central brain structures involved in decision making, many subsequent neuroimaging studies have opted for simpler designs, to allow for delineation of the neural bases of the various underlying cognitive processes.
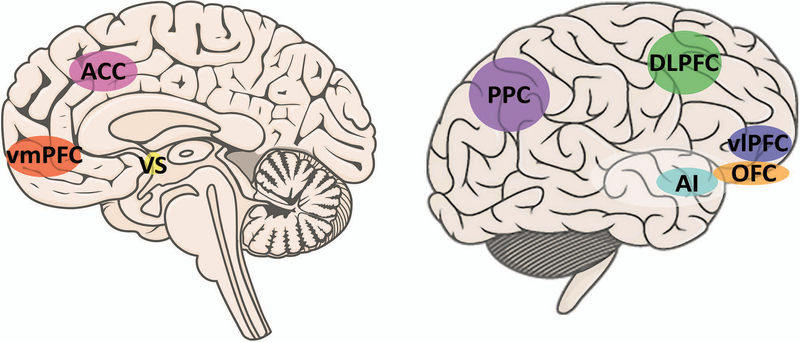
Figure 1.
A schematic representation of central brain areas involved in risk taking behavior. ACC, anterior cingulate cortex; AI, anterior insula; DLPFC, dorsolateral prefrontal cortex; OFC, orbitofrontal cortex; PPC, posterior parietal cortex; vlPFC, ventrolateral prefrontal cortex; vmPFC, ventromedial prefrontal cortex; VS, ventral striatum.
Some of these subsequent studies eliminated the learning aspect of the task. For example, Preuschoff and colleagues (Preuschoff, Bossaerts and Quartz 2006) asked participants to bet on whether the first or second of two consecutively presented cards would be of a higher number (Figure 2A). Cards were withdrawn from a deck of 10 cards, numbered 1–10, with no repetition. In this way, participants experienced varying levels of uncertainty during the anticipatory period between the presentation of the first and second cards. For example, if the number on the first card was 1 or 10, participants could predict with certainty that the second card would bear a higher or lower number respectively. Conversely, uncertainty was maximal if the first card was numbered 5 or 6. Tobler and colleagues (Tobler, O’Doherty, Dolan and Schultz 2007) used a simple paradigm, in which unique stimuli were associated with a particular reward and a particular probability (Figure 2B) in an initial training session. Following training, single stimuli, with fully established associations, were presented in the main experiment. Knutson and colleagues (Knutson, Taylor, Kaufman, Peterson and Glover 2005) devised a task in which reward was obtained if participants pressed a button within a particular time window. The timing of the task was adjusted individually for each participant, yielding trials with varying probabilities for success, and therefore varied probabilities for reward.
Figure 2.
Examples of stimuli used to elicit risk attitudes. (A) Betting on whether the second of two consecutively presented cards will be higher or lower (adapted from Preuschoff et al, 2006). (B) Presentation of stimuli that were previously associated with outcomes of particular magnitudes and probabilities (adapted from Tobler et al, 2007). (C) Choice between a lottery and a certain amount. The lottery could be of varying outcome probability (left) and magnitude (adapted from Gilaie-Dotan et al, 2014).
Several studies adopted the behavioral economics approach of “revealed preference” (Samuelson 1948). In these studies participants choose between options that vary on their potential outcomes, as well as on the probability for obtaining these outcomes, and thus need to tradeoff reward and probability (Figure 2C). Based on these observed choices, the researchers can estimate individual risk attitudes (Box 1). Consider for example the choice between receiving $5 for sure and playing a lottery that offers 50% chance of winning $10 (but also 50% of winning nothing). Both options are of the same expected value (Box 1), but the lottery is risky. An individual who is not affected by risk (risk neutral) will be indifferent between these options. Conversely, a risk-averse individual would prefer the sure $5, whereas a risk-seeking individual would opt for the lottery. To simplify the design and the interpretation of the neural results, some of these studies keep one of the options constant across trials, such that any change in neural activation from trial to trial can be directly related to changes in only one option. For example, Levy and colleagues (Levy, Snell, Nelson, Rustichini and Glimcher 2010) asked participants to make a series of choices between risky options. One option was always a probability of 0.5 to win $5, whereas the other option varied in its outcome probability (0.13–0.38) and outcome magnitude ($5-$65). No feedback was provided during the experiment. Instead, after the completion of the scan a few trials were randomly selected and played for real money. This experimental feature incentivizes participants to reveal their true preferences (Hertwig and Ortmann 2001) - since participants do not know beforehand which trials would be selected, they have to treat each and every trial as if they will be paid according to their choice on that trial. Using these various techniques, functional and structural MRI studies have begun to unravel the neural processing of uncertainty and the neuroanatomical substrates of individual differences in uncertainty processing.
Functional studies of decision under risk
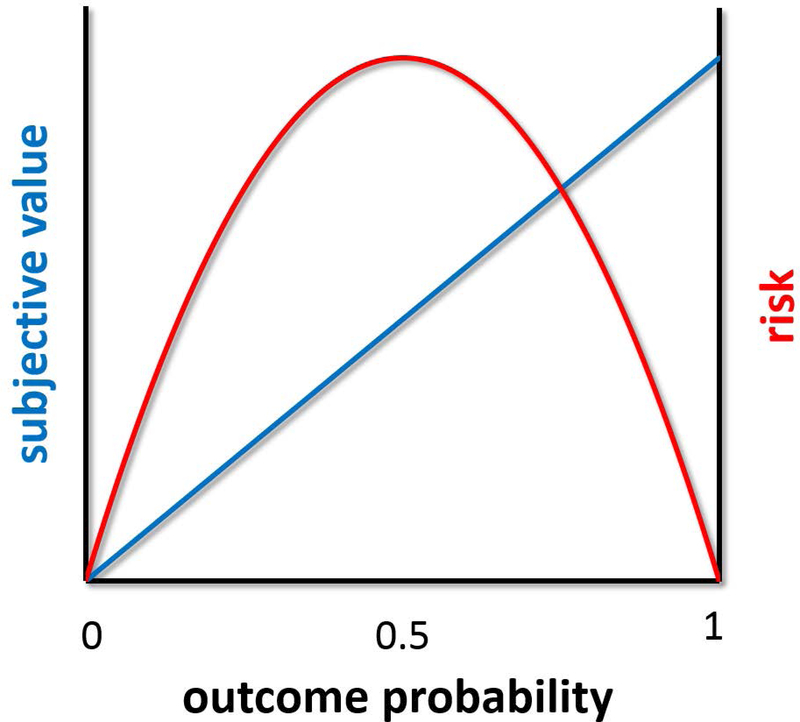
Several brain areas were implicated in the processing of risk, or uncertainty with known probabilities (Box 1). First, outcome probability is reflected in activation magnitude in ventral striatum (Tobler, O’Doherty, Dolan and Schultz 2007), and vmPFC (Knutson, Taylor, Kaufman, Peterson and Glover 2005). Activity in both of these brain areas scales positively with probability, compatible with the role of these areas in the encoding of subjective value (Box 1) or the desirability of anticipated outcomes. Of course, increasing the probability for obtaining reward (or decreasing the probability for incurring a punishment) will make an available option more desirable, naturally leading to enhanced activation in value-related regions. In theory, this effect of probability may be incorporated in the way the subjective value of an option is perceived, and does not require a separate neural encoding of probability. There is, however, evidence for neural encoding of the level of risk regarding outcome receipt, which is separate from the encoding of value. As seen in Figure 3, these two quantities can be distinguished from each other if measured over the full range of probabilities. As probability increases from 0 to 0.5, both subjective value and risk increase monotonically. Conversely, when probability increases beyond 0.5, risk decreases monotonically, while subjective value continues to increase. Increased activation to increased risk was observed in several brain regions (Figure 1), including the lateral orbitofrontal cortex (OFC) / ventrolateral prefrontal cortex (vlPFC) (Tobler, O’Doherty, Dolan and Schultz 2007; Huettel, Song and McCarthy 2005), as well as in bilateral ventral striatum (Preuschoff, Bossaerts and Quartz 2006), anterior insula (Huettel, Song and McCarthy 2005), and posterior parietal cortex (PCC; Huettel, Song and McCarthy 2005).
Figure 3.
Risk and subjective value as a function of outcome probability. While subjective value increases monotonically with reward probability, risk first increases and then decreases. This can be used to distinguish between neural encoding of probability (or value) and neural encoding of risk.
The studies described so far focused on commonalities across participants to highlight brain circuits that participate in the processing of probability and risk. To understand the neural basis of individual propensity to engage in risky behavior, however, it is crucial to examine individual differences in activation patterns. To do this, the idiosyncratic risk attitude of each participant is estimated based on their behavior on the experimental task (Box 1), allowing researchers to look for psychometricneurometric matches, or correspondence between behavior and neural patterns across participants. Not surprisingly, value-related activation patterns in lateral (Tobler, Christopoulos, O’Doherty, Dolan and Schultz 2009) and medial (Levy, Snell, Nelson, Rustichini and Glimcher 2010) prefrontal regions were modulated by individual risk attitudes. Interestingly, activity in left PPC in response to choices that involved risky lotteries was also correlated with risk preference across participants (Huettel, Stowe, Gordon, Warner and Platt 2006). Although in humans the PPC has not received much attention in the context of risk-taking and decision making, substantial evidence from electrophysiological studies in monkeys support its role in these processes (Louie and Glimcher 2010; Louie, Grattan and Glimcher 2011; Platt and Glimcher 1999; Sugrue, Corrado and Newsome 2004). This notion is reinforced by recent structural MRI findings, as described below.
Risk and brain structure
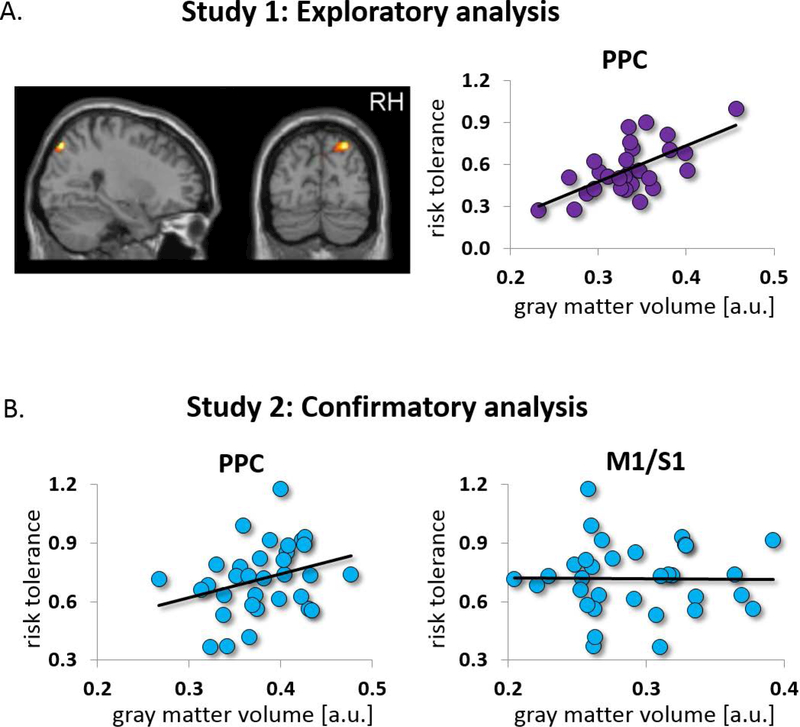
Recent studies have begun to unravel neuroanatomical features that are predictive of individual traits and capabilities (Kable and Levy 2015; Kanai and Rees 2011). Such associations between structure and behavior are important, because unlike functional activation patterns, structural measures do not depend on the particular experimental paradigm, and are therefore likely to represent stable behavioral traits. Using voxel-based morphometry (VBM), Gilaie-Dotan and colleagues (Gilaie-Dotan and others 2014) identified a region in right PPC whose gray-matter volume is predictive of individual risk attitudes. Two groups of participants made a series of choices between risky options (Figure 2C) and went through anatomical MRI scans. The first group was tested in New York, and provided data for a whole-brain exploratory analysis. Based on participants’ choice behavior, their risk attitudes were estimated with a standard economic model (Box 1). These attitudes were then used in a whole-brain VBM analysis, which revealed a single region, within right PPC, whose volume was significantly correlated with risk attitudes. Individuals with more gray-matter volume in this region were more tolerant of risk (or less risk averse) (Figure 4A). A similar result was obtained when risk attitudes were estimated simply based on the proportion of trials in which participants chose the risky option, demonstrating that the results did not depend on the specific assumptions used to calculate risk attitudes. Following the exploratory analysis, data from a second, independent group of participants, scanned in Philadelphia, was used for a confirmatory analysis (Figure 4B). The gray matter volume from the region identified in the first group was measured in participants of the second group and used to successfully predict these participants’ risk attitudes. Conversely, the gray-matter volume of a control area in the vicinity of primary motor / primary somatosensory cortex did not yield significant predictions. This finding is consistent with behavioral reports suggesting that, at least to some extent, risk preferences are stable across time (Harrison, Johnson, McInnes and Rutstrom 2005). One should be cautious, however, in making inferences from these VBM results about the underlying neural architecture, as the relationships between the microstructure and structural MRI measures are still poorly understood (Kanai and Rees 2011). It should also be stressed that, just like fMRI results, VBM results cannot inform us about causality. While it could be the case that structure gives rise to risk attitudes, it is also possible that environmental factors affect both, or even that behavior shapes structure.
Figure 4.
A region in right posterior parietal cortex (PPC) predicts individual risk attitudes. (A) Exploratory analysis. Left: whole-brain VBM revealed a single brain region whose volume correlates with individual risk tolerance. Right: illustration of the association between gray-matter volume and risk tolerance. (B) Confirmatory analysis in an independent group of subjects. The gray-matter volume from the region identified in the exploratory analysis predicted risk attitudes in the new sample (left), while gray-matter volume from a control area did not (right).
Subjective probability and ambiguity
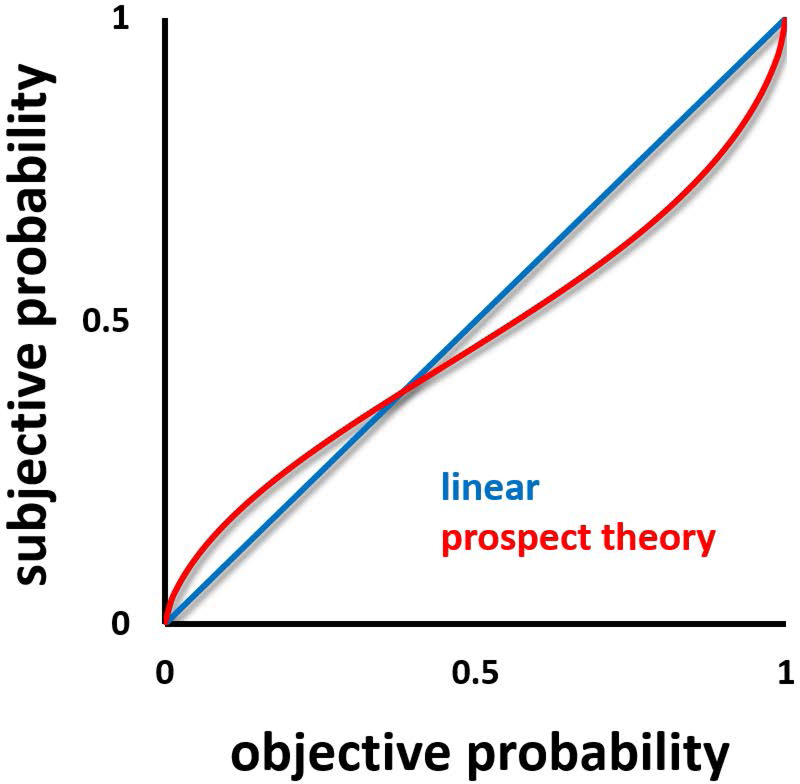
An individual’s willingness to take risks relies on how they balance the magnitude of a potential outcome with the probability that the outcome will occur. But how the individual perceives outcome probability will also contribute to her willingness to take a risk. Substantial research in economics suggests that people typically weigh outcome probabilities in a non-linear manner. As Kahneman and Tversky described in their Prospect Theory (Kahneman and Tversky 1979; Tversky and Kahneman 1992), when participants are presented with explicit probabilities (e.g. “50% chance”) they tend to overweigh low probabilities and underweigh high probabilities, in the form of an inverted S-shaped function (Figure 5). When probabilities are learned by experience, from repeated sampling, an opposite effect is observed, where small probabilities are underestimated and large probabilities overestimated, in the form of an S-shaped function (Hertwig, Barron, Weber and Erev 2004). fMRI data suggest that the inverted s-shaped non-linear weighting of explicit probabilities is reflected in striatal activation (Hsu, Krajbich, Zhao and Camerer 2009), compatible with the role of this brain area in representation of subjective value. There is also evidence for a similar effect in the left dorsolateral prefrontal cortex (DLPFC; Tobler, Christopoulos, O’Doherty, Dolan and Schultz 2008). Interestingly, after some experience with these outcome probabilities, nonlinear s-shaped probability weighting is observed in ventrolateral prefrontal cortex (Tobler, Christopoulos, O’Doherty, Dolan and Schultz 2008).
Figure 5.
Subjective probability. When probability information is explicitly conveyed, low probabilities are typically overestimated, whereas high probabilities are underestimated.
Note that in most of the studies surveyed so far outcome probabilities were precisely known. In these studies participants either saw explicit symbolic presentations of the probability information (Gilaie-Dotan and others 2014), or acquired this information in a training procedure, in which they experienced the potential outcomes repeatedly (Tobler, O’Doherty, Dolan and Schultz 2007). In some of the studies, however, outcome probability was not precisely known (Knutson, Taylor, Kaufman, Peterson and Glover 2005). Indeed, in real life we can rarely estimate the probabilities for potential outcomes of our actions in a precise way. We know the probability of getting heads when tossing a coin, and can calculate the precise probability for choosing the winning numbers in the New York Mega Millions lottery, but what is the chance that the chosen course at the restaurant will be satisfying? That drinking and driving will end in an accident? While some probability estimates can usually be generated, these estimates are seldom exact. Rather, outcome probabilities are usually at least somewhat ambiguous (Box 1). From a decision-theory point of view, ambiguity, or the precision in which outcome probabilities are stated, should not affect choice. A long line of research, however, suggests that in many cases individuals are strongly affected by the presence of ambiguity. In particular, when choosing between possible gains, many individuals tend to avoid ambiguity, even at a large financial or other costs (Ellsberg 1961; Camerer and Weber 1992; Fox and Tversky 1995; Heath and Tversky 1991; Trautmann, Vieider and Wakker 2008). Importantly, how an individual treats known probabilities (what economists call “risk”) tells us very little about how she treats ambiguity (Cohen, Jaffray and Said 1987; Tymula, Rosenberg Belmaker, Ruderman, Glimcher and Levy 2013). Similarly, ambiguity and risk attitudes seem to follow separate, independent, developmental trajectories (Blankenstein, Crone, van den Bos and van Duijvenvoorde 2016; Tymula and others 2012). This suggests that ambiguity attitudes (attitudes towards unknown probabilities) may contribute to risk-taking behavior, independently from pure risk attitudes (attitudes towards known probabilities).
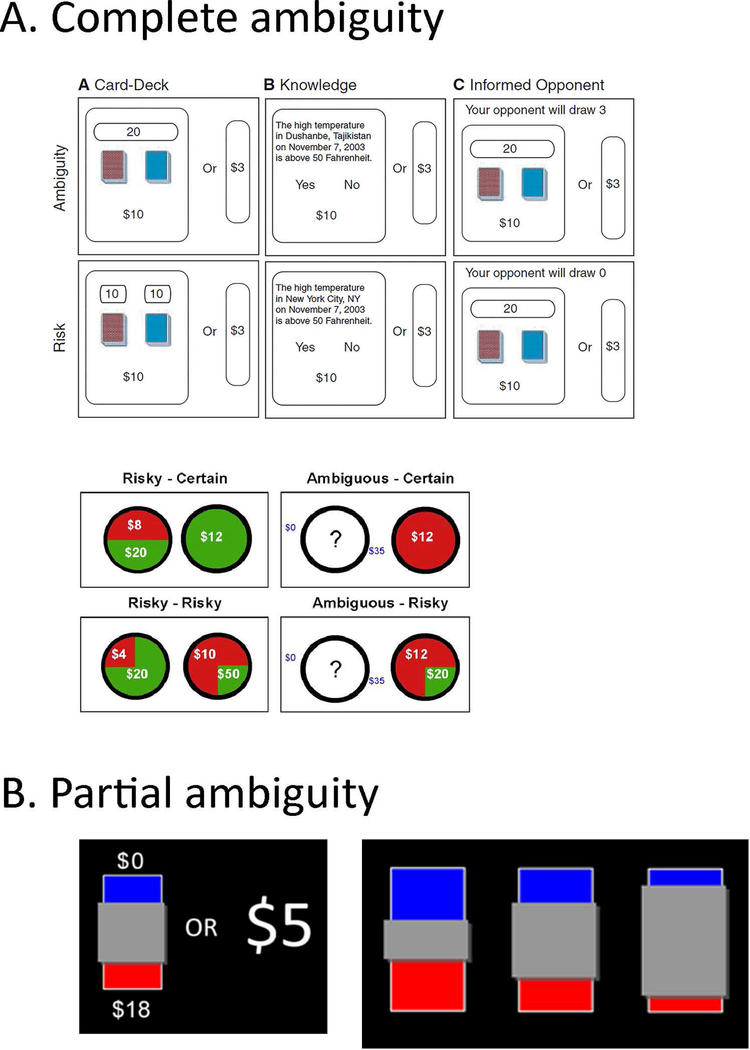
Several neuroimaging studies have examined decision making under conditions of ambiguity. Ambiguous options are created by withholding some of the information about outcome probability, either by providing partial information, or by physically occluding a graphic stimulus that conveys outcome probability (Figure 6). Some of these studies compared complete ambiguity (i.e. no information about outcome probability) to no ambiguity (i.e. full information about outcome probability; Figure 6A; Hsu, Bhatt, Adolphs, Tranel and Camerer 2005; Huettel, Stowe, Gordon, Warner and Platt 2006). There is evidence for increased processing of ambiguity compared to risk in lateral OFC, which is correlated with the level of ambiguity aversion across participants (Hsu et al 2005). Taken together with the involvement of lateral OFC in encoding the level of risk (Tobler, O’Doherty, Dolan and Schultz 2007; Huettel, Song and McCarthy 2005), these findings suggest a general role for lateral OFC in uncertainty processing. In experimental designs that resolved ambiguity at the end of each trial, activity in the neighboring region of ventrolateral prefrontal cortex (vlPFC) was associated with ambiguity preference across participants (Huettel 2006; Bach, Hulme, Penny and Dolan 2011), pointing to a potential role of this brain region in resolving ambiguity or in attempting to make sense of an ambiguous situation (although the direction of the correlation was inconsistent across studies). More recent studies included conditions of partial ambiguity by parametrically modulating the information provided in each trial (Figure 6B). Compatible with the effect of ambiguity on value, there is evidence for decreased activation in response to increasing ambiguity in the value-related vmPFC (Pushkarskaya, Smithson, Joseph, Corbly and Levy 2015). Moreover, activity in the vmPFC, as well as in the striatum, is correlated with subjective value, which takes into account individual ambiguity (as well as risk) attitudes (Levy, Snell, Nelson, Rustichini and Glimcher 2010).
Figure 6.
Stimuli used to elicit ambiguity attitudes. (A) Complete ambiguity. Comparison of ambiguity with no ambiguity. Top: adapted from Hsu et al, 2005; Bottom: adapted from Huettel et al, 2006. (B) Partial ambiguity. By occluding part of a risky lottery, some of the information about outcome probability is withheld, creating partial ambiguity (adapted from Gilaie-Dotan et al, 2014).
Learning under uncertainty
In the studies described above each choice situation (trial) was examined in isolation from other choices. To prevent learning, many of these studies also intentionally refrained from providing information about decision outcomes. While this feature of the experimental design is important for a clean delineation of uncertainty attitudes, real-life decisions are affected by context and changes in the environment, including the previous choices that the individual made and the outcomes she has experienced. Failing to adapt to changes in the environment and to learn from the outcomes of previous decisions may lead to unnecessary risk-taking. If consuming a certain food, for example, leads to nausea, it may be wise to avoid that particular food, and if a financial investment pays off, it may be worthwhile to make similar future investments. Kuhnen and Knutson (Kuhnen and Knutson 2005) examined how neural activation is associated with switches between subsequent decisions in an investment paradigm. In that paradigm, participants chose on each trial whether to invest in risky stocks or in a safe bond whose outcome was certain. The good stock was superior to the bad one in that it was more likely to lead to a positive outcome and less likely to lead to a negative outcome. Participants knew all this, but did not know which of the two stocks was the good one – they had to learn from experience. During this learning process, activity in ventral striatum was associated with switching from choosing a bond on the previous trial to choosing a stock on the current trial. Conversely, activity in anterior insula increased the likelihood of choosing a bond, but only when the prior choice was a stock. Thus it is possible that the ventral striatum facilitates switching from risk averse to risk seeking behavior, whereas anterior insula facilitates the opposite switch. Kuhnen and Knutson have also compared the choices of their participants to the ones that an ideal Bayesian learning, aimed at maximizing expected value, would make. This comparison showed that ventral striatum activity increased the likelihood of choosing a stock where the ideal learner would choose the bond (risk-seeking “mistake”), after previously choosing the bond, whereas anterior insula activity increased the likelihood of a risk-averse “mistake” (choosing a bond where the ideal learner would choose a stock), following a stock choice. Moreover, individual differences in the frequency of switching from a stock to a bond, as well as the frequency of “risk-averse” mistakes are reflected in anterior insula activation. Interestingly, anterior cingulate cortex (ACC) exhibited increased activity when the model predicted maximal response conflict – i.e. when it was unclear whether the stock or the bond had higher expected value. More recent studies of learning under uncertainty implicated the same brain area in encoding the level of ambiguity in the environment (Behrens, Woolrich, Walton and Rushworth 2007; Payzan-LeNestour, Dunne, Bossaerts and O’Doherty 2013) (those studies used the term “estimation uncertainty” for ambiguity). These findings in the ACC are consistent both with a long line of research implicating the ACC in conflict and error monitoring (Carter and others 1998; Shenhav, Straccia, Cohen and Botvinick 2014) and with recent studies that implicated the ACC in foraging decisions (Kolling, Behrens, Mars and Rushworth 2012). The latter function is especially interesting in the context of risk-taking behavior, because, rather than a choice between a limited number of options, as in the typical laboratory experiment, risk behavior often entails a decision to explore multiple novel options (foraging). Indeed, a recent study suggested that the same brain area is involved in adapting risk attitudes to changing conditions (Kolling, Wittmann and Rushworth 2014).
Reward and punishment sensitivity
Risk taking is influenced not just by how the individual perceives and treats uncertainty, but also by how she perceives and treats the potential rewards and punishments that may result from the risky behavior. If the reward of a risky action is perceived as highly positive, or the potential punishment as only slightly negative, the subjective value of the action will be higher. For example, a person who emphasizes the positive effect of a drug of abuse and plays down its potential harms will be more likely to engage in drug abuse compared to someone who focuses on the negative potential results of drug use. There is now substantial evidence for a “valuation system” in the brain that encodes the desirability of expected and experienced outcomes (Bartra, McGuire and Kable 2013; Clithero and Rangel 2014; Levy and Glimcher 2012). Activity in this systems, that consists of at least the vmPFC and ventral striatum, scales with the value of both rewards and punishments (Tom, Fox, Trepel and Poldrack 2007) of various categories (Chib, Rangel, Shimojo and O’Doherty 2009; FitzGerald, Seymour and Dolan 2009; Levy and Glimcher 2011; Lin, Adolphs and Rangel 2012; McNamee, Rangel and O’Doherty 2013). Other factors that affect the subjective desirability of a prospect are also reflected in activation of these areas, including the delay to reward (Kable and Glimcher 2007), self-control (Hare, Camerer and Rangel 2009), and, as mentioned above, risk and ambiguity (Levy, Snell, Nelson, Rustichini and Glimcher 2010). There is evidence for integration of potential gains and losses in these areas. Thus, when choosing whether or not to accept risky gambles with 50–50 chance of a gain or a loss, activity in both vmPFC and ventral striatum scales with both the potential gain and the potential loss (Tom, Fox, Trepel and Poldrack 2007). Importantly, individual ratios of sensitivity to gains and sensitivity to losses correlated with the ratio of neural modulation by gain and loss magnitudes. Further support for the notion of value integration in vmPFC comes from a study showing interactive integration of the values of probabilistic combinations of monetary rewards and electric shocks, which participants chose whether to accept (Park, Kahnt, Rieskamp and Heekeren 2011). While the neural mechanisms of value integration are not fully understood, this integration mechanism may play a role in risk-taking behavior.
Summary and open questions
A network of brain areas contribute to risk-taking behavior. Not surprisingly, individual preferences for risk and ambiguity, as well as for rewards and punishments, are reflected in activation patterns in value-related areas, most notably the vmPFC and the ventral striatum. While this modulation of the subjective value representation may have practical implications for improving predictions of future risk taking, a more interesting question from a neurobiological point of view is what sources provide input to the valuation areas. A series of functional neuroimaging studies implicated the anterior insula, anterior cingulate cortex, PPC and lateral OFC / vlPFC in processing various aspects of uncertainty, and showed that individual uncertainty attitudes are reflected in activation patterns in these areas. Moreover, the neuroanatomy of the PPC is itself predictive of individual risk attitudes, suggesting that this brain region may be associated with stable trait-like risk-taking propensity. Several additional neural structures that have not been surveyed here are likely also linked to risk-taking behavior. This includes the right DLPFC, whose disruption with transcranial magnetic stimulation (TMS) has been shown to increase risky choices (Knoch and others 2006), and the amygdala, which has a central role in emotional processing (Phelps and LeDoux 2005). Future research will need to identify the parts of this network that play a causal role in risky behavior, and characterize the orchestrated contribution of the various components to such behavior. Many additional questions remain open. Most importantly, it is not clear whether the findings from the laboratory experiments, most of which employed paradigms with either monetary or hypothetical “points” outcomes, can generalize to more realistic decisions and to other decision domains. While there is some evidence for consistent risk attitudes across rewards domains, specifically food and money (Levy and Glimcher 2011), there is also behavioral evidence for domain-specific uncertainty attitudes (Weber, Blais and Betz 2002), and substantial evidence for different risk and ambiguity attitudes for gains and losses (Tymula, Rosenberg Belmaker, Ruderman, Glimcher and Levy 2013). Whether the same neural mechanisms support vastly different risk-taking behaviors, such as financial investments and medical decisions remains to be seen. Another interesting question is how much of this neural architecture is hard-wired, and how much may be shaped by experience. As decreased or increased risk-taking behavior is closely linked to psychopathology and substance abuse, understanding the relevant neural circuitry, its variations in pathological conditions, and how it may be modified by behavioral and pharmacological interventions is of high public-health value.
Acknowledgments
Funding
IL was supported by NIH grants R21MH102634 and R21AG049293.
Footnotes
Note that this economic definition of risk is separate, and narrower, from the day-to-day usage of the word. In this review I use the term “risk” in this narrow economic sense, and the terms “risk taking” or “risk behavior” for the broader meaning of risk.
References
- Bach DR, Hulme O, Penny WD, Dolan RJ. 2011. The known unknowns: neural representation of second-order uncertainty, and ambiguity. J Neurosci 31(13):4811–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartra O, McGuire JT, Kable JW. 2013. The valuation system: a coordinate-based meta-analysis of BOLD fMRI experiments examining neural correlates of subjective value. NeuroImage 76:412–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Tranel D, Damasio AR. 1997. Deciding advantageously before knowing the advantageous strategy. Science 275(5304):1293–5. [DOI] [PubMed] [Google Scholar]
- Behrens TE, Woolrich MW, Walton ME, Rushworth MF. 2007. Learning the value of information in an uncertain world. Nat Neurosci 10(9):1214–21. [DOI] [PubMed] [Google Scholar]
- Bernoulli D 1738/1954. Exposition of a new theory on the measurement of risk. Econometrica 22(1):23–36. [Google Scholar]
- Blankenstein NE, Crone EA, van den Bos W, van Duijvenvoorde AC. 2016. Dealing With Uncertainty: Testing Risk- and Ambiguity-Attitude Across Adolescence. Dev Neuropsychol:1–16. [DOI] [PubMed] [Google Scholar]
- Camerer C, Weber M. 1992. Recent Developments in Modeling Preferences - Uncertainty and Ambiguity. Journal of Risk and Uncertainty 5(4):325–370. [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. 1998. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science 280(5364):747–9. [DOI] [PubMed] [Google Scholar]
- Chib VS, Rangel A, Shimojo S, O’Doherty JP. 2009. Evidence for a common representation of decision values for dissimilar goods in human ventromedial prefrontal cortex. J Neurosci 29(39):12315–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clithero JA, Rangel A. 2014. Informatic parcellation of the network involved in the computation of subjective value. Soc Cogn Affect Neurosci 9(9):1289–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M, Jaffray JY, Said T. 1987. Experimental Comparison of Individual Behavior under Risk and under Uncertainty for Gains and for Losses. Organizational Behavior and Human Decision Processes 39(1):1–22. [Google Scholar]
- Donohew L, Zimmerman R, Cupp PS, Novak S, Colon S, Abell R. 2000. Sensation seeking, impulsive decision-making, and risky sex: implications for risk-taking and design of interventions. Personality and Individual Differences 28(6):1079–1091. [Google Scholar]
- Ellsberg D 1961. Risk, Ambiguity, and the Savage Axioms. Quarterly Journal of Economics 75(4):643–669. [Google Scholar]
- FitzGerald TH, Seymour B, Dolan RJ. 2009. The role of human orbitofrontal cortex in value comparison for incommensurable objects. J Neurosci 29(26):8388–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox CR, Tversky A. 1995. Ambiguity Aversion and Comparative Ignorance. Quarterly Journal of Economics 110(3):585–603. [Google Scholar]
- Gilaie-Dotan S, Tymula A, Cooper N, Kable JW, Glimcher PW, Levy I. 2014. Neuroanatomy predicts individual risk attitudes. J Neurosci 34(37):12394–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glimcher PW. 2008. Understanding risk: a guide for the perplexed. Cogn Affect Behav Neurosci 8(4):348–54. [DOI] [PubMed] [Google Scholar]
- Glimcher PW, Fehr E. 2014. Neuroeconomics : decision making and the brain Amsterdam: Elsevier/Academic Press; xxviii, 577 pages p. [Google Scholar]
- Hare TA, Camerer CF, Rangel A. 2009. Self-control in decision-making involves modulation of the vmPFC valuation system. Science 324(5927):646–8. [DOI] [PubMed] [Google Scholar]
- Harrison GW, Johnson EI, McInnes MM, Rutstrom EE. 2005. Temporal stability of estimates of risk aversion. Applied Financial Economics Letters 1:31–35. [Google Scholar]
- Heath C, Tversky A. 1991. Preference and Belief - Ambiguity and Competence in Choice under Uncertainty. Journal of Risk and Uncertainty 4(1):5–28. [Google Scholar]
- Hertwig R, Barron G, Weber EU, Erev I. 2004. Decisions from experience and the effect of rare events in risky choice. Psychological Science 15(8):534–539. [DOI] [PubMed] [Google Scholar]
- Hertwig R, Ortmann A. 2001. Experimental practices in economics: A methodological challenge for psychologists? Behavioral and Brain Sciences 24(3):383–403. [DOI] [PubMed] [Google Scholar]
- Hsu M, Bhatt M, Adolphs R, Tranel D, Camerer CF. 2005. Neural systems responding to degrees of uncertainty in human decision-making. Science 310(5754):1680–3. [DOI] [PubMed] [Google Scholar]
- Hsu M, Krajbich I, Zhao C, Camerer CF. 2009. Neural response to reward anticipation under risk is nonlinear in probabilities. J Neurosci 29(7):2231–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huettel SA, Song AW, McCarthy G. 2005. Decisions under uncertainty: probabilistic context influences activation of prefrontal and parietal cortices. J Neurosci 25(13):3304–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huettel SA, Stowe CJ, Gordon EM, Warner BT, Platt ML. 2006. Neural signatures of economic preferences for risk and ambiguity. Neuron 49(5):765–75. [DOI] [PubMed] [Google Scholar]
- Kable JW, Glimcher PW. 2007. The neural correlates of subjective value during intertemporal choice. Nat Neurosci 10(12):1625–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kable JW, Levy I. 2015. Neural markers of individual differences in decision-making. Current Opinion in Behavioral Science 5:100–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahneman D, Tversky A. 1979. Prospect Theory - Analysis of Decision under Risk. Econometrica 47(2):263–291. [Google Scholar]
- Kanai R, Rees G. 2011. The structural basis of inter-individual differences in human behaviour and cognition. Nat Rev Neurosci 12(4):231–42. [DOI] [PubMed] [Google Scholar]
- Knoch D, Gianotti LR, Pascual-Leone A, Treyer V, Regard M, Hohmann M and et al. 2006. Disruption of right prefrontal cortex by low-frequency repetitive transcranial magnetic stimulation induces risk-taking behavior. J Neurosci 26(24):6469–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Taylor J, Kaufman M, Peterson R, Glover G. 2005. Distributed neural representation of expected value. J Neurosci 25(19):4806–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolling N, Behrens TE, Mars RB, Rushworth MF. 2012. Neural mechanisms of foraging. Science 336(6077):95–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolling N, Wittmann M, Rushworth MF. 2014. Multiple neural mechanisms of decision making and their competition under changing risk pressure. Neuron 81(5):1190–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhnen CM, Knutson B. 2005. The neural basis of financial risk taking. Neuron 47(5):763–70. [DOI] [PubMed] [Google Scholar]
- Levy DJ, Glimcher PW. 2011. Comparing apples and oranges: using reward-specific and reward-general subjective value representation in the brain. J Neurosci 31(41):14693–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy DJ, Glimcher PW. 2012. The root of all value: a neural common currency for choice. Curr Opin Neurobiol 22(6):1027–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy I, Snell J, Nelson AJ, Rustichini A, Glimcher PW. 2010. Neural representation of subjective value under risk and ambiguity. J Neurophysiol 103(2):1036–47. [DOI] [PubMed] [Google Scholar]
- Lin A, Adolphs R, Rangel A. 2012. Social and monetary reward learning engage overlapping neural substrates. Soc Cogn Affect Neurosci 7(3):274–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louie K, Glimcher PW. 2010. Separating value from choice: delay discounting activity in the lateral intraparietal area. J Neurosci 30(16):5498–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louie K, Grattan LE, Glimcher PW. 2011. Reward value-based gain control: divisive normalization in parietal cortex. J Neurosci 31(29):10627–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maner JK, Richey JA, Cromer K, Mallott M, Lejuez CW, Joiner TE and et al. 2007. Dispositional anxiety and risk-avoidant decision-making. Personality and Individual Differences 42(4):665–675. [Google Scholar]
- March JG, Shapira Z. 1987. Managerial Perspectives on Risk and Risk-Taking. Management Science 33(11):1404–1418. [Google Scholar]
- Markowitz HM. 1991. Foundations of Portfolio Theory. Journal of Finance 46(2):469–477. [Google Scholar]
- McNamee D, Rangel A, O’Doherty JP. 2013. Category-dependent and category-independent goal-value codes in human ventromedial prefrontal cortex. Nat Neurosci 16(4):479–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SQ, Kahnt T, Rieskamp J, Heekeren HR. 2011. Neurobiology of value integration: when value impacts valuation. J Neurosci 31(25):9307–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascal B 1966. Pensées. Harmondsworth: Penguin Books; 359 p. p. [Google Scholar]
- Payzan-LeNestour E, Dunne S, Bossaerts P, O’Doherty JP. 2013. The neural representation of unexpected uncertainty during value-based decision making. Neuron 79(1):191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit P 2002. To Reach the Clouds: My High-Wire Walk Between the Twin Towers. New York NY: North Point Press. [Google Scholar]
- Phelps EA, LeDoux JE. 2005. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron 48(2):175–87. [DOI] [PubMed] [Google Scholar]
- Platt ML, Glimcher PW. 1999. Neural correlates of decision variables in parietal cortex. Nature 400(6741):233–238. [DOI] [PubMed] [Google Scholar]
- Preuschoff K, Bossaerts P, Quartz SR. 2006. Neural differentiation of expected reward and risk in human subcortical structures. Neuron 51(3):381–90. [DOI] [PubMed] [Google Scholar]
- Pushkarskaya H, Smithson M, Joseph JE, Corbly C, Levy I. 2015. Neural Correlates of Decision-Making Under Ambiguity and Conflict. Front Behav Neurosci 9:325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pushkarskaya H, Tolin D, Ruderman L, Kirshenbaum A, Kelly JM, Pittenger C and et al. 2015. Decision-making under uncertainty in obsessive-compulsive disorder. J Psychiatr Res 69:166–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy LF, Lee J, Davis MC, Altshuler L, Glahn DC, Miklowitz DJ and et al. 2014. Impulsivity and risk taking in bipolar disorder and schizophrenia. Neuropsychopharmacology 39(2):456–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruderman L, Ehrlich DB, Roy A, Pietrzak RH, Harpaz-Rotem I, Levy I. 2016. Posttraumatic Stress Symptoms and Aversion to Ambiguous Losses in Combat Veterans. Depress Anxiety 33(7):606–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rzhetsky A, Foster JG, Foster IT, Evans JA. 2015. Choosing experiments to accelerate collective discovery. Proceedings of the National Academy of Sciences of the United States of America 112(47):14569–14574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelson PA. 1938. A note on the pure theory of consumer behavior. Economia 1:61–71. [Google Scholar]
- Samuelson PA. 1948. Consumption Theory in Terms of Revealed Preference. Economica-New Series 15(60):243–253. [Google Scholar]
- Shenhav A, Straccia MA, Cohen JD, Botvinick MM. 2014. Anterior cingulate engagement in a foraging context reflects choice difficulty, not foraging value. Nat Neurosci 17(9):1249–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugrue LP, Corrado GS, Newsome WT. 2004. Matching behavior and the representation of value in the parietal cortex. Science 304(5678):1782–7. [DOI] [PubMed] [Google Scholar]
- Tobler PN, Christopoulos GI, O’Doherty JP, Dolan RJ, Schultz W. 2008. Neuronal distortions of reward probability without choice. J Neurosci 28(45):11703–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobler PN, Christopoulos GI, O’Doherty JP, Dolan RJ, Schultz W. 2009. Risk-dependent reward value signal in human prefrontal cortex. Proc Natl Acad Sci U S A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobler PN, O’Doherty JP, Dolan RJ, Schultz W. 2007. Reward value coding distinct from risk attitude-related uncertainty coding in human reward systems. J Neurophysiol 97(2):1621–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tom SM, Fox CR, Trepel C, Poldrack RA. 2007. The neural basis of loss aversion in decision-making under risk. Science 315(5811):515–8. [DOI] [PubMed] [Google Scholar]
- Trautmann ST, Vieider FM, Wakker PP. 2008. Causes of ambiguity aversion: Known versus unknown preferences. Journal of Risk and Uncertainty 36(3):225–243. [Google Scholar]
- Tversky A, Kahneman D. 1992. Advances in Prospect-Theory - Cumulative Representation of Uncertainty. Journal of Risk and Uncertainty 5(4):297–323. [Google Scholar]
- Tymula A, Rosenberg Belmaker LA, Roy AK, Ruderman L, Manson K, Glimcher PW and et al. 2012. Adolescents’ risk-taking behavior is driven by tolerance to ambiguity. Proc Natl Acad Sci U S A 109(42):17135–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tymula A, Rosenberg Belmaker LA, Ruderman L, Glimcher PW, Levy I. 2013. Like cognitive function, decision making across the life span shows profound age-related changes. Proc Natl Acad Sci U S A 110(42):17143–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Neumann J, Morgenstern O. 1944. Theory of games and economic behavior. Princeton: Princeton university press; xviii, 625 p. [Google Scholar]
- Wagner MK. 2001. Behavioral characteristics related to substance abuse and risk-taking, sensation-seeking, anxiety sensitivity, and self-reinforcement. Addictive Behaviors 26(1):115–120. [DOI] [PubMed] [Google Scholar]
- Weber EU, Blais AR, Betz NE. 2002. A domain-specific risk-attitude scale: Measuring risk perceptions and risk behaviors. Journal of Behavioral Decision Making 15(4):263. [Google Scholar]
- Zuckerman M, Kuhlman DM. 2000. Personality and risk-taking: Common biosocial factors. Journal of Personality 68(6):999–1029. [DOI] [PubMed] [Google Scholar]