Abstract
This trial was designed to evaluate the off-label use of ceftiofur with Marek’s vaccine in one-day-old broiler chicks, a prophylactic treatment that has been done in some commercial hatcheries, on the emergence of extended-spectrum beta-lactamase producing Escherichia coli (ESBL-E. coli). A total of 168 chicks (Cobb500®) were used in a completely randomized design. Birds were assigned to two treatments (Marek’s vaccine plus saline vs Marek’s vaccine plus ceftiofur) and six repetitions, with 14 animals each. Cloacal swabs were collected from 1 to 14 days post-hatch. The majority (86%; p<0.0001) of the ESBL-producing isolates harboring blaCTX-M and blaSHV genes originated from animals receiving the antimicrobial. None of the isolates were positive for plasmid-mediated AmpC betalactamase genes (blaACC, blaCMY-2, blaDHA, blaFOX, blaMOX and blaMIR). These findings indicate that the off-label use of ceftiofur with Marek’s vaccine is associated with the short-term increase in ESBL-producing Escherichia coli in the gut of chicks.
Introduction
The non-therapeutic use of antimicrobial drugs in farm animals is a common practice for disease control and prevention, or to enhance performance. Previous reports demonstrated the link between the off-label antibiotic use of antimicrobials in animals and the increase in antimicrobial resistance [1,2]. Third-generation cephalosporins are among the highest priority critically important antimicrobials to human health [3] and their indiscriminate use is of great concern to public health.
Ceftiofur is commonly administered to day-one chicks together with Marek’s vaccine in certain commercial hatcheries to prevent disease in broilers [4]. Use of ceftiofur in poultry production has been responsible for the increase of resistant E. coli and Salmonella Heidelberg isolates in Canada [5]. The use of ceftiofur has also been reported to be related to the increase of the blaCTX-M gene in E. coli isolates from swine and cattle fecal samples [6]. The potential spread of resistant isolates of E. coli or even their encoding plasmids through direct contact or ingestion of contaminated food pose a worrying public health risk. The objective of this study was to experimentally investigate the effects of the administration of ceftiofur together with Marek’s vaccine in one-day-old chicks on the emergence of extended-spectrum beta-lactamases (ESBL) producing Escherichia coli (E. coli) in the gut.
Materials and methods
The experimental proposal was submitted and approved by the Ethics Committee on Animal Use of the Federal University of Paraiba (CEUA/UFPB). Two hundred fertile eggs were obtained from a commercial hatchery (Guaraves Alimentos Ltda, Guarabira, PB, Brazil), originated from Cobb500 breeders with 44 weeks of age. They were placed in egg incubators with standard temperature (37.7°C) and humidity (60%) conditions and automatic turning at each two hours. After hatch, a total of 168 chicks were weighed individually (mean weight 47.0 ± 0.5g) and used in a completely randomized design with two treatments and six repetitions, with 14 animals per repetition. The chicks were kept into boxes (50 x 50 x 50 cm). Each box was equipped with feeder and drinker and was covered with nylon to avoid contamination between boxes by vectors such as flies. Thermo-hygrometers (Oregon Scientific, Portland, EUA) were used to monitor temperature and relative humidity in the room.
A corn-soybean meal diet was formulated for the initial phase with the following levels: 22.2% crude protein, 2,950 kcal of metabolizable energy/kg of diet, 1.31% digestible lysine, 0.94% digestible methionine + cystine and 0.852% digestible threonine. Animals in the control group (CG) were administered 0.2 mL of Marek’s vaccine suspended in sterile saline solution subcutaneously, whereas the animals in the antimicrobial-administered group (AG) received 0.2 mL of Marek’s vaccine suspended in sterile ceftiofur solution (0.2 mg ceftiofur sodium).
Cloacal swabs were randomly collected from two animals per repetition before vaccination (day 0) and at 3, 5, 7, 9, 11 and 14 days post-hatching. After swab collection the animals were euthanized. The swabs were placed into Luria-Bertani broth (Himedia, India) supplemented with ceftiofur (2mg/L) and incubated at 37°C for 24 h. Then, a 20μL aliquot was spread onto MacConkey Agar (Acumedia, EUA) supplemented with ceftiofur (2mg/L) and incubated at 37°C for 24 h. Lactose fermenting colonies (four per plate) showing characteristics of E. coli were transferred to Eosin Methylene Blue Agar (EMB) (Himedia, India) and further confirmed as E. coli by the following biochemical tests: Triple Sugar Iron Agar (TSI) (Himedia, India), Lysine Iron Agar (LIA) (Himedia, India), Sulfide Indole Motility (SIM) (Acumedia, EUA), Simmons Citrate Agar (Oxoid, UK) and Urea Agar Base (Oxoid, UK).
The Clinical Laboratory Standards Institute (CLSI) disk diffusion method [7] was used to test E. coli- confirmed colonies for antimicrobial susceptibility to the following drugs: amoxicillin/clavulanate (Amx/Clv, 20/10 μg, Cecon, São Paulo, Brazil), aztreonam (ATM, 30 μg, Cecon) cefotaxime (CTX, 30 μg, Cecon), ceftazidime (CAZ, 30 μg, Cecon), ceftriaxone (CRO, 30 μg, Cecon), ciprofloxacin (CIP, 5 μg, Cecon), chloramphenicol (C, 30 μg, Cecon), gentamicin (GM, 10 μg, Cecon), sulfisoxazole / trimethoprim (SXT, 23.75 / 1.25 μg, Cecon) and tetracycline (Te, 30 μg, Cecon).
From each plate, all isolates showing different resistance patterns were taken for further confirmation, therefore, in some cases more than one isolate was recovered per sample (bird). We also determined the minimum inhibitory concentration (MIC) of ceftiofur (CTF) by the broth microdilution method [7] using 96-well microtiter plates containing final ceftiofur concentrations ranging from 0.5 μg/mL to 256 μg/mL. CLSI [7] criteria were used to interpret MIC results as susceptible (MIC ≤2 mg/L), intermediate (4 mg/L), or resistant (≥8 mg/L).
Phenotypic ESBL detection was carried out by double-disk synergy test using CTX, CAZ and CRO disks placed at a distance of 20 mm concentrically to the Amx/Clv disk [7]. E. coli isolates were also tested by PCR targeting the ESBL genes (blaCTX-M, blaCTX-M-1, blaCTX-M-2, blaCTX-M-8 and blaSHV) as well as plasmid-mediated AmpC genes (blaACC, blaCMY-2, blaDHA, blaFOX, blaMOX and blaMIR), using primer sequences and conditions as previously described [8–11] and DNA extracted by phenol/chloroform/isoamyl-alcohol (25:24:1) as described by Fritsch et al. [12]. Afterwards, the DNA extracted from confirmed ESBL-producing E. coli was adjusted to 50 ng/ μL using a microvolume spectrometer (Colibri, Titertek Berthold, Germany) and Enterobacterial Repetitive Intergenic Consensus PCR (ERIC-PCR) was used as genotyping method, as previously described [13]. Shortly, reactions were performed in 25 μL containing 1 pmol of primer, 200 mM of each dNTP, 3 mM of MgCl2, 100 ng of genomic DNA, and 1U of Taq DNA polymerase (Invitrogen, Brazil). Amplification was performed in a thermal cycler (TPersonal Thermocycler, Biometra, Germany). Products were analyzed by electrophoresis in 2% agarose gel (LGC Biotechnology, Brazil) stained with GelRed (Biotium, USA). The presence or absence of bands was analyzed visually under ultraviolet light. ERIC-PCR band patterns were scanned and analyzed using the Dice product moment correlation coefficient (2% tolerance) by BioNumerics software (Version 7.1, Applied Maths, Belgium). Clustering analysis was carried out by the unweighted pair group method with arithmetic averages (UPGMA). E. coli ATCC 25922 was used as internal control (outgroup). Details on the DNA extraction and PCR protocols that were used in this study are described in the Supplementary material (S1 Text and S1 Table). The discriminatory power (D-value) was calculated as described by Hunter [14].
Fisher’s exact test at 5% probability was used to compare the overall frequency of phenotypic ESBL-E. coli isolates between control group (CG) and antimicrobial-administered group (AG). A Bayesian binomial logistic regression (BLR) approach with 8,000 repetitions was used to infer the probability of the ESBL occurrence between the treatment groups along the experimental period. Statistical analyses were performed in R environment [15] using brms package obtained from CRAN (https://cran.r-project.org/web/packages/brms/index.html)
Results and discussion
A total of 57 ceftiofur-resistant E. coli isolates were obtained and confirmed by means of the disk-diffusion method. Out of them, 52 (91.2%) were recovered from the AG group in days 5 (10; 17.5%), 7 (12; 21.1%), 9 (9; 15.8%), 11 (7; 12.3%), and 14 (19; 33.3%). Five (8.8%) ceftiofur-resistant E. coli were cultured from CG birds; all from the last sampling day (14 days).
According to the antimicrobial susceptibility test, high resistance rates were observed for CTX (100%), Te (100%), CRO (94.7%), STX (54.4%), ATM (42.1%) and CIP (40.4%), corroborating previous reports showing increased antimicrobial resistance rates in bacteria from broilers and layers [1] and pigs [16] treated with third generation cephalosporins.
The lowest resistance rates were seen against CAZ (17.5%), Amx/Clv (14%) and GM (8.8%) (Table 1). All 57 recovered E. coli isolates were resistant to CTF (MIC ≥ 8 μg/mL), 21% (12/57) of them showing MIC ≥ 128 μg/mL (S3 Table). We observed a perfect agreement between CFT and CTX resistance. This finding supports a previous report comparing MIC values of CFT versus other cephalosporins, including CAZ, in 118 E. coli from different food-producing animals [17]. Therefore, the use of CTX seems to be a successful approach to assess ceftiofur susceptibility in enterobacteria. That could be of special interest for investigations comparing antimicrobial resistance between isolates from animal and human sources.
Table 1. Antimicrobial resistance of 57 E. coli cultured from chicks receiving ceftiofur added to Marek’s vaccine (AG) and chicks receiving the vaccine only (CG).
Isolates were recovered from MacConkey agar supplemented with ceftiofur (2mg / L).
| Antimicrobials | Frequency of resistance | ||
|---|---|---|---|
| AG* | CG# | Total (%) | |
| Amx/Clv (20 / 10 μg) | 8 | 0 | 8 (14) |
| ATM (30 μg) | 22 | 2 | 24 (42.1) |
| CTX (30 μg) | 52 | 5 | 57 (100) |
| CAZ (30 μg) | 10 | 0 | 10 (17.5) |
| CRO (30 μg) | 49 | 5 | 54 (94.7) |
| CIP (5 μg) | 22 | 1 | 23 (40.4) |
| C (30 μg) | 19 | 1 | 20 (35.1) |
| GM (10 μg) | 5 | 0 | 5 (8.8) |
| SXT (23.75 / 1.25 μg) | 29 | 2 | 31 (54.4) |
| Te (30 μg) | 52 | 5 | 57 (100) |
Antimicrobials tested: Amx/Clv = amoxicillin + clavulanate; ATM = aztreonam; CTX = cefotaxime; CAZ = ceftazidime; CRO = ceftriaxone; CIP = ciprofloxacin; C = chloramphenicol; GM = gentamicin; SXT = sulfisoxazole + trimethoprim; Te = tetracycline.
* antimicrobial-administered group
# control group
Based on the antimicrobial susceptibility test, 63.2% (36/57) of all E. coli isolates were resistant to three or more different classes of drugs and therefore considered multidrug resistant (MDR) (S2 and S3 Tables). A total of 14 isolates (24.6%) showed resistance to more than five antimicrobial classes.
A total of 24 (42.1%) isolates phenotypically confirmed as ESBL were recovered throughout the experiment and further investigated by PCR (Table 2). Only 3/24 (12.5%) isolates were obtained from CG, whereas 21 (87.5%) were recovered from AG. From the positive isolates, 14 (58.3%) harbored the genes blaCTX-M and blaSHV; 12 out of the 14 originated from AG. Only 2 out of 14 ESBL-E. coli recovered from CG harbored those genes. blaCTX-M-1 was the most frequent CTX-M type observed (13/14), whereas only one isolate harbored blaCTXM-8 (1/14). No blaCTX-M-2 has been detected among the isolates. Similarly to the findings of our study, E. coli harboring blaCTX-M genes have been shown to be frequently resistant to CTF and CTX but susceptible to CAZ [18], which is possibly related to the fact that CTX-M β-lactamase cannot hydrolyze CAZ as efficiently as CTX [19].
Table 2. Number of animals positive for ESBL-producing E. coli identified phenotypically by the double-disk synergy test.
| Sampling daya | Experimental groups | P* | |||||
|---|---|---|---|---|---|---|---|
| AG | CG | AG vs. CG | |||||
| Positive ESBL-E. coli chicksb | MICc | Genesd | Positive ESBL-E. coli chicks | MIC | Genes | ||
| Day 5 | 5/12 | 64–256 | blaCTX-M-8; blaSHV | 0/12 | - | - | * |
| Day 7 | 5/12 | 16–256 | blaCTX-M-1; blaSHV | 0/12 | - | - | * |
| Day 9 | 2/12 | 8 | blaCTX-M-1; blaSHV | 0/12 | - | - | * |
| Day 11 | 1/12 | 16 | - | 0/12 | - | - | * |
| Day 14 | 8/12 | 32–64 | blaCTX-M-1; blaSHV | 3/12 | 64 | blaCTX-M-1; blaSHV | * |
a No ESBL-E. coli recovered at 0 and 3 days
b Frequency of animals harboring ceftiofur-resistant E. coli
c Range of minimum inhibitory concentration (MIC) of ceftiofur (ug/mL)
d Resistant genes detected by PCR in the isolates
* Significantly different confidence intervals of the predicted probabilities of ESBL E. colli by Bayesian Binomial Logistic Regression analysis.
The genes blaCTX-M, blaCTX-M-1, blaCTX-M-2, blaCTX-M-8 and blaSHV were not detected in the 33 ceftiofur-resistant E. coli that were phenotypically negative for the double-disk synergy test for ESBL detection. Furthermore, none of the 57 CFT-resistant E. coli isolates harbored any of the plasmid-mediated AmpC genes investigated in the present study, including blaCMY-2, which has been reported in CFT-resistant E. coli from animal origin in different countries [20–24]. Based on these findings and on the phenotypic results, we assume that the 33 CFT-resistant isolates could harbor other cephamycinase genes that were not investigated in our study or maybe a mutation in the chromosomal ampC associated with the hyperproduction of cephamycinase. This last hypothesis is more probable, as cefoxitin-resistance in E. coli has been less commonly associated to plasmid-mediated AmpC β-lactamases than to hyperproduction of chromosomal AmpC β-lactamase [25].
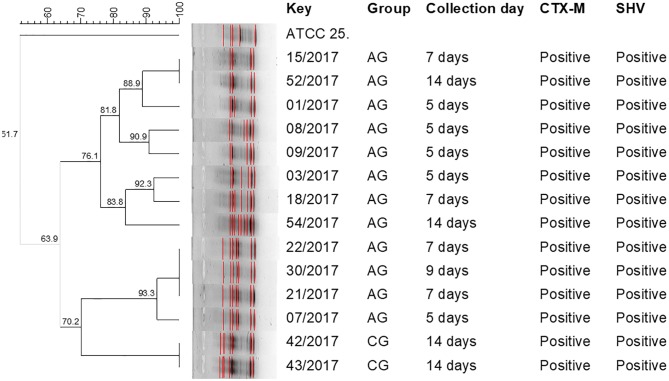
Interestingly, distinct genotypic patterns among ESBL-producing E. coli isolates from AG and CG were observed, indicating that isolates of CG animals emerged from a different population. This finding also indicates that there was no cross-contamination of ESBL-E. coli from AG to CG animals (Fig 1). The ERIC-PCR showed a D-value of 0.96, indicating that the test was highly discriminatory.
Fig 1. ERIC-PCR genotyping of ESBL-E. coli cultured from broiler chick cloacal swabs.
Dendrogram showing the genotypic similarities among ESBL-positive E. coli by means of ERIC-PCR. Key = Identification of isolate; AG = antimicrobial-administered group—0.2mL (2 mg/L) of ceftiofur; CG = control group—0.2mL of saline solution.
Our study demonstrated that the off-label administration of a third-generation cephalosporin is associated (p<0.001) with the presence ESBL-producing E. coli in the gut of chicks. The Bayesian binomial logistic regression indicated that the probability of ESBL-E. coli occurrence was significantly higher in treated chicks at 7 and 14 days compared to the probabilities of ESBL-E. coli across other experimental days, although significant differences were observed between AG and CG groups at 5, 7, 9, 11 and 14 days of age (S1 Fig).
Although the clonal spread of ESBL-E. coli to humans through the food chain has not been demonstrated yet [26], the most common ESBL found in bacteria from chicken meat and humans, including blood culture specimens, have been shown to be identical [27]. Therefore, foods contaminated by antimicrobial resistant Enterobacteriaceae become reservoirs of ESBL genes [26–30] that can be ultimately acquired by pathogenic bacteria, reducing the efficacy of antimicrobials in veterinary and human medicine. Furthermore, ESBL-producing enterobacteria can disseminate among animals and occupationally exposed workers, as recently demonstrated in swine abattoirs [31].
To our knowledge, this is the first experimental report demonstrating that the common practice of administering ceftiofur to day-one chicks increases the short-term shedding of ESBL-producing E. coli. The real impact of that on the spread of antimicrobial resistant bacteria to the food chain, final consumers, and environment needs to be further investigated.
Supporting information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Analysis performed by means of Bayesian binomial logistic regression analysis using 8,000 repetitions in R environment and brms package.
(PDF)
Acknowledgments
This study was supported by the Coordination for the Improvement of Higher Education Personnel (CAPES; https://www.capes.gov.br/) that provided scholarships to graduate students (MMSS) and post-docs (ALBMF, NMVS). This study was also granted by The Brazilian National Council for Scientific and Technological Development (CNPq: http://cnpq.br/in-english-summary) (CJBO) and The Institute of International Education / Global Innovation Initiative / S-ECAGD-13-CA 149 DT (IIE: https://www.iie.org/) (WAG). The project has been conceptualized, written and submitted during CJBO and PENG time as visiting scholars at the Department of Animal Science of The University of Illinois at Urbana-Champaign under senior fellowship grants from CAPES (BEX 1910/14-0 and BEX 1853/14-7, respectively. Fertile eggs were provided by Guaraves Alimentos Ltda, Guarabira-PB. We are in debt with Prof. Walter Esfrain Pereira (CCA/UFPB) for the valuable support in the statistical analyses, Prof. Eloiza H. Campana (CCS/UFPB) for providing the primers and the positive controls for the PCR assays, and also for critically reviewing the manuscript, and Priscylla C. Vasconcelos (PDIZ/CCA/UFPB) for performing the MICs of ceftiofur of the E. coli isolates.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported by the Coordination for the Improvement of Higher Education Personnel (CAPES; https://www.capes.gov.br/) that provided scholarships to graduate students (MMSS) and post-docs (ALBMF, NMVS). This study was also granted by The Brazilian National Council for Scientific and Technological Development (CNPq: http://cnpq.br/in-english-summary) (CJBO) and The Institute of International Education / Global Innovation Initiative / S-ECAGD-13-CA 149 DT (IIE: https://www.iie.org/) (WAG). The project has been conceptualized, written and submitted during CJBO and PENG time as visiting scholars at the Department of Animal Science of The University of Illinois at Urbana-Champaign under senior fellowship grants from CAPES (BEX 1910/14-0 and BEX 1853/14-7, respectively).
References
- 1.Baron S, Jouy E, Larvor E, Eono F, Bougeard S, Kempf I. Impact of third-generation-cephalosporin administration in hatcheries on fecal Escherichia coli antimicrobial resistance in broilers and layers. Antimicrob Agents Chemother. 2014. September 1;58(9):5428–34. 10.1128/AAC.03106-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gibbons JF, Boland F, Egan J, Fanning S, Markey BK, Leonard FC. Antimicrobial resistance of faecal Escherichia coli isolates from pig farms with different durations of in-feed antimicrobial use. Zoonoses Public Health. 2016. May 1;63(3):241–50. 10.1111/zph.12225 [DOI] [PubMed] [Google Scholar]
- 3.Collignon PC, Conly JM, Andremont A, McEwen SA, Aidara-Kane A, Agerso Y, et al. World Health Organization ranking of antimicrobials according to their importance in human medicine: a critical step for developing risk management strategies to control antimicrobial resistance from food animal production. Clin Infect Dis. 2016. October 15;63(8):1087–93. 10.1093/cid/ciw475 [DOI] [PubMed] [Google Scholar]
- 4.Webster P. The perils of poultry. CMAJ. 2009. July 7;181(1–2):21–4. 10.1503/cmaj.091009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dutil L, Irwin R, Finley R, Ng LK, Avery B, Boerlin P, et al. Ceftiofur resistance in Salmonella enterica serovar heidelberg from chicken meat and humans, Canada. Emerg Infect Dis. 2010. January 16(1):48–54. 10.3201/eid1601.090729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horton RA, Randall LP, Snary EL, Cockrem H, Lotz S, Wearing H, et al. Fecal carriage and shedding density of CTX-M extended-spectrum β-lactamase-producing Escherichia coli in cattle, chickens, and pigs: implications for environmental contamination and food production. Appl Environ Microbiol. 2011. June 1;77(11):3715–9. 10.1128/AEM.02831-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.CLSI. Performance standards for antimicrobial disk and dilution susceptibility tests for bacteria isolated from animals- Third Edition CLSI document VET01S. Wayne, PA, USA: CLSI; 2015. [Google Scholar]
- 8.Warjri I, Dutta TK, Lalzampuia H, Chandra R. Detection and characterization of extended-spectrum β-lactamases (blaCTX-M-1 and blaSHV) producing Escherichia coli, Salmonella spp. and Klebsiella pneumoniae isolated from humans in Mizoram. Vet World. 2015. May 8(5):599–604. doi: 10.14202/vetworld.2015.599-604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu H, Wang Y, Wang G, Xing Q, Shao L, Dong X, et al. The prevalence of Escherichia coli strains with extended spectrum beta-lactamases isolated in China. Front Microbiol. 2015. April 21;6(1):1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dropa M, Balsalobre LC, Lincopan N, Matté GR, Matté MH. Complex class 1 integrons harboring CTX-M-2-encoding genes in clinical Enterobacteriaceae from a hospital in Brazil. J Infect Dev Ctries. 2015. August 29;9(8):890–897. 10.3855/jidc.6241 [DOI] [PubMed] [Google Scholar]
- 11.Pérez-Pérez FJ, Hanson ND. Detection of plasmid-mediated AmpC β-lactamase genes in clinical isolates by using multiplex PCR. J Clin Microbiol. 2002. June 1;40(6):2153–2162. 10.1128/JCM.40.6.2153-2162.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fritsch EF, Maniatis T, Sambrook J. Molecular cloning: a laboratory manual. 2nd ed New York: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 13.Ye Y, Wu Q, Zhang J, Lu J, Lin L. Isolation of Salmonella from meat samples and characterization by enterobacterial repetitive intergenic consensus–polymerase chain reaction and antibiotics test. Foodborne Pathog Dis. 2011. March 28;8(8):935–7. 10.1089/fpd.2010.0799 [DOI] [PubMed] [Google Scholar]
- 14.Hunter PR. Reproducibility and indices of discriminatory power of microbial typing methods. J Clin Microbiol. 1990. September 28(9):1903–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.R Core Team (2017). R: A language and environment for statistical computing. R Foundation for Statistic Computing; Vienna Austria: URL https://www.R-project.org/. [Google Scholar]
- 16.Beyer A, Baumann S, Scherz G, Stahl J, von Bergen M, Friese A, et al. Effects of ceftiofur treatment on the susceptibility of commensal porcine E. coli–comparison between treated and untreated animals housed in the same stable. BMC Vet Res. 2015. October 15(11):265 10.1186/s12917-015-0578-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hiki M, Shimizu Y, Kawanishi M, Ozawa M, Abo H, Kojima A, Koike R, Suzuki S, Asai T, Hamamoto S. Evaluation of the relationship between the minimum inhibitory concentration of ceftiofur and third-generation cephalosporins in Escherichia coli isolates from food-producing animals. J Vet Diagn Invest. 2017. June 29(5):716–720. 10.1177/1040638717713794 [DOI] [PubMed] [Google Scholar]
- 18.Kojima A, Ishii Y, Ishihara K, Esaki H, Asai T, Oda C, et al. Extended-spectrum-beta-lactamase-producing Escherichia coli strains isolated from farm animals from 1999 to 2002: report from the Japanese Veterinary Antimicrobial Resistance Monitoring Program. Antimicrob Agents Chemother. 2005. August 49(8):3533–3537. 10.1128/AAC.49.8.3533-3537.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Queenan AM, Foleno B, Gownley C, Wira E, Bush K. Effects of inoculum and beta-lactamase activity in AmpC- and extended-spectrum beta-lactamase (ESBL)-producing Escherichia coli and Klebsiella pneumoniae clinical isolates tested by using NCCLS ESBL methodology. J Clin Microbiol. 2004. January 42(1):269–275. 10.1128/JCM.42.1.269-275.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao S, White DG, McDermott PF, Friedman S, English L, Ayers S, et al. Identification and expression of cephamycinase blaCMY genes in Escherichia coli and Salmonella isolates from food animals and ground meat. Antimicrob Agents Chemother. 2001. 45:3647–3650. 10.1128/AAC.45.12.3647-3650.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heide LC, Hoet AE, Wittum TE, Khaitsa ML, Love BC, Huston CL, et al. Genetic and phenotypic characterization of the bla(CMY) gene from Escherichia coli and Salmonella enterica isolated from food-producing animals, humans, the environment, and retail meat. Foodborne Pathog Dis. 2009. December;6(10):1235–40. 10.1089/fpd.2009.0294 [DOI] [PubMed] [Google Scholar]
- 22.Martin LC, Weir EK, Poppe C, Reid-Smith RJ, Boerlin P. Characterization of blaCMY-2 plasmids in Salmonella and Escherichia coli isolates from food animals in Canada. Appl Environ Microbiol. 2012. February;78(4):1285–7. 10.1128/AEM.06498-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fang LX, Sun J, Li L, Deng H, Huang T, Yang QE, et al. Dissemination of the chromosomally encoded CMY-2 cephalosporinase gene in Escherichia coli isolated from animals. Int J Antimicrob Agents. 2015. August;46(2):209–13. 10.1016/j.ijantimicag.2015.04.003 [DOI] [PubMed] [Google Scholar]
- 24.Touzain F, Le Devendec L, de Boisséson C, Baron S, Jouy E, Perrin-Guyomard A, et al. Characterization of plasmids harboring blaCTX-M and blaCMY genes in E. coli from French broilers. PLoS One. 2018. January 23;13(1):e0188768 10.1371/journal.pone.0188768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jacoby G. A. 2009. AmpC beta-lactamases. Clin Microbiol Rev. January 22(1):161–182. 10.1128/CMR.00036-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Börjesson S, Ny S, Egervärn M, Bergström J, Rosengren Å, Englund S, et al. Limited dissemination of extended-spectrum β-lactamase–and plasmid-encoded AmpC–producing Escherichia coli from food and farm animals, Sweden. Emerg Infect Dis. 2016. April;22(4):634–40. 10.3201/eid2204.151142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Overdevest I, Willemsen I, Rijnsburger M, Eustace A, Xu L, Hawkey P, et al. Extended-Spectrum β-Lactamase genes of Escherichia coli in chicken meat and humans, the Netherlands. Emerg Infect Dis. 2011. July; 17(7): 1216–1222. 10.3201/eid1707.110209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Casella T, Rodríguez MM, Takahashi JT, Ghiglione B, Dropa M, Assunção E, et al. Detection of blaCTX-M-type genes in complex class 1 integrons carried by Enterobacteriaceae isolated from retail chicken meat in Brazil. Int J Food Microbiol. 2015. March 16;197:88–91. 10.1016/j.ijfoodmicro.2014.12.001 [DOI] [PubMed] [Google Scholar]
- 29.Kruse H, Sørum H. Transfer of multiple drug resistance plasmids between bacteria of diverse origins in natural microenvironments. Appl Environ Microbiol. 1994. November 1;60(11):4015–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Randall LP, Lodge MP, Elviss NC, Lemma FL, Hopkins KL, Teale CJ, et al. Evaluation of meat, fruit and vegetables from retail stores in five United Kingdom regions as sources of extended-spectrum beta-lactamase (ESBL)-producing and carbapenem-resistant Escherichia coli. Int J Food Microbiol. 2017. January 16;241(Supplement C):283–90. [DOI] [PubMed] [Google Scholar]
- 31.Founou LL, Founou RC, Allam M, Ismail A, Djoko CF, Essack SY. Genome sequencing of Extended-Spectrum β-Lactamase (ESBL)-producing Klebsiella pneumoniae isolated from pigs and abattoir workers in Cameroon. Front Microbiol. 2018. February; 9: 188 10.3389/fmicb.2018.00188 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Analysis performed by means of Bayesian binomial logistic regression analysis using 8,000 repetitions in R environment and brms package.
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.