Supplemental Digital Content is available in the text
Keywords: continuous glucose monitoring system, critically ill, glucose control, glucose variability, time in range
Abstract
Background:
The purpose of this study was to determine whether subcutaneous continuous glucose monitoring systems (CGMS) could improve glucose management in critically ill patients compared with frequent and conventional point-of-care (POC) glucose measurements.
Methods:
A total of 144 patients with an expected length of stay in the ICU of at least 72 hours and with an admission glucose or two random glucose values of >10.0 mmol/L within 24 hours after admission, were randomly assigned to the CGMS group (n = 74) or the conventional group (C group, n = 70). Both groups used the same insulin algorithm to reach the same glucose target range (8.0–10.0 mmol/L).
Results:
Time in range (TIR, 8.0–10.0 mmol/L), which is our primary outcome measure, was higher in the CGMS group than in the C group (51.5% vs. 29.0%, P < .001). Glucose variability (coefficient of variation, CV; standard deviation, SD; glucose lability index, and GLI) was improved by CGMS (all P < .05). Mean glucose level (MGL) (9.6 vs. 10.3 mmol/L, P = .156) and the proportion of patients with hypoglycemia did not differ between CGMS (5.4%) and C (5.7%) (P = 1.000). However, duration of hypoglycemia was reduced in the CGMS group (15 vs. 28 minutes, P = .032). Clinical outcomes were similar between groups except for the fewer usage of CRRT and lower peak plasma urea nitrogen level in the CGMS group.
Conclusion:
The use of CGMS, compared with POC glucose measurement, could improve the TIR, GV and duration of hypoglycemia.
1. Introduction
The principal domains of glucose control in critically ill patients include correcting hyperglycemia and avoiding hypoglycemia and glucose fluctuation, because they have been associated with increased mortality and poor prognosis.[1–3] However, we still do not know to what extent these domains should be controlled. New guidelines have recommended that blood glucose (BG) level should not exceed 180 mg dL−1 (10.0 mmol L−1); however, these guidelines also point out that a stricter range (110–140 mg dL−1) may be appropriate for selected patients if achievable.[4] Moreover, new glucose control parameters are also emerging, such as glucose variability (GV),[5] time in range (TIR),[6] etc, enriching traditional glucose control parameters.
These years, researchers in the area of glucose control find that the way to achieve their desired glucose target is as important as finding the proper glucose target, when they try to find the reasons for conflicting results in glucose control areas in the intensive care unit (ICU).[7] Frequent and timely glucose measurement and a safe and efficient insulin protocol are core to glucose control in critically ill patients. Moreover, glucose measurement is a prerequisite for insulin adjustment.[8,9] However, in most ICU settings, insulin is guided by conventional intermittent BG measurement, which is inevitably labor-intensive and time-consuming. Moreover, conventional intermittent BG measurement may ignore the hypoglycemia episodes between 2 measurements.[10] Most nurses endorse tight glycemic control, but they also think that the conventional glucose measurement is burdensome and costly, thus, they need easier clinical methods for glucose monitoring.[11]
Continuous glucose monitoring system (CGMS), which automatically provides glucose values every few minutes, might theoretically increase the effectiveness of glucose control and minimize hypoglycemia risk.[12] To date, 5 randomized controlled trials (RCTs) have provided insights into the effects of CGMS on glucose control in ICU settings. In 2 of these studies, glucose was controlled by CGMS combined with a software-guided insulin algorithm; thus, the attributive effects were ambiguous.[13,14] In the other 3 studies, hypoglycemic events, nursing workload, and daily costs were reduced by CGMS, rather than the mean glucose level (MGL), TIR, or GV. However, clinical outcomes have not been studied in most of these studies. Therefore, we carried out the present investigation to assess whether CGMS could improve glucose control more aggressively in a mixed population of critically ill patients, compared with frequent point-of-care (POC) BG measurements. Clinical outcomes were also evaluated in this study.
2. Methods
2.1. Trial design
This was a singer-center, randomized control, and open-label clinical trial performed in a 52-bed general ICU in West China Hospital. The protocol was approved by the ethics committees of West China hospitals and registered in ClinicalTrials.gov (NCT01992965).
2.2. Patients
Adult patients (>18 and <65 years old) were recruited from July 2015 to December 2016. Patients with an expected length of stay in the ICU of at least 72 hours and with admission glucose or 2 random glucose values of >10.0 mmol L−1 were screened for eligibility. We excluded patients above 65 years old because glucose control will be complicated by their clinical and functional heterogeneity. Other major exclusion criteria were lack of informed consent; pregnant; glycosylated hemoglobin (HbA1c) of >8% (HbA1c test was done only in patients who met the inclusion criterion); contraindications for use of CGMS due to damaged skin; admission glucose and 2 random BG levels of <10.0 mmol L−1 within 24 hours after admission; moribund on admission; and participation in another trial or previous participation in this trial.
2.3. Randomization and masking
Patients were randomized as a ratio of 1:1 to either using CGMS (CGMS group) or conventional POC BG measurements (C group). The random numbers were generated by Excel and then sealed in opaque envelopes, which were kept by an independent investigator. Outcome assessors were blinded to treatment allocation.
2.4. The CGMS system
Nurses were trained professionally about how to connect, setup, and calibrate the CGMS before the formal trial started. The CGMS system[15] (DGMS, San MediTech Medical Technology Co. Ltd, Huzhou, Zhejiang, China) was composed of 2 parts: the device monitor and glucose sensor, which could be used for up to 7 days. The sensor was inserted into the right chest wall by the nurses and then connected to the device monitor. CGMS needed a 3-hour initiation period before starting. Each sensor had 2 glucose oxidase-based probes, which sensed subcutaneous glucose concentration and transmitted chemical signals through the processor cable to the monitor every 10 seconds. The CGMS averaged these glucose values at every 3-minute interval, displayed the mean values on the monitors, and ended with 480 CGMS glucose values daily. The valid range of glucose was 1.7 to 25.0 mmol L−1. At the end of the study, the device monitors were connected to a computer and the data were downloaded for analysis.
In critically ill patients, some studies have shown good accuracy and reliability of CGMS.[16,17] The CGMS used in our trial was the same as that in the study of Yue et al,[15] in which the CGMS demonstrated a high accuracy and reliability. Thus, we did not dwell on the accuracy and reliability of CGMS.
2.5. The insulin protocol
Our insulin protocol, which was mainly adopted from the Yale protocol[18] and based on the BG change rate, was shown in Table S1 (Supplemental Content, which illustrates how to initiate and adjust insulin dosage). In brief, this insulin protocol contained 2 steps. First, the nurse determined the current BG level. Second, the nurse calculated the hourly rate of BG change by subtracting the current BG level from the previous BG value; this rate would guide him/her to find a specific instruction of insulin in the insulin protocol. If any of the following situations occurred in both groups (any change or cessation of nutrition; significant change in clinical condition, cessation of glucocorticoid therapy, transportation of patients, abnormal current which refers to current values < 60 nA on the CGMS monitor), nurses adjusted insulin on the basis of his/her expertise and recorded this change as “violation from insulin suggestion.”
2.6. The glucose range and nutrition strategy
According to the actual glucose target range of patients assigned to conventional glucose control in the NICE-SUGAR study and the recommendation from American Diabetes Association in 2012,[19,20] we chose 8.0 to 10.0 mmol L−1 (144–180 mg dL−1) as the target range. Insulin (Novo Nordisk, Bagsvaerd, Denmark), in concentrations of 50 units in 50 mL of normal saline, was continuously infused using the syringe infusion system. Nutrition in both groups was started as early as possible and followed the same guidelines.[21] For patients with severe acute pancreatitis (SAP), a nasojejunal tube was placed upon starting enteral nutrition. We defined acute pancreatitis and SAP according to the Atlanta criteria[22] (Supplemental Content, which lists the Atlanta criteria of acute pancreatitis and SAP)
2.7. The intervention
In CGMS group, when the CGMS readings stayed in the target range, CGMS readings were taken every 2 hours to adjust the insulin. In addition, an alarm was set in order to give an alert when the CGMS readings were out of the target range. The frequency of CGMS readings and insulin adjustments would be changed to every 15 minutes when the alarm sounded until the CGMS readings returned to the target range.
In the C group, CGMS probes were also placed on the right chest wall and continuous CGMS readings were recorded; however, nurses were prohibited to use CGMS readings for insulin adjustment. Simultaneously, no alarm was set to remind that the glucose readings were out of the target range. BG was measured by conventional POC glucose measurements (ACCU-CHEK Active; Roche Diagnostics GmbH, Mannheim, Germany) every 2 hours, which was the minimum monitoring interval in our clinical routine in adjusting the insulin, to adjust insulin according to the same insulin protocol.
A 5-day study period started after CGMS initiation. Within 5 days, glucose control would be terminated upon patient death or discharge from the ICU, or technical failure of the CGMS device (current < 60 nA or sensor was removed by accident). If 5 days passed and none of the 3 conditions (patient's death, discharge from the ICU, or technical failure of the CGMS device) occurred, the CGMS sensor would be removed from the patients by our researchers, and the trial would end. The patients in both groups would be given usual clinical care, and the glucose level would be measured with POC. The frequency of glucose measure would be decided by the patients’ responsible doctors.
2.8. Primary and secondary outcomes
All glucose metric analyses in both groups were calculated using CGMS data. The primary outcome was quality of glucose control, as evaluated by the percentage of TIR, which was defined as the duration in which BG stayed within the target range.[6]
Secondary outcomes included time above range (TAR, >10.0 mmol L−1), time below range (TBR, <8.0 mmol L−1), MGL (mmol L−1), incidence of hypoglycemia (<4.0 mmol L−1), and severe hypoglycemia (<2.2 mmol L−1) (calculated as number of patients suffering from hypoglycemia or severe hypoglycemia divided by total number of patients in each group), the duration of hypoglycemia or severe hypoglycemia if it happened according to CGMS records; GV was also assessed by 3 indices: standard deviation (SD, mmol L−1), coefficient of variation (CV), and glucose lability index (GLI, mmol L−1)2 h−1 d−1). GLI was calculated as the square of the difference between consecutive glucose measurements per unit of actual time among the whole glucose values (GLI = Σ[{[Delta]glucose (mmol L−1)}2 h−1] d−1).[23]
Adherence to the study was also recorded. In the C group, the fixed time point (every 2 hours) was autogenerated in our patient management system in advance. Once the time interval elapsed, the nurses checked the fingertip BG; recorded the present glucose value, present insulin rate, and the changed insulin rate. In CGMS group, the recording time point was unfixed because of the additional time point (every 15 minutes of recording once the CGMS reading was outside the target range). Nurses in this group were told to record in the patient management system the specific time point at which time they changed the insulin rate, as well as the present glucose value, present insulin rate, and changed insulin rate. The investigator would mark “violation” if the recorded changed insulin rate was different from the insulin rate calculated by the investigator. A specific investigator checked the records daily and asked the reasons for the violations from the assigned nurses.
Clinical data were extracted from the patient management system. History of diabetes mellitus (DM) and renal diseases were diagnosed as they were mentioned from patients’ medical history in the electronic medical record. Duration of mechanical ventilation, lengths of stay in the ICU and in the hospital, and 28-day mortality rate were our prespecified secondary outcomes. In the post hoc analysis, we added mortality in the ICU and in hospital, daily sequential organ failure assessment (SOFA) in days 1, 7, 14, 21, and 28 after trial start, need for mechanical ventilation, vasopressor therapy, continuous renal replacement therapy (CRRT), glucocorticoid and blood transfusion, antibiotics days, and peak plasma creatinine and peak plasma urea nitrogen as secondary outcomes. New organ injuries in the ICU were defined as when patients experienced an episode wherein the change of sole organ SOFA exceeds 3. New infections in the ICU were diagnosed according to the criteria for critically ill patients from consensus conferences.[24,25]
2.9. Statistical analysis
According to the literature, a total sample size of 120 patients conferred 80% power, with a 2-sided P = .05, to detect 80% TIR in the CGMS group and 57% TIR in the C group. Another 12 patients in each group were needed to correct an expected 10% drop out.[26]
All the data were analyzed according to the intention-to-treat principle. Results were expressed as percentages for categorical variables, mean, and SD for continuous normally distributed variables and median and interquartile range for the variables in skewness distribution. Groups were compared by using Fisher exact test, Student t test, or Mann–Whitney rank-sum test as appropriate. A 2-tailed P < .05 was considered significant. All statistical analyses were performed in SPSS 20.0 (IBM Corp, Armonk, NY).
3. Results
A total of 144 patients were randomized to either CGMS group (n = 74) or C group (n = 70). No patient deteriorated or died during the CGMS initiation period. A total of 128 patients completed the 5-day study period; 10 patients withdrew (9 patients were transferred to local hospitals and 1 patient died rapidly after the study started); and 6 patients stopped the study due to sensor failure (2 sensors with poor current and 4 sensors were removed accidentally) (Fig. 1).
Figure 1.
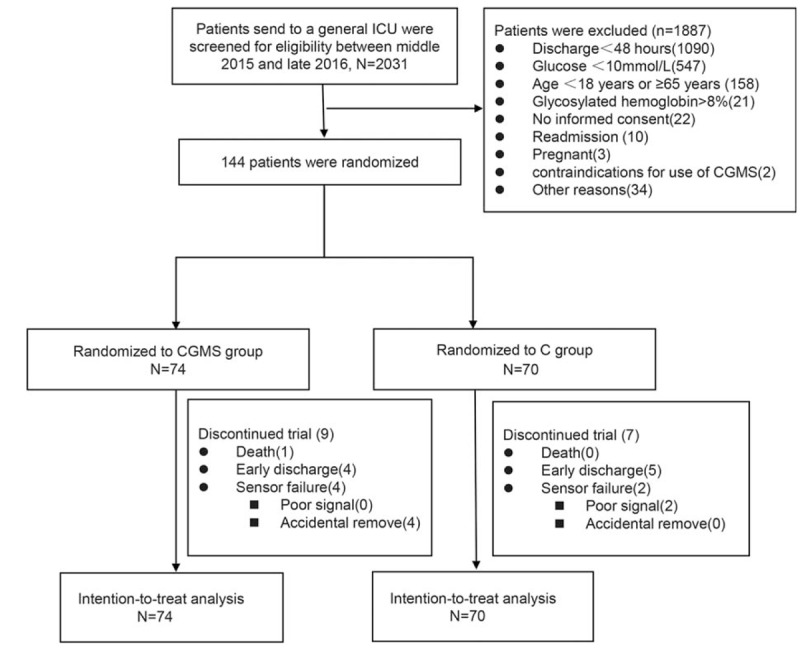
Consolidated Standards of Reporting Trials diagram of enrollment. Among these 16 patients who did not complete the 5-day study period, 13 patients had study periods <72 hours. Early discharge refers to discharged from the intensive care unit to general wards or home or another hospital; poor signal refers to sensor current <60 nA. C = conventional point-of-care, CGMS = continuous glucose monitoring system.
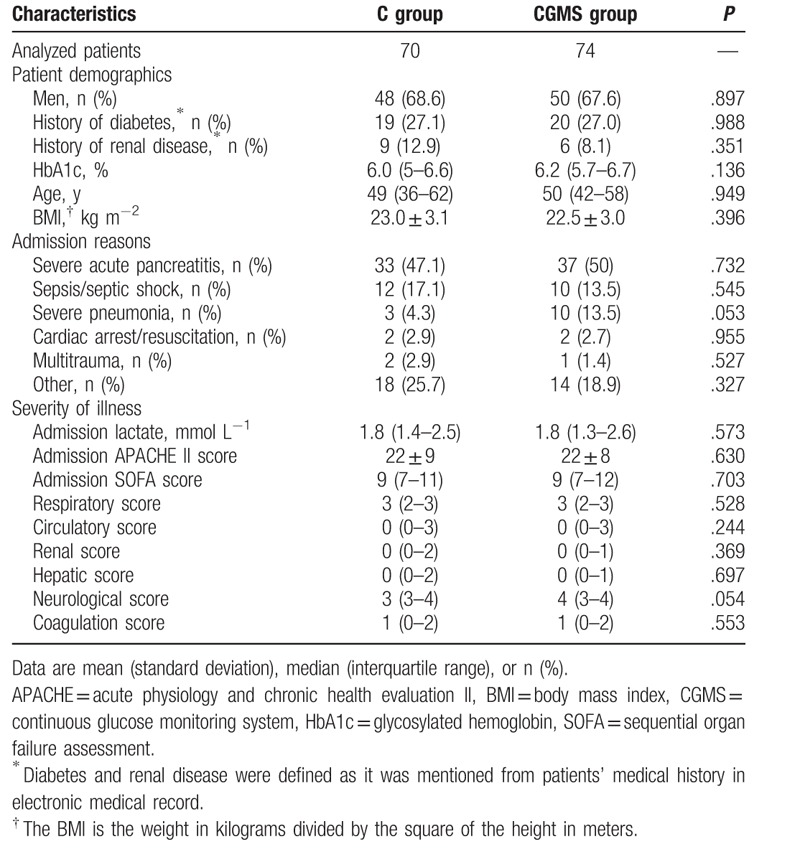
The top 3 admission reasons were SAP (70/48.6%), sepsis/septic shock (22/15.3%), and severe pneumonia (13/9.0%). The median age was 49 (37–60) years; the percentage of male patients was 68.1%; the body mass index was 22.8 ± 3.0 kg m−2; the median admission APACHE II and SOFA scores were 22 (16–27) and 10 (7–13); and the percentage of patients with diabetic and renal diseases were 27.1% and 10.4%, respectively. There was no difference in baseline characteristics between the 2 groups (Table 1).
Table 1.
The demographics, admission reason, and severity of illness in the real-time continuous glucose monitoring system group versus conventional group.
A total of 50% of the patients in our study were diagnosed with SAP, and we showed the demographic characteristics in patients with or without SAP in both groups (see Tables S2 and S3, Supplemental Content, which illustrate the demographic characteristics of patients with or without SAP in both groups).
The median study period in each group was both 5 days. During the study, a total of 165,686 (CGMS group) and 156,215 (C group) CGMS measurements were generated. The glucose values used for insulin adjustment (CGMS readings in the CGMS group and POC measurements in the C group) were 7681 and 3979, respectively. The adherence rates between the CGMS and C groups were not highly different (85.4% vs. 85.0%, P = .841). Among the 2041 violations from insulin recommendations, the main reason was fear of hypoglycemia.
Time in each BG range for the 5-day study period is shown in Fig. 2A. The glucose control in both groups is summarized in Table 2. The percentage of TIR (8.0–10.0 mmol L−1) was statistically greater in the CGMS group than that in the C group (51.5% vs. 21.0%, P < .001). The TAR (>10.0 mmol L−1) (27.5% vs. 50.0%, P = .009) and TBR (<8.0 mmol L−1) (10.0% vs. 15.5%, P = .015) were decreased in the CGMS group. Overall glucose profile during the 5-day study period is presented in Fig. 2B. Two individual glucose plots in each group are shown to provide a direct visual image (see Fig. S1A and B, Supplemental Content, which illustrates a schematic representation of the performance of continuous BG concentration vs. time in both groups).
Figure 2.
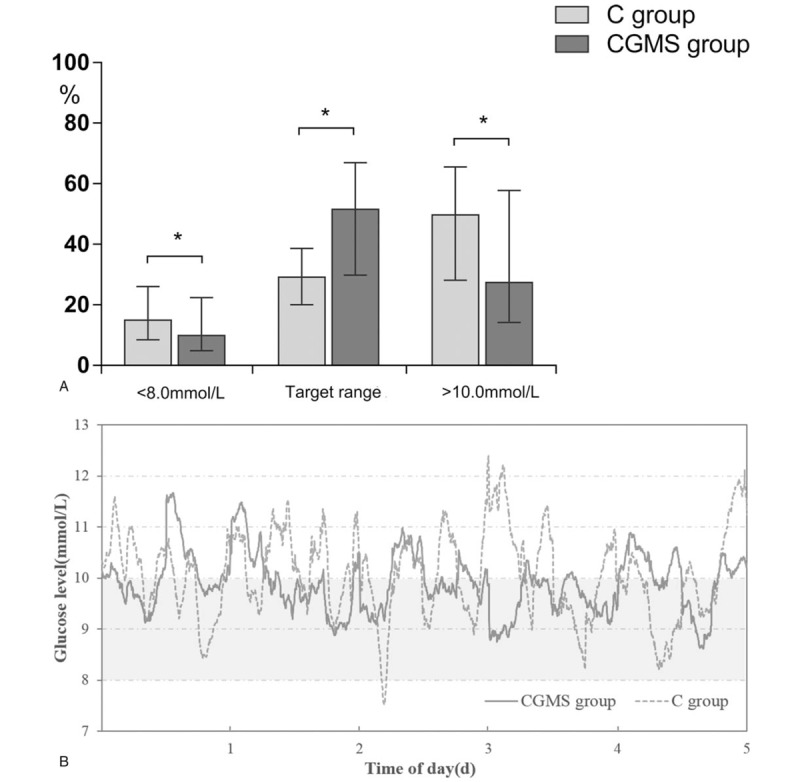
(A) Time in each blood glucose range for the 5-day study period. Target range: 8.0 to 10.0 mmol L−1. ∗P < .05. Data are presented as median (interquartile range). (B) The overall glucose traces for the 5-day study period. Gray area indicates target range (8.0–10.0 mmol L−1). To convert the values for glucose to millimoles per liter, multiplied by 18. C = conventional point-of-care, CGMS = continuous glucose monitoring system.
Table 2.
Blood glucose control in the real-time continuous glucose monitoring system group versus conventional group.
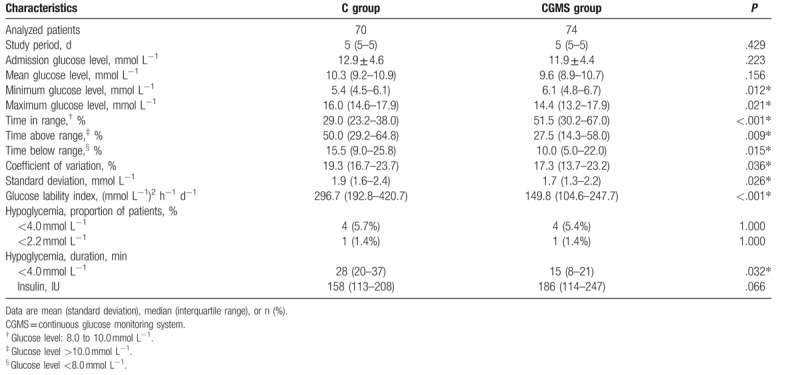
Significantly improved blood GV (CV, SD, and GLI) was observed in the CGMS group (all P < .05). Moreover, daily GLI also was improved in the CGMS group (see Fig. S2A, Supplemental Content, which illustrates the daily GLI in both groups).
A total of 4 patients in each group suffered from hypoglycemia (<4.0 mmol L−1), resulting in 5.7% (C group) and 5.4% (CGMS group) incidence in each group. Severe hypoglycemia (<2.2 mmol L−1) was observed in 1 patient in each group. All patients with hypoglycemic events experienced only 1 episode. The CMGS did not reduce the incidence of hypoglycemia; but the CGMS could reduce the duration. Interestingly, some of the hypoglycemia events still occurred when the insulin rate was 0 IU h−1. Adverse event data are presented in Table S4 (Supplemental Content, which lists the adverse events during the trial).
Total insulin values during the study period were comparable in both groups. Over 5 study days, the daily caloric intake and daily insulin delivery were similar, except for the increased insulin dosage in the CGMS group on the 4th day (see Fig. S2B and C, Supplemental Content, which illustrates the daily caloric intake and daily insulin delivery in both groups).
The TIR, TAR, TBR, and GV were improved by the CGMS in the subgroups of patients with SAP. Moreover, the effect of CGMS was less pronounced in the subgroup of patients without SAP (see Tables S5 and S6, Supplemental Content, which illustrate the glucose control in patients with or without SAP in both groups).
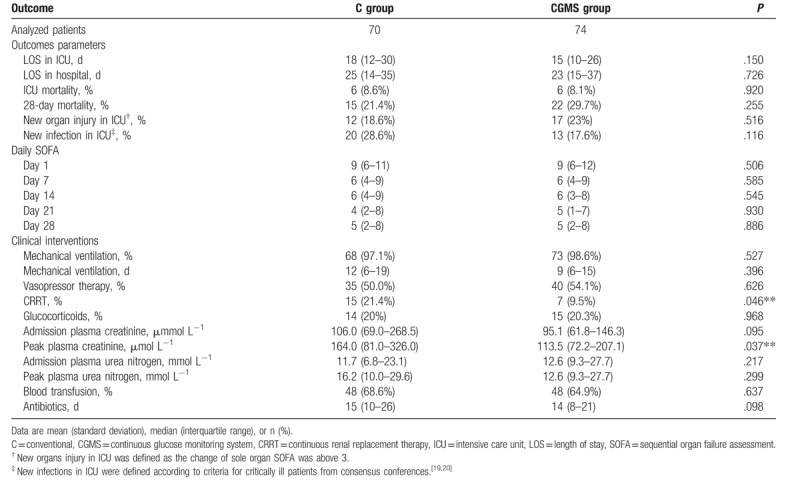
The effects of CGMS were not produced on hospital or ICU stay and ICU or 28-day mortality. Moreover, SOFA scores on days 1, 7, 14, 21, and 28 were similar in each group. Significantly lower peak plasma creatinine levels and fewer patients required CRRT in the CGMS group, whereas the proportions of patients needing other treatments were the same (Table 3). We examined the relationship between TIR and CRRT usage and peak plasma creatinine using Spearman correlation analysis but did not find significant results (the results are not shown). Similarly, the clinical outcome did not differ in patients with or without SAP (see Tables S7 and S8, Supplemental Content, which illustrate the clinical outcomes in patients with or without SAP in both groups).
Table 3.
Outcomes measures in the real-time continuous glucose monitoring system group versus conventional group.
4. Discussion
The operating principle of CGMS enabled us to obtain BG at any time. In addition, setting alarms in CGMS was beneficial to detect the deviation of glucose early and to adjust the insulin at the time point when glucose readings went out of range, thus “pulling” the glucose into the target range in a timely manner. In our CGMS group, the nurses adjusted the insulin not only by checking the CGMS readings every 2 hours, but also every 15 minutes when the alarm sounded. Using CGMS in this mode allowed BG to be adjusted frequently when it was needed to be adjusted most, thus shorting the measurement interval. The shortened measurement interval could be reflected by the total number of CGMS reading used for insulin adjustment and was more than twice as many as POC measurements (7681 and 3979, respectively).
In this present study, CGMS could improve the quality of glucose control, reflected by TIR. The higher the TIR, the longer the BG stayed in the target range, thus making the TAR and TBR in CGMS group less than those in the C group. However, our TIR are lower than those in another 2 studies.[26,27] Nearly 50% of our study patients were diagnosed with SAP, which could have influenced the production of endogenous insulin and caused attributable endocrine and metabolic disturbances. In addition, the cohort of patients appeared to be quite ill and the admission glucose level (12.4 mmol L−1) was quite higher than those in other studies. Namely, the glucose control in our study population was difficult in itself; therefore, controlling the glucose levels and getting them into the target range were more challenging than other studies.
We also found that using CGMS can improve GV, and this is an encouraging result that was never found in previous RCTs. GV is more difficult to control than hyperglycemia or hypoglycemia because it is calculated using glucose values by post hoc analysis, rather than a direct glucose value. GV is usually affected by the frequency of glucose monitoring, and CGMS has made precisely evaluating GV possible.[28] The use mode of CGMS in our investigation was beneficial to stabilize BG and avoid abrupt change of BG. Moreover, the narrow TIR (8.0–10.0 mmol L−1), which allowed a glucose adjust room of only 2.0 mmol L−1, might have contributed to the promoted GV.
Unfortunately, the MGL was not reduced by the CGMS. A relatively high percentage of TBR in the C group may offset the increased percentage of TAR, thus making the MGL similar in both groups. Hypoglycemia is another critical component in glucose control. The CMGS did not reduce the incidence of hypoglycemia; however, it could reduce the duration of hypoglycemia. Detection of hypoglycemia can be difficult with intermittent glucose monitoring even with frequent performances whereas the presence of a lower limit alarm in the CGMS group is expected to have some beneficial contribution. Interestingly, 7 patients were still suffering from hypoglycemia when insulin was 0 IU h−1. Hypoglycemia can be either iatrogenic or spontaneous and can result from underlying medical illnesses, even in the absence of insulin.[29]
Mortality, morbidity, and need for clinical interventions did not differ between CGMS group and C group in the total patients or in patients with or without SAP. Decreased usage of CRRT and peak plasma creatinine was found in the CGMS group, but no association existed between these 2 indicators and TIR. Subsequent analysis of a Glucontrol study showed that a TIR > 50% in the glucose target of 140 to 180 mg dL−1 is independently associated with an increased survival rate.[30] The retrospective analysis of SPRINT study revealed tight glucose control with good quality, as mirrored by the ≥50% cumulative time in the 4.0 to 7.0 mmol L−1 band, and this could resolve organ failure faster.[31] However, the TIR in these 2 studies were calculated by intermittent POC measurements; thus, the true association between TIR and survival or organ failure needs further investigation. Clinical outcomes were not our primary outcomes; therefore, our present sample size was under-powered to detect the differences in those indicators. Larger studies are needed to explore the effects of TIR on clinical endpoints.
The relatively large sample size and the RCT design are the strengths of our study. Relatively severe medical conditions (admission APACHE II of 22) and a high proportion of patients diagnosed with SAP made glucose harder to control than that in previous RCTs, but we achieved a high level of control with CGMS.[26,27,32] However, some important limitations of the study merit further consideration. First, the proportion of DM history was about 27%, SAP was around 50%, and the calculated median HbA1c was 6.1, we also excluded HbA1c >8.0, so CGMS may apply smoothly only in the population of relatively well control DM or acute episode of hyperglycemia. Second, approximately 15% of the insulin suggestions were not followed. The pressure of work in our busy ICU might have weakened the adherence to insulin recommendations. Third, in spite of the significant difference in the primary endpoint, this outcome did not reach our preset criterion; therefore, the interpreting our results should be met with caution. Fourth, conducting the study in a strictly blinded fashion was not feasibly because the frequency of insulin adjustment was obviously different in each group. To minimize bias, we assigned the insulin-adjustment duties to the bed-responsible nurses, with strictly blinded analysis of data. Fifth, the insulin protocol was designed for intermittent POC measurements rather than CGMS. In view of a potential value in continuous glucose monitoring in critically ill patients in the future, a more standardized insulin algorithm pertaining to CGMS is urgently needed.
5. Conclusion
The salient finding of our study is that the use of CGMS, compared with POC glucose measurement, could improve the TIR, GV, and the duration of hypoglycemia. Moreover, the improvement in glucose control provided by the CGMS was observed in patients with or without SAP.
Acknowledgments
The authors acknowledge all the nurses in the Department of Intensive Care Unit at West China Hospital of Sichuan University for their participation and assistance in the study. The authors thank Liang Zhao for data analysis consulting.
Author contributions
Conceptualization: Meizhu Lu, Yanyan Zuo, Yan Kang.
Data curation: Meizhu Lu.
Formal analysis: Meizhu Lu, Yanyan Zuo, Jun Guo.
Funding acquisition: Yan Kang.
Investigation: Meizhu Lu, Xiaoping Wen.
Methodology: Meizhu Lu.
Project administration: Yan Kang.
Supervision: Yan Kang.
Writing – original draft: Meizhu Lu.
Writing – review & editing: Yan Kang.
Supplementary Material
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: BG = blood glucose, CGMS = continuous glucose monitoring system, CRRT = continuous renal replacement therapy, CV = coefficient of variation, DM = diabetes mellitus, GLI = glucose lability index, GV = glucose variability, ICU = intensive care unit, LOS = length of stay, MGL = mean glucose level, SOFA = sequential organ failure assessment, RCT = randomized controlled trial, SAP = severe acute pancreatitis, SD = standard deviation, TAR = time above range, TBR = time below range, TIR = time in range.
Yan Kang has received grant from the San Meditech Medical Technology Co. Ltd in Huzhou, Zhejiang, China. The grant number is 312140352. The funder's website: http://en.sanmeditech.com/. San Meditech Medical Technology Co. Ltd had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The grant was strictly confined to technical support with the device and providing both the device for the duration of the study and the disposable sensors for the study patients, as well as personnel and laboratory costs associated with performing this trial. Meizhu Lu communicated the trial progress to the company and receiving technical support from the company.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Hermanides J, Vriesendorp TM, Bosman RJ, et al. Glucose variability is associated with intensive care unit mortality. Crit Care Med 2010;38:838–42. [DOI] [PubMed] [Google Scholar]
- [2].Hermanides J, Bosman RJ, Vriesendorp TM, et al. Hypoglycemia is associated with intensive care unit mortality. Crit Care Med 2010;38:1430–4. [DOI] [PubMed] [Google Scholar]
- [3].Badawi O, Waite MD, Fuhrman SA, et al. Association between intensive care unit-acquired dysglycemia and in-hospital mortality. Crit Care Med 2012;40:3180–8. [DOI] [PubMed] [Google Scholar]
- [4].Jacobi J, Bircher N, Krinsley J, et al. Guidelines for the use of an insulin infusion for the management of hyperglycemia in critically ill patients. Crit Care Med 2012;40:3251–76. [DOI] [PubMed] [Google Scholar]
- [5].Eslami S, Taherzadeh Z, Schultz MJ, et al. Glucose variability measures and their effect on mortality: a systematic review. Intensive Care Med 2011;37:583–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Omar AS. Time in range: a fourth domain in glycemic control or a glucose variability alternative? Mayo Clin Proc 2016;91:1147. [DOI] [PubMed] [Google Scholar]
- [7].Ng LS, Curley MA. “One more thing to think about…” Cognitive burden experienced by intensive care unit nurses when implementing a tight glucose control protocol. J Diabetes Sci Technol 2012;6:58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wilson M, Weinreb J, Hoo GW. Intensive insulin therapy in critical care: a review of 12 protocols. Diabetes Care 2007;30:1005–11. [DOI] [PubMed] [Google Scholar]
- [9].Shetty S, Inzucchi SE, Goldberg PA, et al. Adapting to the new consensus guidelines for managing hyperglycemia during critical illness: the updated Yale insulin infusion protocol. Endocr Pract 2012;18:363–70. [DOI] [PubMed] [Google Scholar]
- [10].Krinsley JS, Bruns DE, Boyd JC. The impact of measurement frequency on the domains of glycemic control in the critically ill—a Monte Carlo simulation. J Diabetes Sci Technol 2015;9:237–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Aragon D. Evaluation of nursing work effort and perceptions about blood glucose testing in tight glycemic control. Am J Crit Care 2006;15:370–7. [PubMed] [Google Scholar]
- [12].De Block C, Manuel-y-Keenoy B, Rogiers P, et al. Glucose control and use of continuous glucose monitoring in the intensive care unit: a critical review. Curr Diabetes Rev 2008;4:234–44. [DOI] [PubMed] [Google Scholar]
- [13].Leelarathna L, English SW, Thabit H, et al. Feasibility of fully automated closed-loop glucose control using continuous subcutaneous glucose measurements in critical illness: a randomized controlled trial. Crit Care 2013;17:R159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kopecký P, Mráz M, Bláha J, et al. The use of continuous glucose monitoring combined with computer-based eMPC algorithm for tight glucose control in cardiosurgical ICU. Biomed Res Int 2013;2013:186439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Yue XY, Zheng Y, Cai YH, et al. Real-time continuous glucose monitoring shows high accuracy within 6 hours after sensor calibration: a prospective study. PLoS ONE 2013;8:e60070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Holzinger U, Warszawska J, Kitzberger R, et al. Impact of shock requiring norepinephrine on the accuracy and reliability of subcutaneous continuous glucose monitoring. Intensive Care Med 2009;35:1383–9. [DOI] [PubMed] [Google Scholar]
- [17].Brunner R, Kitzberger R, Miehsler W, et al. Accuracy and reliability of a subcutaneous continuous glucose-monitoring system in critically ill patients. Crit Care Med 2011;39:659–64. [DOI] [PubMed] [Google Scholar]
- [18].Goldberg PA, Siegel MD, Sherwin RS, et al. Implementation of a safe and effective insulin infusion protocol in a medical intensive care unit. Diabetes Care 2004;27:461–7. [DOI] [PubMed] [Google Scholar]
- [19].Finfer S, Chittock DR, et al. NICE-SUGAR Study Investigators. Intensive versus conventional glucose control in critically ill patients. N Engl J Med 2009;360:1283–97. [DOI] [PubMed] [Google Scholar]
- [20].American Diabetes Association. Standards of medical care in diabetes—2012. Diabetes Care 2012;35(suppl 1):S11–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].McClave SA, Martindale RG, Vanek VW, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J Parenter Enteral Nutr 2009;33:277–316. [DOI] [PubMed] [Google Scholar]
- [22].Banks PA, Bollen TL, Dervenis C, et al. Classification of acute pancreatitis—2012: revision of the Atlanta classification and definitions by international consensus. Gut 2013;62:102–11. [DOI] [PubMed] [Google Scholar]
- [23].Ali NA, O’Brien JM, Jr, Dungan K, et al. Glucose variability and mortality in patients with sepsis. Crit Care Med 2008;36:2316–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Calandra T, Cohen J. International Sepsis Forum Definition of Infection in the ICU Consensus Conference. The international sepsis forum consensus conference on definitions of infection in the intensive care unit. Crit Care Med 2005;33:1538–48. [DOI] [PubMed] [Google Scholar]
- [25].Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control 2008;36:309–32. [DOI] [PubMed] [Google Scholar]
- [26].Holzinger U, Warszawska J, Kitzberger R, et al. Real-time continuous glucose monitoring in critically ill patients: a prospective randomized trial. Diabetes Care 2010;33:467–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Boom DT, Sechterberger MK, Rijkenberg S, et al. Insulin treatment guided by subcutaneous continuous glucose monitoring compared to frequent point-of-care measurement in critically ill patients: a randomized controlled trial. Crit Care 2014;18:453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Rodbard D. New and improved methods to characterize glycemic variability using continuous glucose monitoring. Diabetes Technol Ther 2009;11:551–65. [DOI] [PubMed] [Google Scholar]
- [29].Hulkower RD, Pollack RM, Zonszein J. Understanding hypoglycemia in hospitalized patients. Diabetes Manag (Lond) 2014;4:165–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Penning S, Chase JG, Preiser JC, et al. Does the achievement of an intermediate glycemic target reduce organ failure and mortality? A post hoc analysis of the Glucontrol trial. J Crit Care 2014;29:374–422. [DOI] [PubMed] [Google Scholar]
- [31].Chase JG, Pretty CG, Pfeifer L, et al. Organ failure and tight glycemic control in the SPRINT study. Crit Care 2010;14:R154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].De Block CE, Gios J, Verheyen N, et al. Randomized evaluation of glycemic control in the medical intensive care unit using real-time continuous glucose monitoring (REGIMEN trial). Diabetes Technol Ther 2015;17:889–98. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


