ABSTRACT
Osteosarcoma (OS) is the commonest primary malignant tumour originating from bone. Previous studies demonstrated that long non-coding RNAs (lncRNAs) could participate in both oncogenic and tumor suppressing pathways in various cancer, including OS. The HOXA cluster antisense RNA2 (HOXA-AS2) plays an important role in carcinogenesis, however, the underlying role of HOXA-AS2 in OS progression remains unknown. The aim of the present study was to evaluate the expression and function of HOXA-AS2 in OS. The qRT-PCR analysis was to investigate the expression pattern of HOXA-AS2 in OS tissues. Then, the effects of HOXA-AS2 on cell proliferation, cell cycle, apoptosis, migration, and invasion were assessed in OS in vitro. Furthermore, bioinformatics online programs predicted and luciferase reporter assay were used to validate the association of HOXA-AS2 and miR-520c-3p in OS cells. We observed that HOXA-AS2 was up-regulated in OS tissues. In vitro experiments revealed that HOXA-AS2 knockdown significantly inhibited OS cells proliferation by promoting apoptosis and causing G1 arrest, whereas HOXA-AS2 overexpression promoted cell proliferation. Further functional assays indicated that HOXA-AS2 significantly promoted OS cell migration and invasion by promoting epithelial-mesenchymal transition (EMT). Bioinformatics online programs predicted that HOXA-AS2 sponge miR-520c-3p at 3ʹ-UTR with complementary binding sites, which was validated using luciferase reporter assay. HOXA-AS2 could negatively regulate the expression of miR-520c-3p in OS cells. In conclusion, our study suggests that HOXA-AS2 acts as a functional oncogene in OS.
KEYWORDS: HOXA-AS2, LncRNA, Osteosarcoma, miR-520c-3p, EMT, therapeutic target
Background
Osteosarcoma (OS), the most frequently diagnosed bone malignancies, is the leading cause of cancer-related deaths in children and young adolescents [1]. Although the development of multidisciplinary treatments have significantly improved the outcomes of OS patients, the 5-year survival rate of OS remains poor [2]. Therefore, it is urgent to identify the underlying mechanisms for OS development and progression.
As a type of non-coding RNAs (ncRNAs), long non-coding RNAs (lncRNAs) are molecules greater than 200 nt in length, frequently ranging up to 100 kb [3]. LncRNA can act as oncogenes or tumor suppressors in several cancers, as well as OS [4,5]. Recently study reported that Z HOXA cluster antisense RNA 2 (HOXA-AS2), a lincRNA located between and antisense to the human HOXA3 and HOXA4 genes, was shown to be dysregulated in malignant tumors [6]. Nevertheless, whether and how HOXA-AS2 is involved in the pathogenesis of OS is yet to be investigated. The focus of this study was to investigate the expression and roles of HOXA-AS2 in OS.
LncRNAs could act as competing endogenous RNAs (ceRNAs) with microRNAs (miRNAs) to play a post-transcriptional regulatory role in the gene expression [7,8]. Dysregulation of miRNAs including miR-520c-3p influence the biological progression of various cancer cells [9]. Recent study has demonstrated that lncRNA-HOXA-AS2/EZH2/LSD1 complex may function as an oncogene in pancreatic cancer cell proliferation, and also provides a potential therapy target for pancreatic cancer [10]. Tang et al. found that miR-520c-3p suppress the invasion and migration of breast cancer by targeting IL-8 [11]. In addition, miR-520c-3p could mediate migration and invasion of colorectal cancer by targeting S100A4 expression [12]. In this study, we aimed to explore the ceRNA mechanism of HOXA-AS2 though miR-520c-3p and revealed the functional relevance of miR-520c-3p and HOXA-AS2 in OS.
Methods
Clinical samples
A total of 66 samples of OS tissues and paired adjacent noncancerous tissues were obtained from patients who underwent surgery at Department of Orthopaedics, Renji Hospital, Shanghai Jiao Tong University School of Medicine between 2010 and 2016. All the patients were pathologically confirmed and the tissues were collected immediately after they were obtained during the surgical operation, and then stored at −80°C to prevent RNA loss. They were classified according to the WHO criteria and staged according to the tumor-node-metastasis (TNM) classification. Written informed consent was obtained from all patients according to the guidelines approved by the Ethics Committee of Renji Hospital, Shanghai Jiao Tong University School of Medicine.
Cell lines
The human osteosarcoma cell lines (U2OS, Saos-2, HOS and MG-63) and normal human osteoplastic cell line (NHOst) were purchased from the American Type Culture Collection (ATCC, USA). HEK-293 cells were supplied by China Center for Type Culture Collection (CCTCC). All cell lines were maintained in Dulbecco’s Modified Eagle’s Medium (DMEM) medium (Invitrogen, Carlsbad, CA, USA) containing 10% fetal bovine serum (FBS) (Sigma-Aldrich, St. Louis, MO, USA), 100 U/ml penicillin and 100 g/ml streptomycin (Life Technologies, Grand Island, NY, USA) at 37°C in 5% CO2 and 95% air.
RNA extraction and qRT-PCR assays
Total RNA was extracted from tissues or cultured cells using TRIZOL reagent (Invitrogen). For qRT-PCR, RNA was reverse transcribed to cDNA by using a Reverse Transcription Kit (Takara, Dalian, China). Real-time PCR analyses were performed with SYBR Premix Ex Taq (Takara, Dalian China). Results were normalized to the expression of GAPDH. The sequence of the primers were as following: HOXA-AS2 (Forward: 5ʹ-CCCGTAGGAAGAACCGATGA-3ʹ, Reverse: 5ʹ-TTTAGGCCTTCGCAGACAGC-3ʹ) and GAPDH (Forward: 5ʹ-GGGAGCCAAAAGGGTCAT-3ʹ, Reverse: 5ʹ-GAGTCCTTCCACGATACCAA-3ʹ). The qRT-PCR assays were conducted on an ABI 7500, and data collected with this instrument. Our qRT-PCR results were analyzed and expressed relative to threshold cycle (CT) values, and then converted to fold changes.
Transfection
To perform effective lentivirus-mediated suppression of HOXA-AS2 in U2OS and MG-63 cells, the following HOXA-AS2 shRNA and scrambled control shRNA were inserted into the pLVX-tdTomato-Puro vector (Biowit, Shenzhen, China): HOXA-AS2 shRNA#1 forward, 5ʹ- GAGUUCAGCUCAAGUUGAACAUACA-3ʹ and reverse, 5ʹ- UGUAUGUUCAACUUGAGCUGAACUC-3ʹ; shRNA#2 forward, 5ʹ;- AAACCUUGUAGAUAGCUUGAGCUGG-3ʹ and reverse, 5ʹ- CCAGCUCAAGCUAUCUACAAGGUUU-3ʹ; shRNA#3 forward, 5ʹ- CAAGCUUGACAAGUUCAGCUCAA-3ʹ and reverse, 5ʹ- UUGAGCUGAACUCUUGUCAAGCUUG-3ʹ; The full-length complementary DNA of HOXA-AS2 was synthesized by Realgene (Nanjing, China) and subcloned into the pcDNA3.1 (+) vector (Invitrogen) according to the manufacturer’s instructions. At 48 h post-transfection, cells were harvested for qRT-PCR analysis.
Cell counting kit-8 assay
Cell proliferation was monitored by the Cell Counting Kit-8 (CCK8) assay (Promega) every 24 h following the manufacturer’s protocol. In brief, the transfected cells were plated in 96-well plates (3000 cells/well), and then 10 µl of CCK8 solution was added and incubated for 2h. Each solution was measured spectrophotometrically at 450nm.
Colony formation assay
Cells (0.5 × 103 cells per well) were seeded in a six-well plate and cultured for 10 days after treatment. Colonies were then fixed with 10% formaldehyde for 10 min and stained for 5 min with 0.5% crystal violet. Then the number of colonies was counted using ImageJ and images were taken under Olympus microscope (Tokyo, Japan).
Flow cytometric analysis
Cells were harvested directly or 48 h after siRNA transient transfection and washed with ice-cold phosphate-buffered saline (PBS). The PI/RNase staining kits (Multisciences, Hangzhou, China) and annexin V-fluorescein isothiocyanate (FITC) apoptosis detection kits (KeyGEN Biotech, Nanjing, China) were used to detect cell cycle and apoptosis in a FACScan instrument (Becton Dickinson, Mountain View, CA, USA), respectively.
Transwell migration/invasion assay
Transwell chamber was used to measure cell migration and invasion abilities. In brief, culture inserts with 8-mm pore size (Transwell; Corning, NY) were placed into 24-well plates. Before the measurement of invasion ability, the plates were pre-coated with matrigel. 2 h before the addition of matrigel, 500 µL of serum-free medium was independently added to the upper and lower chambers, followed by incubation at 37°C for hydration. Cells were digested by typsin, and resuspended in serum-free medium. The cell density was adjusted to 1 × 105/mL. Then, 200 µL of cell suspension was added into the upper chamber, and 500 µL of DMEM containing 10% FBS into the lower chamber. After incubation at 37°C with 5% CO2 for 24 h, the Transwell chamber was removed, cells were washed with 1× PBS, fixed in paraformaldehyde for 20 min, and then stained with 0.1% crystal violet for 20 min. The cotton swab was used to clean the non-migrated cells in the upper chamber, cells migrating through the membrane were counted in 5 randomly selected fields under a microscope (Nikon) at a magnification of × 100.
Western blot assay
Cells were lysed in lysis buffer containing protease inhibitor cocktail. Protein concentration was determined using a Bio-Rad protein assay system (Bio-Rad, Hercules, CA, USA). Equivalent amounts of proteins were separated by SDS-PAGE, and then transferred onto polyvinylidene difluoride membranes (Bio-Rad). After being blocked in Tris-buffered saline (TBS) containing 5% non-fat milk, the membranes were incubated with specific primary antibody against E-cadherin, N-cadherin, vimentin, (1:1000; Abcam Inc., Cambridge, MA, USA) and GAPDH (1:2000, Santa Cruz Biotechnology) antibodies at 4°C for 12 h and then with horseradish peroxidase-conjugated anti-rabbit antibody for 2 h at room temperature. Proteins were visualised using ECL (Pierce, Rockford, IL, USA) and detected using BioImaging Systems (UVP Inc., Upland, CA, USA).
Luciferase assay
The luciferase assays were carried out using the Dualluciferase Reporter Assay System (Promega, Madison, WI, USA). Briefly, cells were co-transfected with miR-520c-3p or miR-control and pMIR-report luciferase vector containing 3′ UTR of POU2F1, wild-type or mutant HOXA-AS2 fragment, using Lipofectamine 2000 (Invitrogen). Cells were collected and lysed for luciferase detection 48 h after transfection. The relative luciferase activity was normalized against to the Renilla luciferase activity.
Statistical analysis
All statistical analyses were performed using SPSS 17 software (SPSS, USA). All experiments were performed in triplicate and analyzed for significant differences between two groups were analyzed using Student’s t test; One-way ANOVA was performed to analyze the more than two groups. P < 0.05 was considered statistically significant. The data are showed as means ± SD.
Results
LncRNA-HOXA-AS2 was up-regulated in human OS tissues and cell lines
Firstly, we analyzed the expression levels of HOXA-AS2 in human OS tissues by qRT-PCR analysis, and found that HOXA-AS2 expression levels were upregulated in tumor tissues compared with normal tissues (Figure 1(a)). We found that high expression of HOXA-AS2 was corrected with distant metastasis (*P < 0.05, Figure 1(b)). To evaluate the relationship between HOXA-AS2 expression and clinicopathological parameters in 66 patients with OS, we divided the 66 patients into high and low HOXA-AS2 expression groups based on the median value of HOXA-AS2 expression, and we found that expression was significantly associated with distant metastasis (P < 0.0001) and TNM stage (P < 0.0001) but not correlated with other factors such as gender, age and differentiation (Table 1). These results suggested that HOXA-AS2 might be an oncogene in OS.
Figure 1.
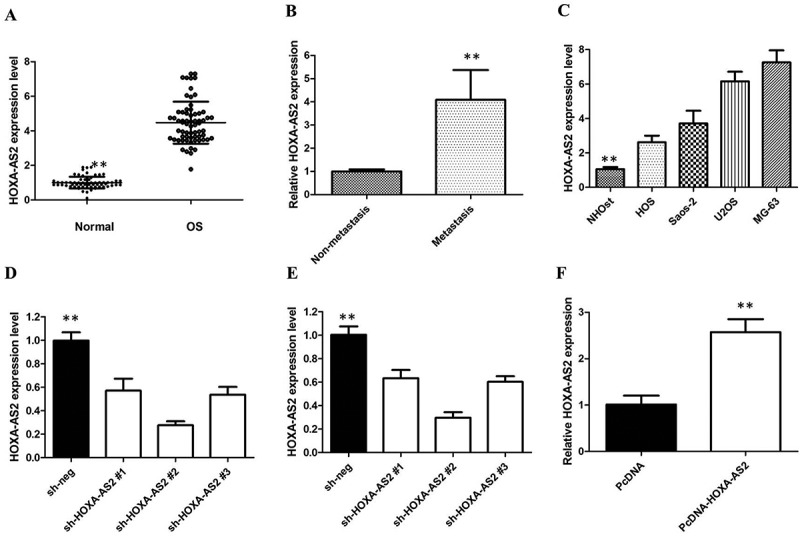
(a) qRT-PCR showing expression level of HOXA-AS2 in OS tissues and adjacent noncancerous tissues; (b) qRT-PCR showing expression level of HOXA-AS2 in metastasis tissues; (c) qRT-PCR showing expression level of HOXA-AS2 in OS cell lines; (d) HOXA-AS2 shRNAs was used to enhance efficiency of HOXA-AS2 knockdown in U2OS cells; (e) HOXA-AS2 shRNAs was used to enhance efficiency of HOXA-AS2 knockdown in MG-63 cells; (f) ectopic overexpression of HOXA-AS2 by transfecting HOS cell lines with pcDNA-HOXA-AS2 expression vector. All tests were at least performed three times. Data were expressed as mean ± SD. **P < 0.01.
Table 1.
Correlation between HOXA-AS2 expression and clinicopathologic characteristics of OS patients.
| HOXA-AS2 |
||||
|---|---|---|---|---|
| Overall (n = 66) |
Low (n = 33) |
High (n = 33) |
P | |
| Gender | ||||
| Male | 40 | 18 | 22 | 0.452 |
| Age, y | ||||
| ≥ 20 | 28 | 15 | 13 | 0.583 |
| Tumor size | ||||
| ≥ 5 | 33 | 15 | 18 | 0.136 |
| Enneking stage | ||||
| IA-IIA | 31 | 21 | 10 | 0.000 |
| Pathologic fracture | ||||
| Yes | 15 | 7 | 8 | 0.834 |
| Distant metastasis | ||||
| Yes | 23 | 8 | 15 | 0.000 |
To investigate whether the expression of HOXA-AS2 was altered in OS, we used the qRT-PCR assay to measure the expression of HOXA-AS2 in four human OS cell lines, namely HOS, Saos-2, U2OS, and MG-63 cells, and in the normal osteoblast cell line NHOst. Our results showed that HOXA-AS2 was increased in all OS cell lines compared with the normal osteoblast cell line NHOst. Furthermore, U2OS and MG-63 cells showed the higher expression of HOXA-AS2, in relation to HOS and Saos-2 (P < 0.01; Figure 1(c)). To analyze the role of HOXA-AS2 in OS, U2OS and MG-63 cells were stably transfected with HOXA-AS2 shRNAs (respectively sh HOXA-AS2 #1, shHOXA-AS2 #2, or shHOXA-AS2 #3) or empty vectors (sh-NC).
We detected HOXA-AS2 expression at 48h post-transfection by qRT-PCR analysis to analyze knockdown efficiency and revealed that shHOXA-AS2 #2 had higher efficiency of interference than shHOXA-AS2 #1 and #3 group (Figure 1(d,e)), so we chose shHOXA-AS2 #2 subsequently for the following experiments. Meanwhile, we induced ectopic overexpression of HOXA-AS2 by transfecting HOS cell lines with pcDNA-HOXA-AS2 expression vector (Figure 1(f)).
HOXA-AS2 promotes OS cell proliferation in vitro
CCK8 assays showed that silencing of HOXA-AS2 expression significantly inhibited cell proliferation of U2OS and MG-63 cells compared with the respective controls (P < 0.01, Figure 2(a,b)). Whereas, stimulated HOXA-AS2 expression promoted HOS cell proliferation (P < 0.01, Figure 2(c)). Furthermore, colony-formation assays also indicated that clonogenic survival was significantly decreased following silencing of HOXA-AS2 in U2OS and MG-63 cells (P < 0.05, Figure 2(d,e)), but markedly increased in HOS cells after overexpression of HOXA-AS2 (P < 0.05, Figure 2(f)).
Figure 2.
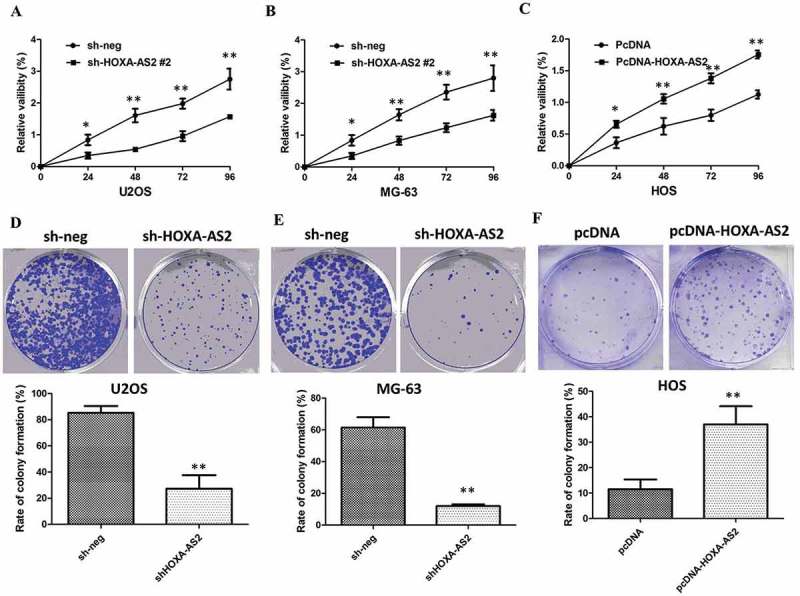
(a) CCK8 assay showing knockdown of HOXA-AS2 inhibited cell proliferation of U2OS cells; (b) CCK8 assay showing knockdown of HOXA-AS2 inhibited cell proliferation of MG-63 cells; (c) CCK8 assay showing overexpression of HOXA-AS2 promoted cell proliferation of HOS cells; (d) Colony-formation assays showed that silencing of HOXA-AS2 significantly inhibited the colony-forming ability of U2OS cells; (e) Colony-formation assays showed that silencing of HOXA-AS2 significantly inhibited the colony-forming ability of MG-63 cells; (f) Colony-formation assays showed that overexpression of HOXA-AS2 significantly promoted the colony-forming ability of HOS cells; All tests were at least performed three times. Data were expressed as mean ± SD. *P < 0.05, **P < 0.01.
Next, flow cytometric analysis was performed further to evaluate whether HOXA-AS2 could impact proliferation of U2OS and MG-63 cells by altering cell-cycle progression or apoptosis. Compared with control cells, knockdown of HOXA-AS2 caused cell cycle arrest in G0/G1 phase 48 hrs after transfection in U2OS and MG-63 cells (P < 0.01, Figure 3(a,b)). Overexpression of HOXA-AS2 increased the S-phase percentage and decreased G0/G1 phase percentage of HOS cells (P < 0.01, Figure 3(c)). The results of flow cytometry showed that knockdown of HOXA-AS2 resulted in a higher number of apoptotic cells in U2OS and MG-63 cells compared with the controls (P < 0.01, Figure 3(d,e)). As expected, the cell apoptosis was markedly decreased in HOS cells by pcDNA 3.1-HOXA-AS2 (P < 0.01, Figure 3(f)).
Figure 3.
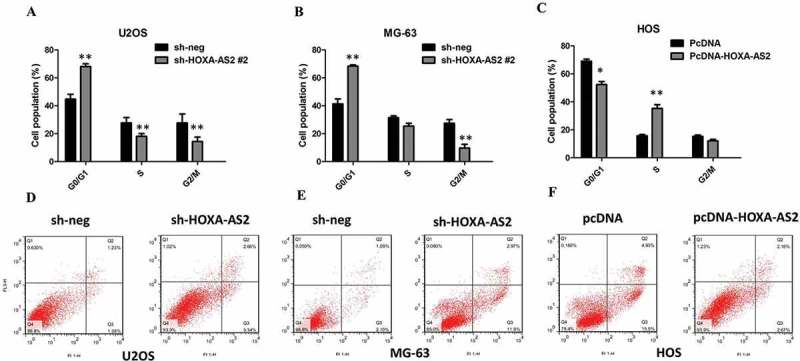
(a) The flow cytometry assay showed that U2OS cells transfected with shHOXA-AS2 #2 had cell-cycle arrest at the G1-G0 phase in comparison with control cells; (b) The flow cytometry assay showed that MG-63 cells transfected with shHOXA-AS2 #2 had cell-cycle arrest at the G1-G0 phase in comparison with control cells; (c) The flow cytometry assay showed that HOS cells transfected with pcDNA 3.1-HOXA-AS2 had cell-cycle arrest at the S phase in comparison with control cells; (d) The flow cytometry assay showed that U2OS cells transfected with shHOXA-AS2 #2 had higher apoptotic rate in comparison with control cells; (e) The flow cytometry assay showed that MG-63 cells transfected with shHOXA-AS2 #2 had higher apoptotic rate in comparison with control cells; (f) The flow cytometry assay showed that HOS cells transfected with pcDNA 3.1-HOXA-AS2 had lower apoptotic rate in comparison with control cells; All tests were at least performed three times. Data were expressed as mean ± SD. *P < 0.05, **P < 0.01.
HOXA-AS2 promotes OS cell migration and invasion via regulating EMT
To further confirm the effect of HOXA-AS2 on OS cell migration and invasion, we investigated cell migration and invasion by transwell assay. The results showed that the migratory and invasive capacities of U2OS and MG-63 cells dramatically decreased after deregulation of HOXA-AS2 (P < 0.05, Figure 4(a,b)). Furthermore, the transwell assay showed that overexpression of HOXA-AS2 treatment significantly promoted the migration and invasive capacity of HOS cells compared to NC (P < 0.05, Figure 4(c)), suggesting that HOXA-AS2 could promote OS cell migration and invasion.
Figure 4.
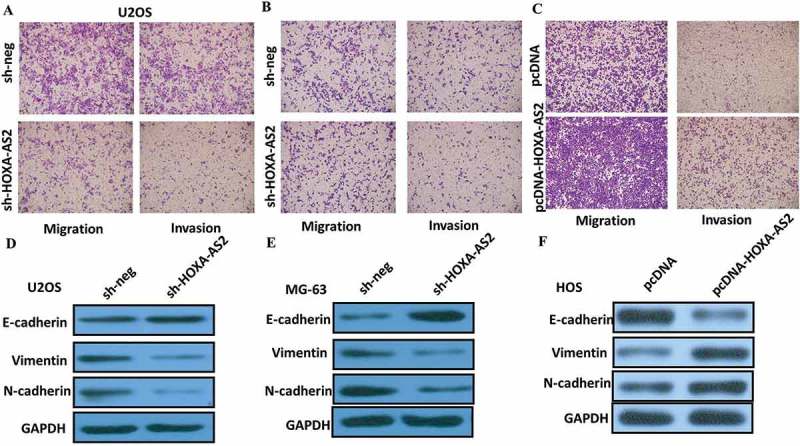
(a) Inhibition of migration and invasion of U2OS cells transfected with shHOXA-AS2 #2; (b) Inhibition of migration and invasion of MG-63 cells transfected with shHOXA-AS2 #2; (c) Overexpression of HOXA-AS2 treatment significantly promoted the migration capacity of HOS cells; (d) Knockdown of HOXA-AS2 reverses EMT in U2OS cells; (e) Knockdown of HOXA-AS2 reverses EMT in MG-63 cells; (f) Overexpression of HOXA-AS2 promotes EMT in HOS cells.
EMT is a process defined by cells losing their junctions, repressing E-cadherin expression, and exhibiting increased cell mobility, with EMT required for the formation of multiple tissues and organs during development. Increasing evidence suggests that EMT process may also be involved in modulating tumor invasion of cancer cells. We next examined whether silencing HOXA-AS2 expression inhibited mesenchymal features. As expected, HOXA-AS2 knockdown decreased the expression of Vimentin and N-cadherin, and increased E-cadherin expression in U2OS and MG-63 cells (Figure 4(c,d)). Overexpression of HOXA-AS2 treatment significantly decreased the expression of E-cadherin, and increased Vimentin and N-cadherin expression in HOS cells. In conclusion, our results suggest that inhibition of HOXA-AS2 decreased the ability of migration and invasion of OS cells, and that this effect likely relies on reversing the EMT process.
HOXA-AS2 inhibited miR-520c-3p expression in OS
To investigate the effect of HOXA-AS2 on the expression of miRNAs, U2OS cells were transfected with either empty vector or shHOXA-AS2 #2 for 48 hrs. As shown in Figure 5(a), miRNA microarray was performed after HOXA-AS2 knockdown, and miR-520c-3p was picked out by setting threshold values (FC <− 2, P < 0.05, n = 3).
Figure 5.
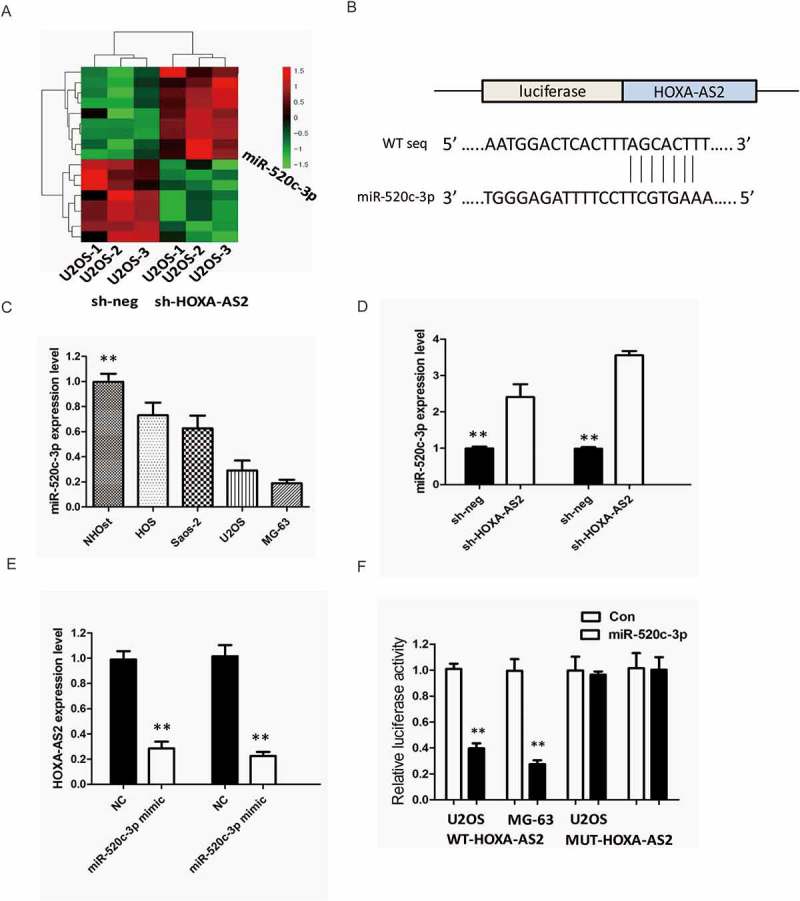
(a) U2OS cells were transfected with shHOXA-AS2 #2 for 48 hrs. Hierarchical clustering revealed systematic variations in the expression of miRNAs. Numerous differentially expressed miRNAs between control and shHOXA-AS2 #2 transfected U2OS cells are shown on a scale from green (low) to red (high); (b) StarBase v2.0 results showing the sequence of HOXA-AS2 with highly conserved putative miR-520c-3p binding sites; (c) Expression levels of miR-520c-3p in different OS cell lines were determined by qRT-PCR; (d) Silencing of HOXA-AS2 increased the expression level of miR-520c-3p in U2OS and MG-63 cells; (e) miR-520c-3p inhibited the expression of HOXA-AS2 in U2OS and MG-63 cells; (f) The wild-type or mutant miR-520c-3p-binding sites in HOXA-AS2 were inserted into pMIR-report luciferase vector. Luciferase activity was detected in OS cells co-transfected with miR-520c-3p or negative control (miR-control) and reporter plasmids containing WT-HOXA-AS2 (wild type) or MUT-HOXA-AS2 (mutant type). The normalized luciferase activity in the miR-control group was used as the relative luciferase activity. All tests were at least performed three times. Data were expressed as mean ± SD. *P < 0.05, **P < 0.01.
Bioinformatics prediction with TargetScan and miRDB of miRNA recognition sequences on HOXA-AS2 revealed the presence of miR-520c-3p binding sites, and the potential binding sites were shown in Figure 5(b). Next, we measured the levels of miR-520c-3p expression in various OS cell lines. As shown in Figure 5(c), the expression of miR-520c-3p was obviously decreased in U2OS and MG-63 cells, indicating the opposite result to HOXA-AS2 expression.
To explore the regulatory relationship between HOXA-AS2 and miR-520c-3p, U2OS and MG-63 cells were transfected with either empty vector or shHOXA-AS2 #2, and qRT-PCR was used to detect the expression level of miR-520c-3p. The results showed that silencing of HOXA-AS2 increased the expression level of miR-520c-3p in U2OS and MG-63 cells (P < 0.01, Figure 5(d)). Meanwhile, miR-520c-3p also inhibited the expression of HOXA-AS2 in U2OS and MG-63 cells (P < 0.05, Figure 5(e)).
For further confirmation, we used the luciferase assay to detect the association between HOXA-AS2 and miR-520c-3p. We cloned the wild type sequence of HOXA-AS2 or its mutant sequence into the pMIR luciferase reporter, and co-transfected into U2OS and MG-63 cells the reporter plasmid (or the corresponding mutant reporter) and miR-520c-3p. The results showed that the relative luciferase activity of the pMIR-HOXA-AS2-wt construct was significantly decreased but was abolished in HOXA-AS2-mt-transfected cells (P < 0.05, Figure 5(e)). All these data demonstrated that HOXA-AS2 associated with the miR-520c-3p and may function as a ceRNA.
miR-520c-3p inhibits OS cell proliferation
Then, we detected the levels of miR-520c-3p in 66 pairs of OS tissues together with adjacent non-tumour tissues by the qRT-PCR assay. It was revealed by our findings that miR-520c-3p expression was considerably downregulated in OS tissues, in comparison with the adjacent non-tumour tissuess (P < 0.01; (Figure 6(a)), and miR-520c-3p expression was inversely correlated with HOXA-AS2 expression in OS tissues (R = -0.175, P = 0.003, Figure 6(b)). In addition, the relationships between miR-520c-3p expression and clinical characteristics of OS patients were analyzed, and we found that lower miR-520c-3p expression was significantly associated with distant metastasis (P = 0.001) and TNM stage (P < 0.001), which has a negative relationship with HOXA-AS2.
Figure 6.
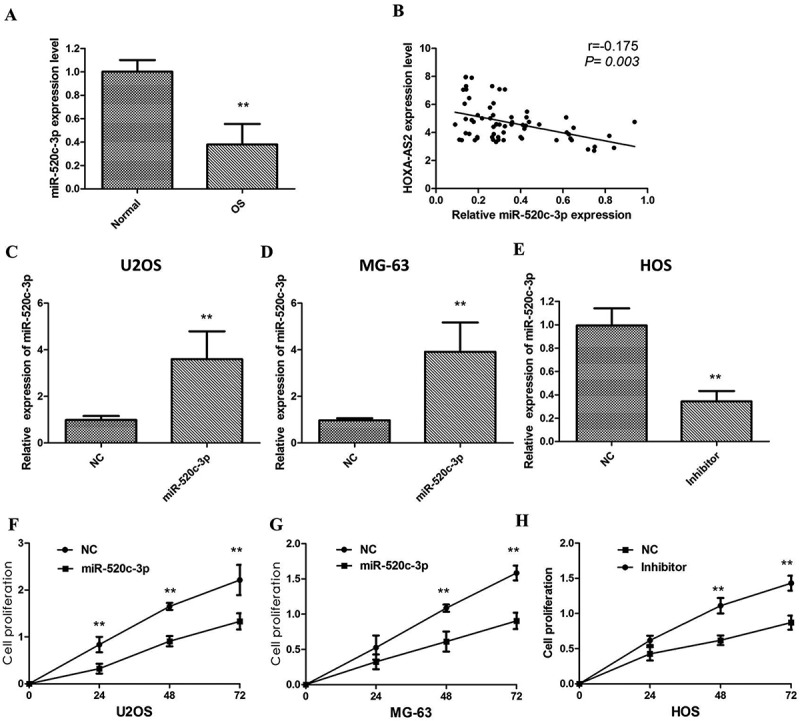
(a) qRT-PCR showing expression level of miR-520c-3pin OS tissues and adjacent noncancerous tissues; (b) miR-520c-3p expression was inversely correlated with HOXA-AS2 expression in OS tissues; (c) Treatment of mimic force the expression of miR-520c-3p in U2OScells; (d) Treatment of mimic force the expression of miR-520c-3p in MG-63cells; (e) miR-520c-3p inhibitor knockdown the expression of miR-520c-3p in HOS cells; (f) CCK8 assay showingoverexpression of miR-520c-3p inhibited cell proliferation of U2OS cells; (g) CCK8 assay showing over expression of miR-520c-3p inhibited cell proliferation of MG-63cells; (h) miR-520c-3p inhibitor promotes cell proliferation of HOS cells.]
To validate whether miR-520c-3p could also inhibit OS cell proliferation, we used the mimic treatment to force the expression of miR-520c-3p in U2OS and MG-63 cells, whereas knockdown the miR-520c-3p level by treatment with miR-520c-3p inhibitor in HOS cells (P < 0.01; Figure 6(c–e)). Functionally, the results of the CCK8 assay revealed that miR-520c-3p overexpression markedly inhibits U2OS and MG-63 cell proliferation, which is consistent with the results of HOXA-AS2 expression knockdown, whereas miR-520c-3p knockdown by specific inhibitor promotes cell proliferation in HOS cells (P < 0.01; Figure 6(f–h)).
Discussion
In this study, we have demonstrated that HOXA-AS2 functioned as a tumor promotor in OS. Specifically, we found that the mechanism by which HOXA-AS2 promotes the progression of OS is mediated by inhibiting miR-520c-3p expression. Expression of HOXA-AS2 in OS and metastatic OS was significantly elevated compared with that in surrounding non-tumor and non-metastatic tissues. The higher HOXA-AS2 levels were associated with enhanced OS invasion, migration, and proliferation. These findings reveal a potential role of HOXA-AS2 in the development of invasion and metastasis of OS.
The evidence proves that there exist lncRNAs in cell proliferation, apoptosis, chromatin modification, RNA processing, genomic reprogramming, and cell cycle. HOXA-AS2, a 1048-bp lncRNA that serves as an apoptosis repressor in all-trans retinoic acid-treated NB4 promyelocytic leukemia cells, has been reported several times in various types of malignant tumours. In multiple cancers including breast, kidney, stomach, ovarian, esophagus, and lung, overexpressed HOXA-AS2 gene is able to promote tumorigenesis and development [10,13–15]. However, understanding of the precise molecular mechanisms underlying OS is needed.
We herein uncover a novel carcinogenic role of HOXA-AS2 in the OS development. Initially, we confirmed that HOXA-AS2 was significantly upregulated in the OS tissues compared with the normal osteogenic tissues. In addition, increased expression of the HOXA-AS2 is associated with distant metastasis and advanced TNM stage. Our subsequent studies demonstrate that knockdown of HOXA-AS2 significantly inhibited OS cell proliferation, whereas HOXA-AS2 overexpression has the opposite results. In addition, HOXA-AS2 knockdown promoted significant arrest in the G0/G1-phase and resulted in an obvious increase of apoptosis in OS cell line.
Several studies are in agreement with our results, indicating that HOXA-AS2 affects the motility of cancer cells, which were key molecules in tumor metastasis [16,17]. In this study, HOXA-AS2 knockdown inhibits the migratory and invasive ability of OS cells, indicating that high expression of HOXA-AS2 could promote OS cell migration and invasiveness ability. HOXA-AS2 might also play an important role in the regulation of EMT. HOXA-AS2 certainly increase the expression level of vimentin and N-cadherin, and inhibit E-Cadherin expression.
LncRNAs are potentially implicated in determining cancer development through binding with miRNAs that have a wide range of targets. Gao et al. found that HOXA-AS2 knockdown inhibited malignant glioma behaviors and VM formation via the miR-373/EGFR axis [18]. To investigate whether HOXA-AS2 can play a role as competitive endogenous RNA (ceRNA) in OS, we searched for its potential interactions with miRNAs by bioinformatic analysis. In support of this notion, we performed a microarray to screen the HOXA-AS2-regulated miRNAs in OS. Bioinformatics analysis and luciferase assays were also used to validate the direct binding ability of the predicted miRNA response elements on the full-length HOXA-AS2 transcript. We confirmed that miR-520c-3p, screened by microarray and bioinformatics analyses, was a HOXA-AS2-regulated miRNA in OS cells.
In current investigation, our finding presents the role of HOXA-AS2 in OS development and demonstrates the underlying mechanism, leading to the development of a potentially novel strategy for OS treatment.
Funding Statement
No funding was received.
Acknowledgments
Not applicable.
Availability of data and materials
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request, but no information infringing on the privacy of the participants will be given.
Authors’ contributions
RX and XH conceived, designed the project and wrote this manuscript. YW, RZ and GC performed most of the experiments. GC and YW collected the clinical and pathological data and analyzed it. All of the authors read and approved the final manuscript.
Consent for publication
Not applicable.
Ethics approval and consent to participate
All procedures performed in these studies involving human participants were in accordance with the ethical standards of the Renji Hospital, Shanghai Jiao Tong University School of Medicine research committee. The study was approved by the ethics committee of Renji Hospital, Shanghai Jiao Tong University School of Medicine. Written informed consent was obtained from all individual participants that were included in the study. All of the animal experiments were performed according to the NIH animal use guidelines on the use of experimental animal. All of the animal experimental protocols were approved by the Animal Care and Use Committee of Renji Hospital, Shanghai Jiao Tong University School of Medicine.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Kager L, Zoubek A, Potschger U, et al. Primary metastatic osteosarcoma: presentation and outcome of patients treated on neoadjuvant Cooperative Osteosarcoma Study Group protocols. J Clin Oncol. 2003;21(10):2011–2018. [DOI] [PubMed] [Google Scholar]
- [2].Lee DH, Qi J, Bradner JE, et al. Synergistic effect of JQ1 and rapamycin for treatment of human osteosarcoma. Int J Cancer. 2015;136(9):2055–2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kornienko AE, Guenzl PM, Barlow DP, et al. Gene regulation by the act of long non-coding RNA transcription. BMC Biology. 2013;11:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ponting CP, Oliver PL, Reik W.. Evolution and functions of long noncoding RNAs. Cell. 2009;136:629–641. [DOI] [PubMed] [Google Scholar]
- [5].Xu XF, Li J, Cao YX, et al. Differential expression of long noncoding RNAs in human cumulus cells related to embryo developmental potential: A microarray analysis. Reprod Sci. 2015;22:672–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Zhao H, Zhang X, Frazao JB, et al. Newburger PE: HOX antisense lincRNA HOXA-AS2 is an apoptosis repressor in all trans retinoic acid treated NB4 promyelocytic leukemia cells. J Cell Biochem. 2013;114:2375–2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Salmena L, Poliseno L, Tay Y, et al. A ceRNA hypothesis: the Rosetta Stone of a hidden RNA language? Cell. 2011;146:353–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].McCarthy N. Regulatory RNA layer by layer. Nat Rev Genet. 2011;12:804. [DOI] [PubMed] [Google Scholar]
- [9].Carthew RW, Sontheimer EJ. Origins and mechanisms of miRNAs and siRNAs. Cell. 2009;136:642–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lian Y, Li Z, Fan Y, et al. The lncRNA-HOXA-AS2/EZH2/LSD1 oncogene complex promotes cell proliferation in pancreatic cancer. Am J Transl Res. 2017. December 15;9(12):5496–5506. eCollection 2017. [PMC free article] [PubMed] [Google Scholar]
- [11].Tang CP, Zhou HJ, Qin J, et al. MicroRNA-520c-3p negatively regulates EMT by targeting IL-8 to suppress the invasion and migration of breast cancer. Oncol Rep. 2017. November;38(5):3144–3152. [DOI] [PubMed] [Google Scholar]
- [12].Mudduluru G, Ilm K, Fuchs S, et al. Epigenetic silencing of miR-520c leads to induced S100A4 expression and its mediated colorectal cancer progression. Oncotarget. 2017. March 28;8(13):21081–21094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wang F, Yang H, Deng Z, et al. HOX antisense lincRNA HOXA-AS2 promotes tumorigenesis of hepatocellular carcinoma. Cell Physiol Biochem. 2016;40:287–296. [PubMed]. [DOI] [PubMed] [Google Scholar]
- [14].Xie M, Sun M, Zhu YN, et al. Long noncoding RNA HOXA-AS2 promotes gastric cancer proliferation by epigenetically silencing P21/PLK3/DDIT3 expression. Oncotarget. 2015;6:33587–601. [PMC free article] [PubMed]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Tong G, Wu X, Cheng B, et al. Knockdown of HOXA-AS2 suppresses proliferation and induces apoptosis in colorectal cancer. Am J Transl Res. 2017. October 15;9(10):4545–4552. [PMC free article] [PubMed] [Google Scholar]
- [16].Fang Y, Wang J, Wu F, et al. Long non-coding RNA HOXA-AS2 promotes proliferation and invasion of breast cancer by acting as a miR-520c-3p sponge. Oncotarget. 2017. July 11;8(28):46090–46103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ding J, Xie M, Lian Y, et al. Long noncoding RNA HOXA-AS2 represses P21 and KLF2 expression transcription by binding with EZH2, LSD1 in colorectal cancer. Oncogenesis. 2017. January 23;6(1):e288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gao Y, Yu H, Liu Y, et al. Long non-coding RNA HOXA-AS2 regulates malignant glioma behaviors and vasculogenic mimicry formation via the MiR-373/EGFR axis. Cell Physiol Biochem. 2017. December 25;45(1):131–147. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request, but no information infringing on the privacy of the participants will be given.


