Abstract
Background:
Several studies have shown that B7-H4 expression is significantly increased in ovarian cancer. However, the role of B7-H4 expression in ovarian cancer remains unclear, and some studies reporting conflicting results. A systematic review of the literature and meta-analysis were conducted to assess the clinicopathologic characteristics and prognostic significance of B7-H4 in ovarian cancer.
Methods:
Eligible studies were searched in the PubMed, MEDLINE, Cochrane Library, and the China National Knowledge Infrastructure databases. The included studies assessed the relationship between B7-H4 expression and clinicopathologic features or prognosis in patients with ovarian cancer through September 2017. A total of 1045 patients in 10 studies were included in the meta-analysis. Stata software version 12.0 was used to analyze the data. We used an odds ratio (OR) or hazard ratio (HR) with a 95% confidence interval (CI) to assess the risk or hazard association.
Results:
B7-H4 expression in ovarian cancer patients was significantly increased (OR: 4.20, 95% CI: 2.85–6.18, Z = 6.91, P < .05), and heterogeneity was low between studies (I2 = 8.2%, P = .366). With respect to the clinicopathologic features, no relation was detected between B7-H4 expression and International Federation of Gynaecology and Obstetricsstages stages (OR: 0.81, 95% CI: 0.64–1.03, Z = 1.70, P = .09), pathologic grade (OR: 0.91, 95% CI: 0.72–1.16, Z = 0.76, P = .45), tumor metastasis (OR: 1.25, 95% CI: 0.90–1.74, Z = 1.34, P = .18), or histologic type (OR: 1.17, 95% CI: 0.85–1.60, Z = 0.96, P = .34) in ovarian cancer. Furthermore, B7-H4 expression was significantly associated with a worse progression-free survival (PFS) (HR: 1.30, 95% CI: 1.17–1.45, Z = 4.79, P < .05).
Conclusion:
B7-H4 expression was related to ovarian cancer, but not to patients’ clinicopathologic characteristics. High B7-H4 expression was negatively correlated with survival outcome, suggesting that B7-H4 plays an essential role in poor prognosis in ovarian cancer patients.
Keywords: B7-H4, meta-analysis, ovarian cancer
1. Introduction
Ovarian cancer is a frequent malignant tumor in the female reproductive system and is often diagnosed late due to the lack of effective early diagnostic methods, which leads to the tumor's higher mortality rates.[1] Due to the lack of early specific clinical symptoms, the diagnosis, treatment, and prognosis of the disease are seriously affected. Therefore, it is clinically essential to find sensitive and specific tumor markers to evaluate the degree of malignancy and improve the prognosis and survival rate of ovarian cancer patients.
B7-H4, also known as B7s1 or B7x, is a newly discovered member of its family that is expressed in antigen-presenting cells.[2] Studies have found that B7-H4 is expressed in many tumor tissues, including ovarian, breast, prostate, and esophageal cancers, and is associated with tumor occurrence, development, and prognosis.[3–5] B7-H4 was overexpressed in ovarian cancer tissue, while it was either not expressed or less expressed in normal ovarian tissue, indicating it is a potential tumor marker for ovarian cancer.[6,7] It is reasonable to speculate that B7-H4 expression may be closely related to the pathogenesis of ovarian cancer. However, the role and the prognostic value of B7-H4 expression in ovarian cancer remain unclear. Therefore, we conducted a systematic review to explore the correlation between B7-H4 expression and ovarian cancer, in addition to determining its clinicopathologic characteristics and prognostic value.
2. Methods
2.1. Search strategy
This meta-analysis was conducted in accordance with the guidelines of the Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. A comprehensive search of PubMed, MEDLINE, Cochrane Library, and the China National Knowledge Infrastructure databases was performed through September 2017. The following search strategy was used: (“B7-H4” OR “V-set domain containing T-cell activation inhibitor 1” OR “B7s1” OR “B7x”) and (“ovarian cancer” OR “ovarian tumor” OR “ovarian neoplasm” OR “cancer of the ovarian”). Subsequently, the eligible literature was included for further screening.
2.2. Study selection
Two researchers independently screened the eligible literature. The inclusion criteria were that the authors measured B7-H4 expression in tumor tissue and reported the clinicopathologic characteristics and survival. Exclusion criteria were the use of animals, reviews, comments, or irrelevant articles, and data that comprised continuous variables or that were incomplete.
2.3. Data extraction
The following items were collected from the included studies: the first author's name, publication year, country in which the study was performed, age, number of patients, cutoff value, detection method for B7-H4 expression, number of B7-H4 positives, recruitment period, and outcome. The hazard ratio (HR) was extracted from survival curve plots when data could not be obtained directly. All authors agreed to the final determinants of the literature to be considered.
2.4. Study quality assessment
According to the guidelines of the Newcastle–Ottawa scale (NOS),[8] 2 independent authors (SLL and JJW) evaluated the quality of the included retrospective studies. The included studies were classified into 2 levels: low quality (0–6) and high quality (7–9).[9] A third investigator (YY) adjudicated when disagreements on the enrolled studies occurred.
2.5. Statistical analysis
The statistical software, Stata version 14.0 (Stata Corp LP, College Station, TX), was used to perform statistical analyses. The B7-H4 cutoff values classified cancer patients into high and low expression groups. Statistical heterogeneity across the eligible studies was inspected by processing the Cochran Q-statistic test (P ≤ .05 were treated as statistically significant) and calculating the I2 statistic.[10,11]I2 values of 25%, 50%, and 75% represented low, medium, and high heterogeneity, respectively.[12] A random-effects model was used when the I2 was >50% and P-value was <.05; otherwise, the fixed effects model was used. Sensitivity analysis was conducted to validate the outcome credibility by deleting individual studies in the meta-analysis. A funnel plot was used to identify evidence of publication bias. Egger linear regression test was used to evaluate funnel plot symmetry. To calculate the effect size of the clinicopathologic characteristics, the summary odds ratios (ORs) with their 95% confidence intervals (CIs) were used for International Federation of Gynaecology and Obstetricsstages (FIGO), pathologic grade, tumor metastasis, and histologic type of B7-H4 expression. The PFS value from each study was determined by combining the HR and its corresponding 95% CI. A P-value of .05 was regarded as statistically significant, and all tests were 2-sided.
3. Results
3.1. Literature search
The study search details are presented in a flow diagram (Fig. 1). In total, 156 relevant studies were identified from a search of the above databases using the search strategy as described earlier. After carefully reading each article, 104 studies were excluded because they were duplicates, letters, reviews, nonhuman studies, or contained limited data. Upon further review, 42 additional studies were excluded because they were irrelevant to B7-H4 or ovarian cancer. Finally, a total of 10 publications were enrolled for the present meta-analysis. The selection process is shown in Figure 1.
Figure 1.
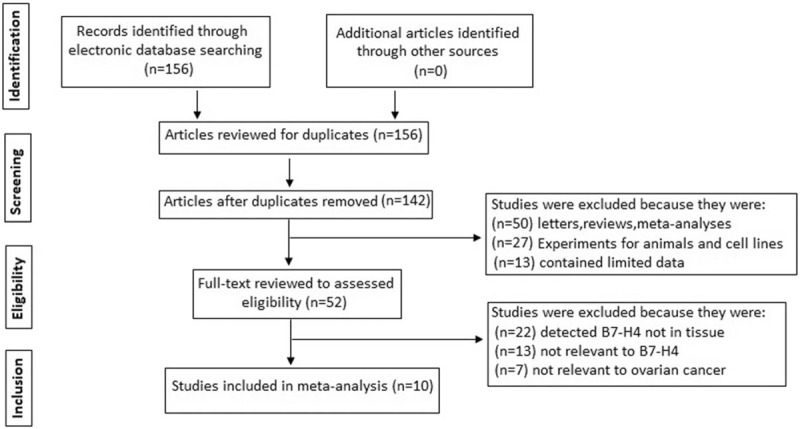
Flow chart showing the study selection procedure.
3.2. Study characteristics
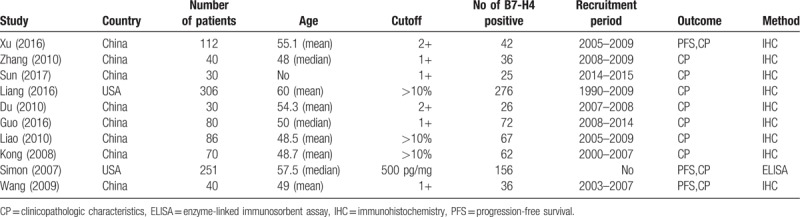
Characteristics of the selected studies are listed in Table 1. The 10 studies were published between 2007 and 2017, including 2 American studies[7,13] and 8 Asian studies.[14–21] A median NOS score of 7 was identified as indicating reliable quality. We explored the correlation between B7-H4 expression and ovarian cancer (7 studies). Furthermore, we compared the positive expression of B7-H4 between the following pairs: FIGO I+II versus III+IV groups (10 studies), high- and medium-differentiation versus low-differentiation groups (9 studies), metastasis versus nonmetastasis (7 studies), serous versus mucinous (9 studies), and the relationship between positive B7-H4 expression and patient PFS (3 studies).
Table 1.
Characteristics of the eligible studies in the meta-analysis.
3.3. Meta-analysis
3.3.1. Correlation between B7-H4 expression and ovarian cancer
We compared the rate of positive B7-H4 expression in the 7 included studies. The pooled OR was 4.20 (95% CI: 2.85–6.18, Z = 6.91, P < .05), and heterogeneity between the studies was low (I2 = 8.2%, P = .366) (Fig. 2).
Figure 2.
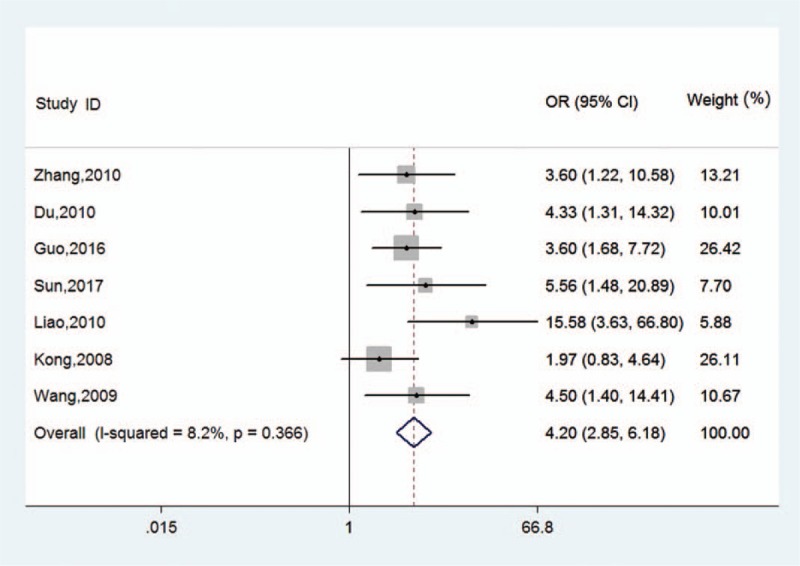
Forest plots for the relationship between B7-H4 expression and prostate cancer. CI = confidence interval, OR = odds ratio.
3.3.2. Association between positive B7-H4 expression and clinicopathologic characteristics
The positive B7-H4 expression rates between the FIGO I+II and FIGO III+IV stages were compared in 10 studies. The pooled OR was 0.81 (95% CI: 0.64–1.03, Z = 1.70, P = .09) with low significant heterogeneity (I2 = 18.1%, P = .28). In addition, the positive expression of B7-H4 between the groups with tumor metastasis and nonmetastasis was compared in 7 studies. The pooled OR was 1.25 (95% CI: 0.90–1.74, Z = 1.34, P = .18), with low significant heterogeneity (I2 = 12.1%, P = .34). Furthermore, B7-H4 was more highly expressed in ovarian cancer patients with high differentiation grade in 3 studies (OR: 0.91, 95% CI: 0.72–1.16, Z = 0.76, P = .45), and medium significant heterogeneity was detected (I2 = 70.5%, P = .001). The results also suggest that there was no statistically significant difference between serous and mucinous ovarian cancer patients in 9 studies (OR: 1.17, 95% CI: 0.85–1.60, Z = 0.96, P = .34) and no evidence of heterogeneity (I2 = 0%, P = .68). We conclude that positive B7-H4 expression was not associated with the clinicopathologic characteristics of ovarian cancer (Fig. 3).
Figure 3.
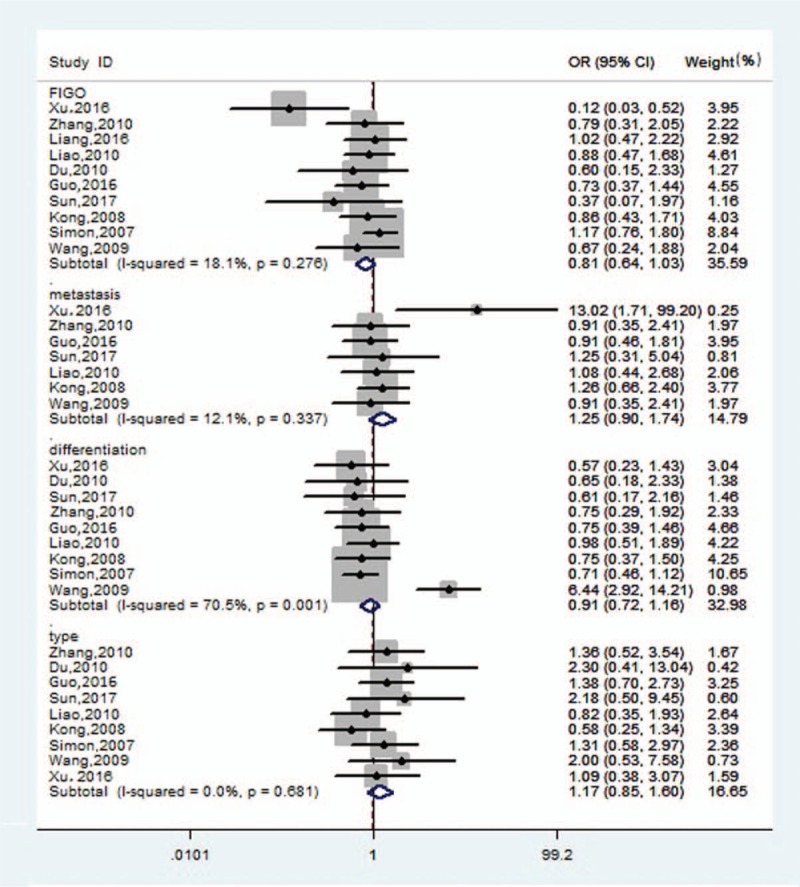
Forest plots for the relationship between B7-H4 expression and clinicopathologic characteristics of ovarian cancer. CI = confidence interval, OR = odds ratio, FIGO = International Federation of Gynaecology and Obstetricsstages.
3.3.3. Meta-analysis of B7-H4 expression and PFS
Three studies provided data on the association between B7-H4 expression and PFS. The analysis results suggested that B7-H4 expression was associated with PFS (HR: 1.30, 95% CI: 1.17–1.45, Z = 4.79, P < .05) with medium significant heterogeneity (I2 = 64.3%, P = .06) (Fig. 4).
Figure 4.
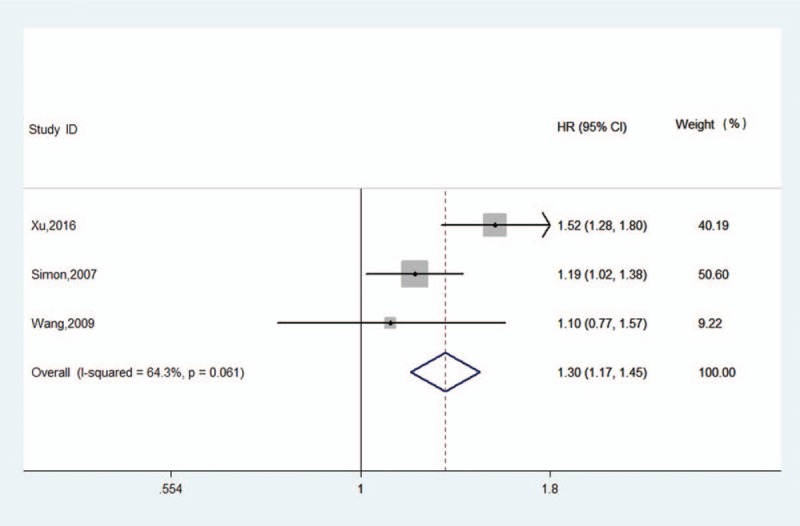
Forest plot of the association between B7-H4 overexpression and ovarian cancer PFS. CI = confidence interval, HR = hazard ratio.
3.3.4. Publication bias and sensitivity analysis
Funnel plots and Egger test were used to evaluate publication bias. The funnel plots of the studies were symmetrical, and Egger test showed no publication bias (Fig. 5). The overall significance did not change when any single study was omitted. Sensitivity analysis showed that the data were relatively stable and reproducible (Fig. 6).
Figure 5.
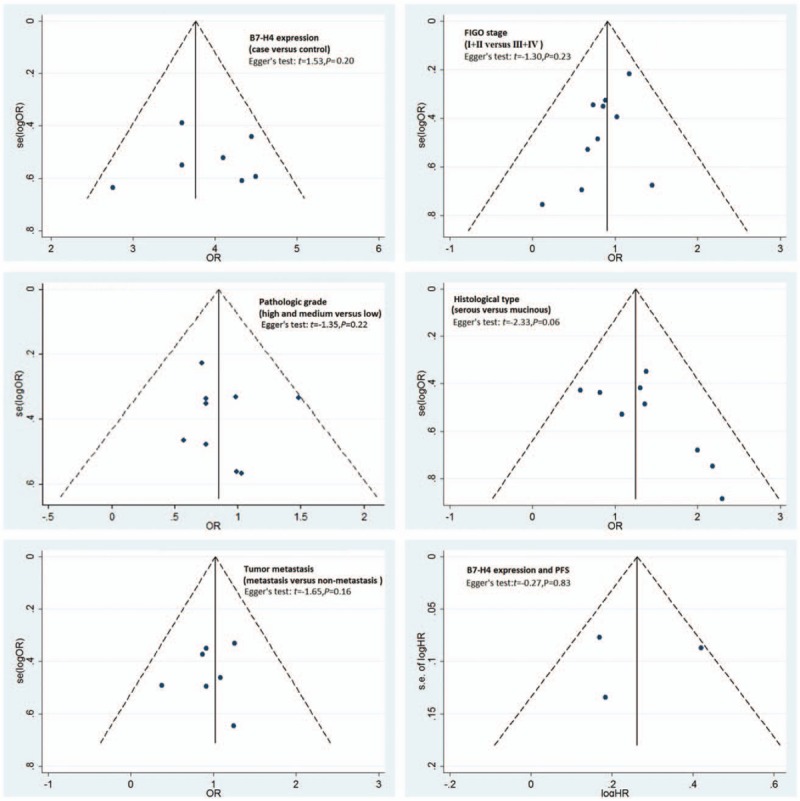
Funnel plot analysis of publication bias. FIGO = International Federation of Gynaecology and Obstetricsstages, HR = hazard ratio, OR = odds ratio, PFS = progression-free survival.
Figure 6.
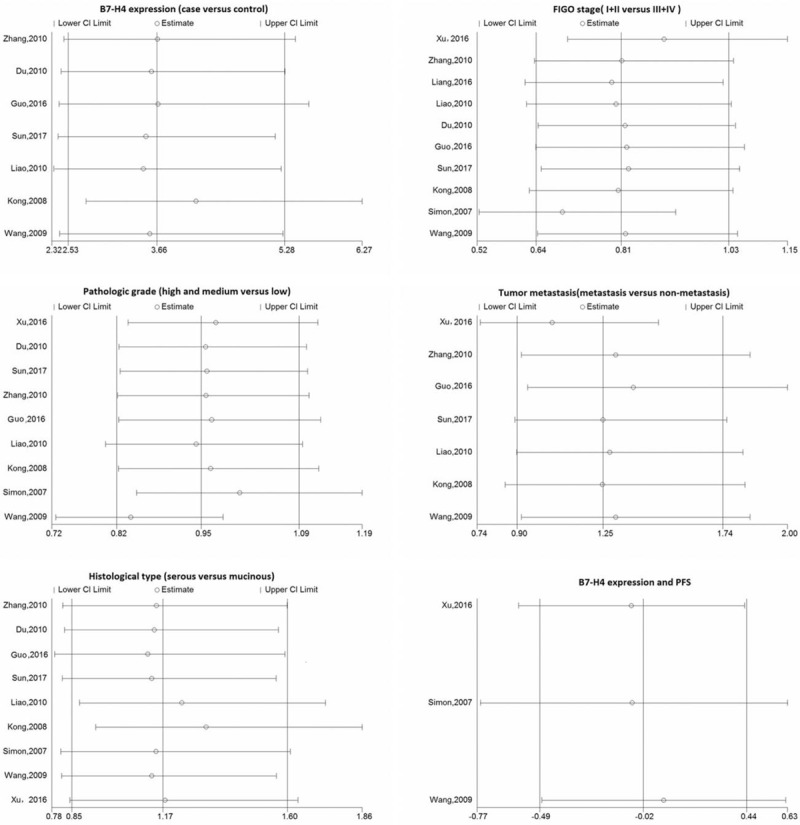
Results of the sensitivity analysis. CI = confidence interval, FIGO = International Federation of Gynaecology and Obstetricsstages, PFS = progression-free survival.
4. Discussion
Ovarian cancer is a malignant tumor of the female reproductive system with high mortality.[22] Because the ovarian tissue is located deep in the pelvic cavity, early symptoms are not obvious, and the lack of effective detection methods means that most patients are in the middle and late stages when seeking medical treatment. Thus, the 5-year survival rate is only 20% to 30%.[23] Therefore, the search for a specific and sensitive tumor molecular marker has important clinical significance for guiding treatment and improving the prognosis of ovarian cancer patients.
As determined bioinformatically in 2003, B7-H4 negatively regulates T-cell-mediated immune response genes.[24] It inhibits T-cell cytokine proliferation and cell cycle processes to negatively regulate T-cell immune responses; therefore, it is highly expressed in various tumor tissues and plays an important role in tumor occurrence and development.[25,26] Enhanced B7-H4 expression results in tumor immune escape and is associated with ovarian cancer occurrence, thus making it a potential new biomarker for early diagnosis and treatment of ovarian cancer.[27] However, the relationship between B7-H4 overexpression and ovarian cancer patient prognosis remains unclear. Additionally, no relevant meta-analysis has yet reported the relationship between B7-H4 expression and clinicopathologic characteristics of ovarian cancer patients. An expanded study is needed to conclude whether B7-H4 expression is a predictive factor. In this meta-analysis, we explored the relationship between B7-H4 expression and the clinicopathologic characteristics and prognosis of ovarian cancer patients.
First, we included 376 patients and 180 controls from 7 studies to analyze the correlation between B7-H4 expression and ovarian cancer. The pooled OR was 4.20 (95% CI: 2.85–6.18, Z = 6.91, P < .05), indicating that B7-H4 expression was associated with PCa, and heterogeneity was low between studies (I2 = 8.2%, P = .366). Next, we evaluated clinicopathologic characteristics of ovarian cancer, such as pretreatment clinical FIGO stages, pathologic grade, tumor metastasis, and histologic type, which have important clinical value. The effect size for clinicopathologic characteristics was calculated, and we found no association between clinicopathologic characteristics of ovarian cancer and positive expression of B7-H4. Finally, we conducted a review to analyze the relationship between B7-H4 expression and ovarian cancer patient prognosis. In our analysis, the pooled HR of B7-H4 on PFS was 1.30 (95% CI: 1.17–1.45, P < .05), which verified that B7-H4 indicates a worse outcome for ovarian cancer patients.
Simon found that the tumor malignancy degree increased as B7 levels increased.[28] However, Zhang et al reported that the expression of B7 in ovarian cancer was unrelated to clinicopathologic factors such as patient age and lymph node metastasis.[15] Based on the results of our meta-analysis, we assume that B7-H4 expression is a biomarker for ovarian cancer, but it is uncorrelated with clinicopathologic characteristics. The previous meta-analysis investigated whether high B7-H4 expression influenced the prognosis of solid tumor patients.[29] In our study, we found that high expression of B7-H4 was an independent prognostic indicator of poor survival. However, certain limitations to our study should be considered. First, continuous variable data were not included. Second, most of the included studies were conducted in the Asian population. Third, HRs were extracted from survival curves when directly reported HR values were lacking, which may have introduced an element of decreased reliability. In our future research, we plan to design a prospective randomized, controlled trial to explore differences and avoid selection bias.
5. Conclusion
The results of this meta-analysis suggest that B7-H4 expression is related to ovarian cancer and poor prognosis, but unrelated to the clinicopathologic characteristics of ovarian cancer patients.
Acknowledgments
The authors express their gratitude to the study participants and research personnel for their involvement in the study. The authors also thank Professors Xiao-Ke Hao and Yue-Yun Ma for their valuable assistance.
Author contributions
Data curation: Su-Liang Li.
Formal analysis: Yun Ye.
Investigation: Jian-Jun Wang, Fa-Hong Jing.
Methodology: Su-Liang Li, Yun Ye.
Software: Sheng-Yu Wang, Yun Ye.
Supervision: Yun Ye.
Validation: Yun Ye.
Writing – original draft: Su-Liang Li.
Writing – review and editing: Su-Liang Li, Jian-Jun Wang, Yun Ye.
Data curation: Su-Liang Li.
Formal analysis: Sheng-Yu Wang.
Funding acquisition: Jian-Jun Wang.
Methodology: Su-Liang Li.
Software: Fa-Hong Jing.
Visualization: Yun Ye.
Writing – original draft: Fa-Hong Jing.
Writing – review & editing: Yun Ye.
Footnotes
Abbreviations: CI = confidence interval, FIGO = International Federation of Gynaecology and Obstetricsstages, HR = hazard ratio, MOOSE = Meta-analysis of Observational Studies in Epidemiology group, NOS = Newcastle–Ottawa scale, OR = odds ratio, P = P-value of overall effect, PFS = progression-free survival.
YY, S-LL, and J-JW contributed equally to this work.
This study was supported by the Department of Science and Technology of Shaanxi Provincial Government (2014JM2-8195) and the First Affiliated Hospital of Xi’an Medical University (XYFY14-02).
The authors have no conflicts of interest to disclose.
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017;67:7–30. [DOI] [PubMed] [Google Scholar]
- [2].Kryczek I, Wei S, Zou L, et al. Cutting edge: induction of B7-H4 on APCs through IL-10: novel suppressive mode for regulatory T cells. J Immunol 2006;177:40–4. [DOI] [PubMed] [Google Scholar]
- [3].Yee EU, Zaino RJ, Torkko KC, et al. B7-H4 expression in Brenner tumours, a descriptive and comparative study. Histopathology 2010;56:652–4. [DOI] [PubMed] [Google Scholar]
- [4].Zhang J, Zhang M, Jiang W, et al. B7-H4 gene polymorphisms are associated with sporadic breast cancer in a Chinese Han population. BMC Cancer 2009;9:394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kryczek I, Wei S, Zhu G, et al. Relationship between B7-H4, regulatory T cells, and patient outcome in human ovarian carcinoma. Cancer Res 2007;67:8900–5. [DOI] [PubMed] [Google Scholar]
- [6].Tringler B, Liu W, Corral L, et al. B7-H4 overexpression in ovarian tumors. Gynecol Oncol 2006;100:44–52. [DOI] [PubMed] [Google Scholar]
- [7].Simon I, Katsaros D, Rigault de la Longrais I, et al. B7-H4 is over-expressed in early-stage ovariancancer andis independent of CA125 expression. Gynecol Oncol 2007;106:334–41. [DOI] [PubMed] [Google Scholar]
- [8].Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- [9].Zhang P, Zhong ZH, Yu HT, et al. Significance of increased leptin expression in osteoarthritis patients. PLoS One 2015;10:e0123224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Jackson D, White IR, Riley RD. Quantifying the impact of between-study heterogeneity in multivariate meta-analyses. Stat Med 2012;31:3805–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Peters JL, Sutton AJ, Jones DR, et al. Comparison of two methods to detect publication bias in meta-analysis. JAMA 2006;295:676–80. [DOI] [PubMed] [Google Scholar]
- [12].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Liang L, Jiang Y, Chen JS, et al. B7-H4 expression in ovarian serous carcinoma:a study of 306 cases. Hum Pathol 2016;57:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Xu M, Zhang B, Zhang M, et al. Clinical relevance of expression of B7-H1 and B7-H4 in ovarian cancer. Oncol Lett 2016;11:2815–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Zhang LL, Shao SL, Wu Y. Expressions of osteopontin and B7-H4 in epithelial ovarian neoplasm and their significance [in Chinese]. Chin J Cancer 2010;29:25–9. [PubMed] [Google Scholar]
- [16].Sun SM, Mu YY. The expression and clinical significance of B7-H4 and RRM2 in ovarian tumors. Heilongjiang Med Pharmacy 2017;40:100–3. [Google Scholar]
- [17].Du H, Xu CL, Zuo HL, et al. Expression of B7-H4 protein in ovarian epithelial cancer and its clinical significance. J Hebei Med Univ 2010;31:291–3. [Google Scholar]
- [18].Guo LX, Zhang LL. Expressions of B7-H4 and GLUT-1 in epithelial ovarian tumor and the significance. Matern Child Health Care China 2016;31:180–2. [Google Scholar]
- [19].Liao LH, Wu FG, Yu XH. Expression of B7-H4 in epithelial ovarian carcinoma and its clinicopathological significance. J Nanchang Univ 2010;50:12–5. [Google Scholar]
- [20].Kong FR, Wang LM, Dai SZ, et al. Expression of B7-H4 on epithelial ovarian cancer and it's significances. Shandong Med J 2008;48:32–3. [Google Scholar]
- [21].Wang YL, Liang JF, Zheng HX, et al. Expression of B7-H4 in epithelial tumor of ovarian. J Changzhi Med Coll 2009;23:91–4. [Google Scholar]
- [22].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7–30. [DOI] [PubMed] [Google Scholar]
- [23].Gadducci A, Cosio S, Gargini A, et al. Sex-steroid hormones, gonadotropin and ovarian carcinogenesis: a review of epidemiological and experimental data. Gynecol Endocrinol 2004;19:216–28. [DOI] [PubMed] [Google Scholar]
- [24].Sica GL, Choi IH, Zhu G, et al. B7-H4, a molecule of the B7 family, negatively regulates T cell immunity. Immunity 2003;18:849–61. [DOI] [PubMed] [Google Scholar]
- [25].Prasad DV, Richards S, Mai XM, et al. B7S1, a novel B7 family member that negatively regulates T cell activation. Immunity 2003;18:863–73. [DOI] [PubMed] [Google Scholar]
- [26].Zang X, Loke P, Kim J, et al. B7x:a widely expressed B7 family member that inhibits T cell activation. Proc Natl Acad Sci U S A 2003;100:10388–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Simon I, Liu Y, Krall KL, et al. Evaluation of the novel serum markers B7-H4, Spondin 2, and DcR3 for diagnosis and early detection of ovarian cancer. Gynecol Oncol 2007;106:112–8. [DOI] [PubMed] [Google Scholar]
- [28].Simon I, Zhuo S, Corral L, et al. B7-h4 is a novel membrane-bound protein and a candidate serum and tissue biomarker for ovarian cancer. Cancer Res 2006;66:1570–5. [DOI] [PubMed] [Google Scholar]
- [29].Song X, Shao Y, Gu W, et al. Prognostic role of high B7-H4 expression in patients with solid tumors: a meta-analysis. Oncotarget 2016;7:76523–33. [DOI] [PMC free article] [PubMed] [Google Scholar]


