Abstract
Background:
Pediatric chronic pain is relatively common in the world. Although cognitive behavior therapy (CBT) has been shown to be efficacious in children and adolescents, it is generally recognized that availability and accessibility of CBT are limited. While Internet-delivered cognitive-behavioral therapy (ICBT) performs better in these areas.
Objectives:
This systematic review aims to evaluate the clinical effects of ICBT for chronic pain in youth when compared with the control treatments.
Methods:
We searched electronic databases to identify randomized controlled trials that compared ICBT with the control therapy for pediatric chronic pain. The primary outcomes were 95% confidence intervals and mean difference or standardized mean difference in change of pain intensity and activity limitations.
Results:
Four trials met the inclusion criteria with a total of 404 participants of whom 208 received ICBT. Compared with pretreatment, children reported significant, medium to large benefits on pain intensity, activity limitations, and parental protective behaviors after receiving ICBT immediately. Significant small to medium effects were found for outcomes of depressive symptoms, anxiety, and sleep quality from baseline to post-treatment in the ICBT group. But most measures of ICBT did not show statistically significant superiority to those of the control conditions, except parental protective behaviors. Generally children and their parents were highly acceptable and satisfied with ICBT.
Conclusion:
ICBT for physical and psychological conditions in youth with chronic pain is a full potential therapy; it can be successful on clinically effects and socioeconomic benefits. However, only limited data supported the conclusion, we require further methodologically robust trials.
Systematic review registration:
PROSPERO CRD42017069811.
Keywords: children and adolescents, chronic pain, Internet-delivered cognitive-behavioral therapy, meta-analysis, systematic review
1. Introduction
Although precise definitions of chronic pain are varied, it is usually considered as any constant ache that lasts longer than expected (arbitrarily defined as >3 months) or any recurrent pain that arises at least 3 times throughout a period of 3 months.[1] Pediatric chronic pain is a significant problem conservatively estimates that posit 20% to 35% of children and adolescents around the world.[2,3] It is not only a common problem among the general population of children—one that often demands medical attention, but also a serious disease which could negatively affects the overall health status and daily activities of the children and their families.[4] Children and adolescents with a chronic pain syndrome are frequently associated with severe pain, impaired functioning, emotional disorder, and disturbed sleep, they may miss school and withdraw from social activities, or even tend to develop internalizing symptoms by virtue of their pain.[5–7] Statistics indicate that the total cost for caring them in the United States has been estimated at about $19.5 billion annually,[8] and approximately 3.8 billion pounds per year in the United Kingdom,[9,10] the cost will continue to grow with the increasing prevalence.[11]
The injuries associated with chronic pain pervade every aspect of the children's lives and emphasize the need to develop and provide convenient and effective interventions. One of the broad family of therapies for chronic pain is behavioral treatment. Especially, this type of psychological interventions compare favorably to pharmacological treatments.[12] In the past 20 years, cognitive behavioral therapy (CBT) has been shown to yield improvements in the treatment of chronic pain in children and adolescents.[13,14] The purpose of CBT is to help them understand the environment, increase their self-awareness, and enhance their self-control. CBT established a series of strategies about pain-related cognition, emotion, physiology, and behavior to rescue chronic pain, they mainly include the following: enhance patients’ sense of control of pain, focus on cultivating self-efficacy, eliminate the original negative coping model, strengthen the skills training, promote relaxation, and so forth.[13–16]
Although many children and adolescents have benefited from CBT, a UK survey of child and adolescent mental health services suggests that the availability of CBT is doubtful.[17] There are some significant barriers to prevent youth with chronic pain from accessing pain care due to limited approaches to trained instructors, the potential risk of stigma involved in visiting a therapist, geographical distance from treatment centers, and long waiting times,[18–21] only a small proportion of children and adolescents could receive effective psychological treatments. To improve availability and accessibility of treatments for patients with pain conditions and solve other barriers, various technologies (e.g., audiotapes,[22] the telephone,[23] CD-ROMs,[24] handheld wireless devices,[25] videoconferencing,[26] and the Internet[23]) have been applied and evaluated with the development of information and communication technology. Emerging evidence from these remotely delivered studies have demonstrated different degrees of beneficial efficacy on pain and disability.
However, Internet-delivered CBT (ICBT) is a markedly different method of delivery and holds important advantages over the rest technologies. Compared with other interventions, the areas ICBT performs better are the flexibility, time- and cost-saving possibilities, ability to update information in real time, convenience in downloading data, and swift dissemination of time-efficient information.[21,27] Aided by the convenience and constant access provided by mobile devices, 89% of households in Great Britain (23.7 million) had Internet access,[28] 86% of all households in Australia,[29] in the US, 92% of youths go online daily,[30] and approximately 80% of adults have either a smartphone or a home broadband subscription.[31] Given that the Internet is very widely used in the world, ICBT is a full potential and accessible therapy, its effects worth being investigated separately. To the best of our knowledge, notwithstanding there have been systematic reviews and meta-analysis on the ICBT, no earlier reviews has targeted ICBT for children and adolescents with chronic pain; therefore, we need to systematically analyze efficacy of ICBT for youth and put forward treatment advice and improvement direction in the future.
Our primary objective was to systematically review the literature on ICBT and present meta-analyses to examine therapeutic effects of ICBT for the management of chronic pain in children and adolescents. Specifically, we aimed to determine the clinical effectiveness of ICBT in pain intensity, activity limitations, emotion functioning, sleep quality, parental protective behaviors, and treatment acceptability and satisfaction. The secondary objective was to describe the methodological quality of the studies and identify confounding factors that may limit the estimated treatment efficacy on the measured endpoints.
2. Methods
This review was registered on the PROSPERO register of systematic reviews (registration number: CRD42017069811). The Preferred Reporting Items for Systematic Reviews and Meta-Analyses guideline was used to guide reporting. All analyses were based on previous published studies; thus, no ethical approval and patient consent are required.
2.1. Search strategy
The following databases were searched by the first and second authors: PubMed (including Medline), Ovid, Clinical Trial, and Cochrane Library. We included publications from their inception through October 2017 using combinations of the following medical subject headings and keywords: online, Web, e-health, computer, Internet, cognitive behavioral treatment, cognitive behavioral therapy, chronic pain, chronic ache, children, adolescent, youth, teenager, and youngster.
2.2. Study inclusion and exclusion criteria
Eligibility criteria for the present analyses required that included articles be participants included children and adolescents with chronic pain between the ages of 11 and 17 years; the investigated intervention was ICBT; ICBT focused on symptom reduction of both physical and psychological conditions or problems; a randomized controlled trial (RCT) published in a peer-reviewed journal; original data presented on continuous outcome measures was not used in other studies; and full-text articles were available. Exclusion criteria should be the intervention or the outcome of interest was not clearly defined, such as published abstracts, letters, commentaries, or editorials; trials that did not report the specific outcomes; and unavailability of full text.
2.3. Data extraction and assessment of study quality
Two investigators (W-XT and L-FZ) independently extracted data from each included article into standardized tables and resolved discrepancies by consensus. If we could not come to an agreement, the conclusion might be determined by the corresponding author. Extracted details relate to primary author, year of publication, the design of the study, the participants, diagnosis, treatment intervention, outcome measures, and outcome data for computation of effect sizes.
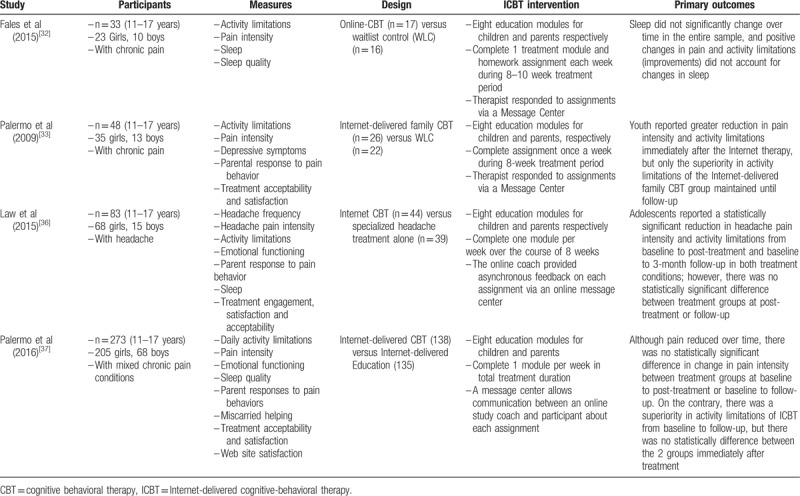
When several publications reported the same participants, we chose studies according to the sample size and the outcomes to avoid duplication of information. Among the included articles, the patients of Fales et al[32] were a subset of youth from Palermo et al,[33] but their concerned measurements are different, so we included both of them to evaluate different outcomes. We will contact authors as feasible if additional information is needed. Table 1 summarizes the characteristics of the included studies.
Table 1.
Characteristics of included trials.
We estimated efficacy of ICBT by primary and secondary outcome measures. The primary outcomes were pain intensity and activity limitations, prespecified secondary outcomes included emotional functioning, sleep quality, parental protective behaviors, and treatment acceptability and satisfaction. The Collaboration's recommended tool for assessing risk of bias is a domain-based evaluation which considered 7 different domains[34]: random sequence generation (selection bias); allocation concealment (selection bias); blinding of participants and personnel (performance bias); blinding of outcome assessment (detection bias); incomplete outcome data (attrition bias); selective reporting (reporting bias); and other bias. The risk assessment is provided in Figure 7.
Figure 7.
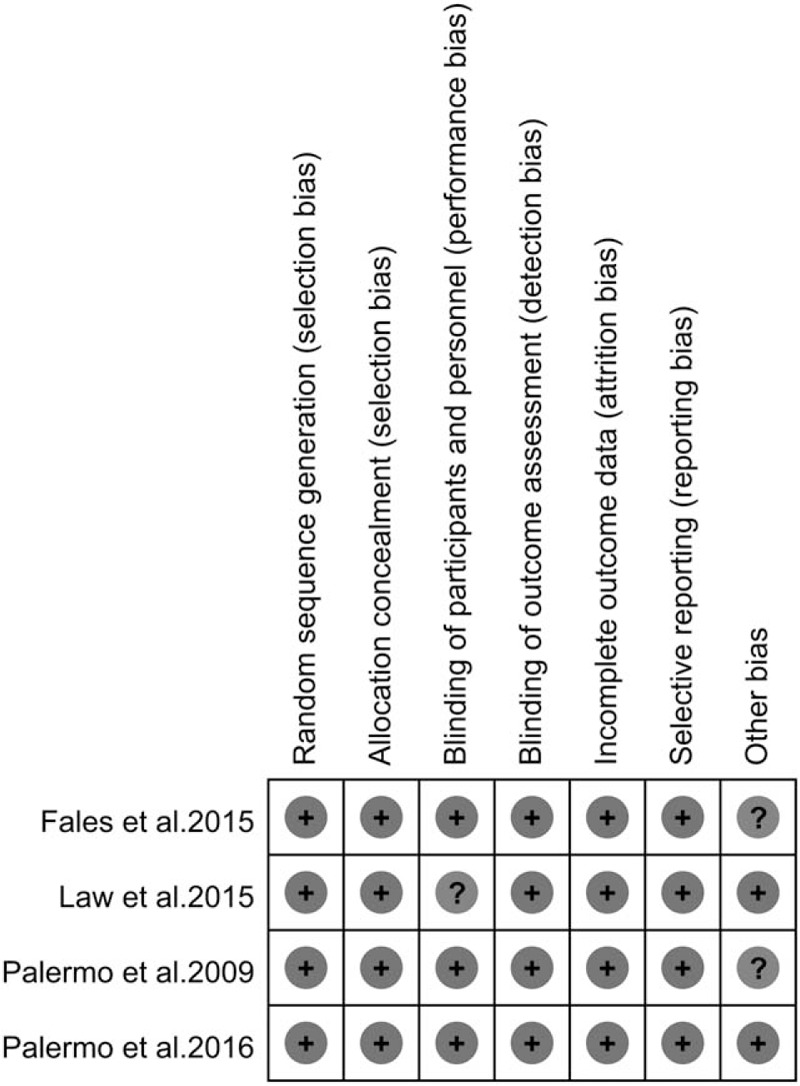
Quality assessment: The Collaboration's recommended tool for risk of bias[35] (+ indicates low risk of bias, ? indicates unclear risk of bias).
2.4. Assessment of heterogeneity
χ2 and I2 in the forest plots are used to quantify heterogeneity of intervention effects. The latter describes the percentage of the variability in effect estimates that is attributable to heterogeneity rather than sampling error (chance). A value of 0% to 40% indicates heterogeneity might not be important, 30% to 60% may represent moderate heterogeneity, and 75% to 100% shows considerable heterogeneity. When heterogeneity could be ignored, the intervention effect was estimated by the fixed effects model; when heterogeneity could not be explained, we only used the random effects model to present results. Sensitivity analyses were performed for each outcome with an I2 confidence interval (CI) that included 40% or greater to investigate the degree to which the main findings of a systematic review are affected by changes in its methods or in the data used from individual studies.
2.5. Assessment of reporting biases
We could not use a test for asymmetry of the funnel plot proposed by Egger et al[35] to assess publication bias, because the biggest number of articles included in the analyses of all the outcomes is 3. General considerations suggest that the use of the method with substantially fewer than 10 studies would be unwise.[34] We did not have enough studies to yield a reliable funnel plot.
2.6. Statistical analysis
The random effects meta-analysis was performed using Review Manager (version 5.3) and the chosen outcomes in the respective studies, the main outcomes are reported in Tables 2 and 3. Data were pooled if at least 2 trials were comparable for an outcome. The pooled effect sizes were calculated by within-group comparisons and between-group effectiveness respectively. Within-group comparisons was calculated based on the pre- and post-treatment estimates for groups receiving ICBT, whereas between-group effectiveness were calculated using effect sizes on the chosen outcomes at post-treatment in the ICBT group in comparison to the control group.
Table 2.
Within-group main outcomes.
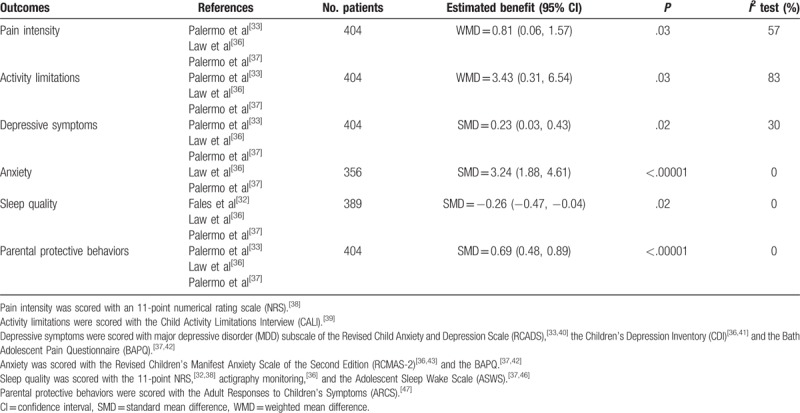
Table 3.
Between-group main outcomes.
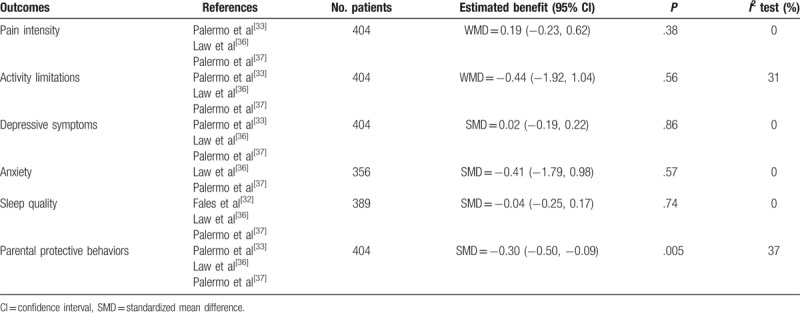
Outcomes were presented as 95% CIs) and standardized mean difference (SMD) or mean difference (MD) as appropriate, and we considered the CI including zero or a P value >.05 was not statistically significant. When trials have used different instruments to measure the same construct, we used an SMD in meta-analysis for combining continuous data owing to that the SMD expresses the intervention effect in standard units rather than the original units of measurements.
Heterogeneity was assessed by I2 test. If there is heterogeneity (I2 > 40%), the random effects method will be used; if heterogeneity could be ignored (I2 ≤ 40%), we will use the fixed effects method, the CI for the average intervention effects will be smaller and the corresponding P values will be more significant compared with the random effects method.
Missing standard deviations (SDs) is a common feature of meta-analyses of continuous outcome data. According to the Cochrane Handbook,[34] if most studies in meta-analysis have missing SDs, these values should not be imputed. After all, all imputation techniques involve making assumptions about unknown statistics, and it is best to avoid using them wherever possible. Moreover, all the included trials were randomized; thus, we treated MD and SDs at post-treatment as value effects to conduct between-group comparisons.
3. Results
3.1. Description of studies
The flow of studies through the selection process is displayed in Figure 1. The database searches yielded 1198 studies, removed 20 duplications, 1178 articles remained. After reviewing the titles and/or abstracts, we identified 55 studies potentially eligible for full-text review. Fifty-one of the remaining articles were excluded by analyzing the full text due to various reasons, only 4 were included in the meta-analysis.
Figure 1.
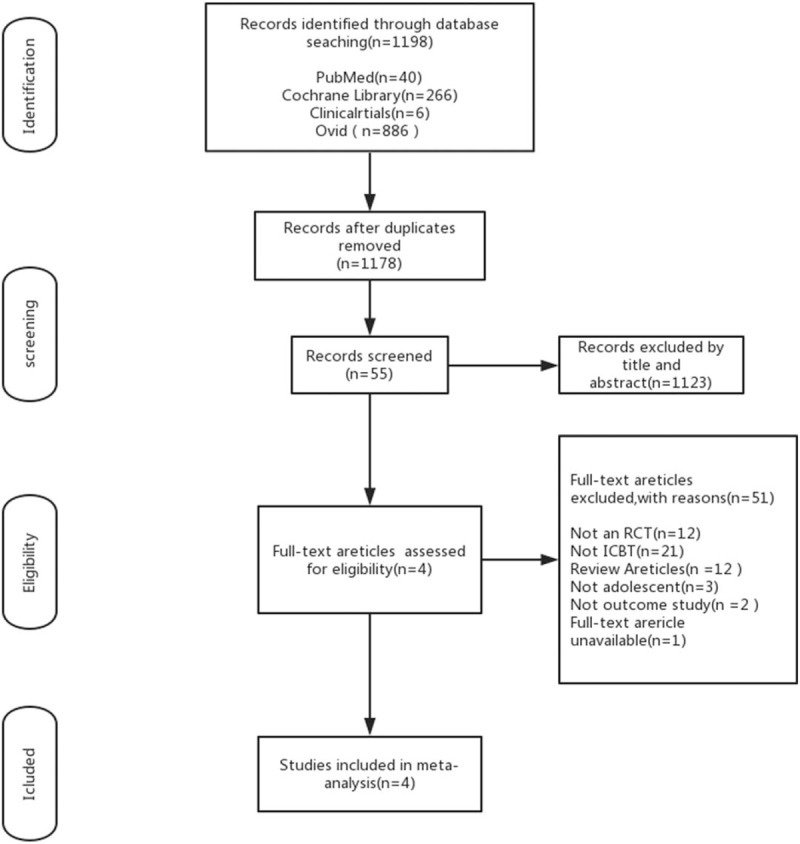
Flow chart of study screening and selection process. On the basis of the search strategy, 1178 studies were identified by the initial search of the medical literature databases and 55 required further assessment. Finally, 4 articles were included in this review. RCT = randomized controlled trial.
The 4 RCTs meeting eligibility criteria included 208 participants randomized to the ICBT group and 196 randomized to the control therapy group. All trials were conducted in the United States and published in the English language. In addition, the interventions in all the trials were very similar, just 2 trials[32,33] compared the ICBT intervention with the waitlist control treatment, and another 2[36,37] randomly assigned patients to the ICBT group and the Internet-delivered Education or specialized headache therapy group. There are 3[32,33,37] trials including participants with different kinds of chronic pain, one[36] only recruited patients with chronic headache, but all the patients’ age ranged from 11 to 17 years.
Figure 2.
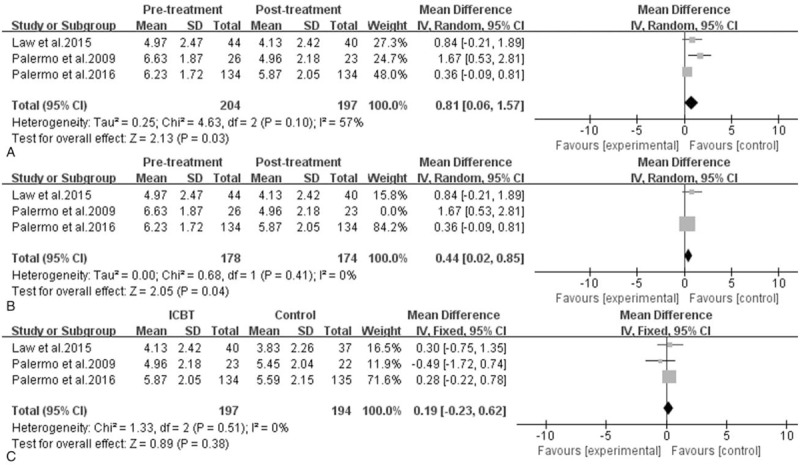
Efficacy of ICBT for children and adolescents with chronic pain: pain intensity. A, Pretreatment versus post-treatment of ICBT, the mean difference was 0.81 (95% CI: 0.06–1.57), I2 = 57%, P = .03. B, the sensitivity analysis of A, the mean difference was 0.44 (95% CI: 0.02–0.85), I2 = 0%, P = .04. C, ICBT is versus the control therapy at post-treatment, the mean difference was 0.19 (95% CI: −0.23–0.62), I2 = 0%, P = .38. CI = confidence interval, ICBT = Internet-delivered cognitive-behavioral therapy, SD = standard deviation.
Adolescents and their parents received not exactly the same 8 modules in destinations respectively, and they both needed complete 1 treatment module and homework assignment each week during therapy period. In addition, they had an online coach or therapist to respond to their assignments and provide advice via a message center. However, every study had a small portion of participants who failed to complete their assignments; they all used intent-to-treat analysis. Among the included trials, patients of Fales et al[32] were a part of youth in Palermo et al,[33] and Fales et al showed outcome measure of sleep quality, whereas Palermo et al did not, so we only included Fales et al for evaluating sleep quality rather than other measures. In addition, follow-up period in 2 studies[33,36] is 3 months which is different from 6 months in Palermo et al,[37] and all of them did not provide accurate data in treatment acceptability and satisfaction; therefore, we could not pool the data at follow-up or in treatment acceptability and satisfaction to enter into meta-analyses.
3.2. Clinical outcomes
3.2.1. Pain intensity
The data on pain intensity from 3[33,36,37] trials were entered into the meta-analysis, it revealed a statistically significant reduction in pain intensity after treatment in the ICBT group (MD = 0.81, 95% CI: 0.06–1.57, P = .03, I2 = 57%). However, as shown in Figure 3, pain intensity was not statistically different between the ICBT group and the control therapy group immediately post-treatment (MD = 0.19, 95% CI: −0.23–0.62, P = .38, I2 = 0%) as well as from baseline to follow-up. Moreover, the sensitivity analysis indicated that the Palermo et al's[33] study was the source of statistical heterogeneity. When this outlier study was removed, the fixed effect model was performed and the effect size was consistent compared with the previous outcome, there was no evidence of heterogeneity in the 2 remaining trials (I2 = 0%), and the statistical significance of 2 P values did not change, it demonstrated that the results were very stable.
Figure 3.
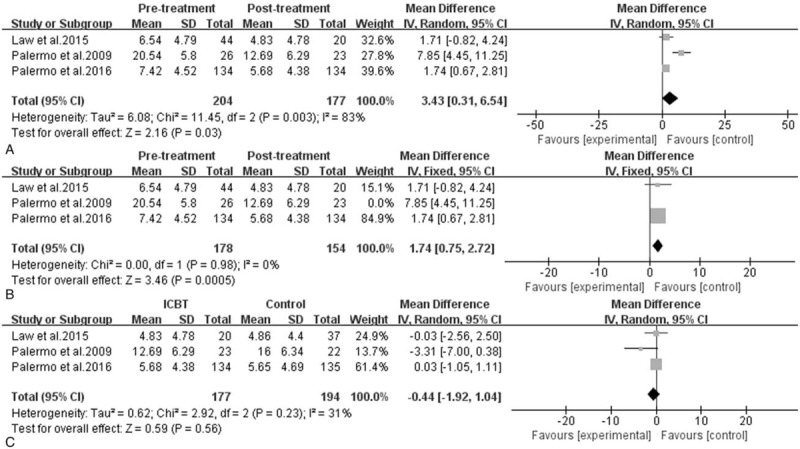
Efficacy of ICBT for children and adolescents with chronic pain: activity limitations. A, Pretreatment versus post-treatment of ICBT, the mean difference was 3.43 (95% CI: 0.31–6.54), I2 = 83%, P = .03. B, The sensitivity analysis of A, the mean difference was 1.74 (95% CI: 0.75–2.72), I2 = 0%, P = .0005. C, ICBT versus the control therapy at post-treatment, the mean difference was −0.44 (95% CI: −1.92–1.04), I2 = 31%, P = .56. CI = confidence interval, ICBT = Internet-delivered cognitive-behavioral therapy, SD = standard deviation.
3.2.2. Activity limitations
Data reporting activity limitations were described in 3 pooled studies,[33,36,37] it presented that at post-treatment, adolescents receiving ICBT achieved great reductions in daily activity limitations (MD = 3.43, 95% CI: 0.31–6.54, P = .03, I2 = 83%), but there was no statistically significant difference between the ICBT group and the control group on change in activity limitations from pretreatment to post-treatment (MD = −0.44, 95% CI: −1.92–1.04, P = .56, I2 = 31%). However, at follow-up, youth in the ICBT group reported greater reductions than did the control group in 2 studies,[33,37] whereas the result of activity limitations in Law et al[36] did not support that. As for heterogeneity of comparison in groups, we could see that Palermo et al's[33] study was still the main reason for the sensitivity analysis shown in Figure 4, and the results were also stable.
Figure 4.
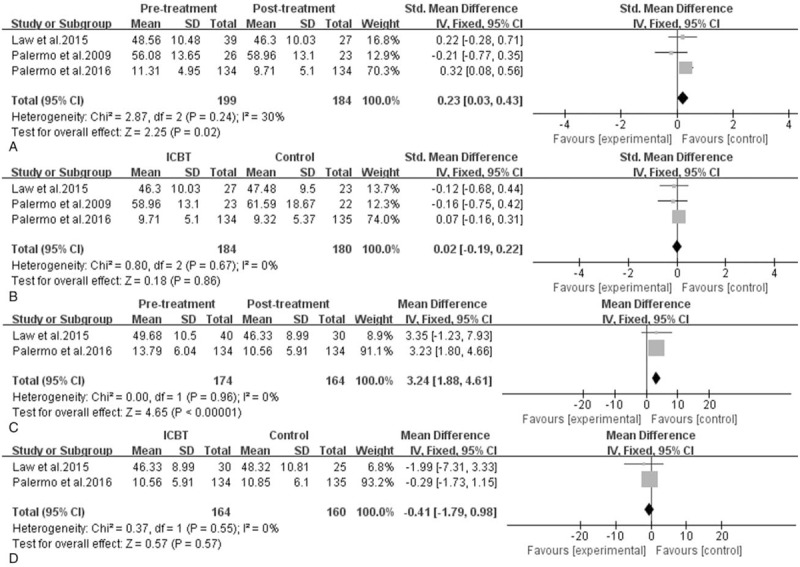
Efficacy of ICBT for children and adolescents with chronic pain: emotional functioning. A, Pretreatment versus post-treatment on depressive symptoms of ICBT, the mean difference was 0.23 (95% CI: 0.03–0.43), I2 = 30%, P = .02. B, ICBT versus the control therapy on depressive symptoms at post-treatment, the mean difference was 0.02 (95% CI: −0.19–0.22), I2 = 0%, P = .86. C, Pretreatment versus post-treatment on anxiety of ICBT, the mean difference was 3.24 (95% CI: 1.88–4.61), I2 = 0%, P < .00001. D, ICBT versus the control therapy on anxiety at post-treatment, the mean difference was −0.41 (95% CI: −1.79–0.98), I2 = 0%, P = .57. CI = confidence interval, ICBT = Internet-delivered cognitive-behavioral therapy, SD = standard deviation.
3.2.3. Emotional functioning
The measurements of emotional functioning are depressive symptoms and anxiety. From Figure 5, depressive symptoms showed a statistically significant decrease from baseline to post-treatment (MD = 0.23, 95% CI: 0.03–0.43, P = .02, I2 = 30%), but between-group differences were not statistically significant (MD = 0.02, 95% CI: −0.19–0.22, P = .86, I2 = 0%). From baseline to follow-up, Law et al[36] reported that there was still a statistically significant reduction in depressive symptoms; however, another 2 trials[33,37] indicated that the effects of treatments were not maintained, so there was insufficient evidence of an effect in either direction at follow-up.
Figure 5.
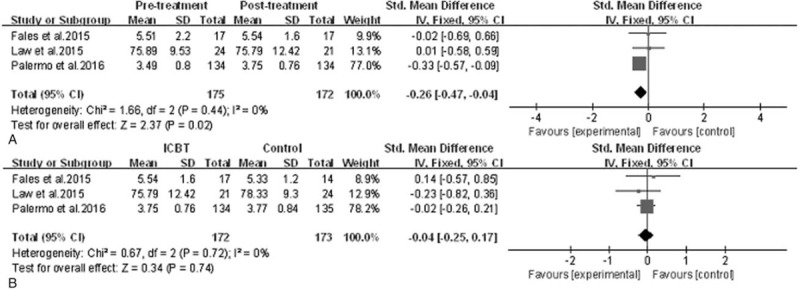
Efficacy of ICBT for children and adolescents with chronic pain: sleep quality. A, Pretreatment versus post-treatment of ICBT, the mean difference was −0.26 (95% CI: −0.47 to −0.04), I2 = 0%, P = .02. B, ICBT versus the control therapy at post-treatment, the mean difference was −0.04 (95% CI: −0.25–0.17), I2 = 0%, P = .74. CI = confidence interval, ICBT = Internet-delivered cognitive-behavioral therapy, SD = standard deviation.
With regard to anxiety, only 2 studies[36,37] reported the assessment. Statistical results indicated that there was significant change in anxiety of ICBT group from baseline to post-treatment (SMD = 3.24, 95% CI: 1.88–4.61, P < .00001, I2 = 0%), but the changes between groups after treatment did not make any difference (SMD = −0.41, 95% CI: −1.79–0.98, P = .57, I2 = 0%). From baseline to follow-up, both of the 2 trials revealed that there was insufficient evidence supporting an effect in ICBT group, between-group differences were also not statistically significant.
3.2.4. Sleep quality
The effect estimates of sleep quality in Figure 6 indicate a statistically significant reduction in sleep quality after treatment immediately in ICBT group (SMD = −0.26, 95% CI: −0.47 to −0.04, P = .02, I2 = 0%), but there was no statistical significant difference in sleep quality between groups at post-treatment (SMD = −0.04, 95% CI: −0.25–0.17, P = .74, I2 = 0%). From baseline to follow-up, patients in ICBT group of Palermo et al[37] achieved a greater magnitude of improvement in sleep quality compared with control group and the effect size was small, whereas another 2 trials did not conduct the sleep assessment at follow-up; therefore, no data were reported for that time point.
Figure 6.
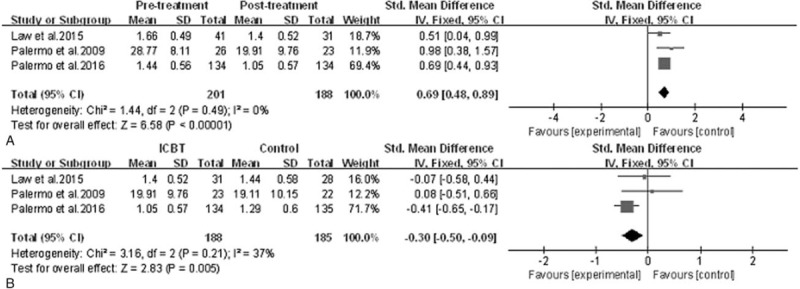
Efficacy of ICBT for children and adolescents with chronic pain: parental protective behaviors. A, Pretreatment versus post-treatment of ICBT, the mean difference was 0.69 (95% CI: 0.48–0.89), I2 = 0%, P < .00001. B, ICBT versus the control treatment at post-treatment, the mean difference was −0.30 (95% CI: −0.50 to −0.09), I2 = 37%, P = .005. CI = confidence interval, ICBT = Internet-delivered cognitive-behavioral therapy, SD = standard deviation.
3.2.5. Parental protective behaviors
Figure 7 summarizes the results of parental protective behaviors in the 3 pooled studies,[33,36,37] it showed ICBT helped to reduce maladaptive parent behaviors significantly (SMD = 0.69, 95% CI: 0.48–0.89, P < .00001, I2 = 0%) from baseline to post-treatment and baseline to follow-up, and parents in the ICBT group reported a significantly greater reduction in their protective behaviors than that of the control group after treatment (SMD = −0.30, 95% CI: −0.5 to −0.09, P = .005, I2 = 37%). At follow-up, 1 trial[37] demonstrated that the efficacy of ICBT was much better than the control therapy, another one[36] showed that there was no statistically significant difference between groups, and the follow-up records of the last one were not sufficient to outline the long-term benefits of ICBT; therefore, there was no strong evidence to make a conclusion.
3.2.6. Treatment acceptability and satisfaction
Children and their parents in the 3 trials[33,36,37] all completed an adapted version of the Treatment Evaluation Inventory-Short Form (TEI-SF)[44,45] to evaluate their acceptability and satisfaction of the treatment program. The results of the 3 studies are very consistent; they revealed that adolescents and parents in the ICBT group were generally acceptable and satisfied with the intervention immediately after treatment, and 2 trials[36,37] clearly indicated that youth and parents rated the ICBT highly in acceptability and satisfaction at follow-up. Only Palermo et al[37] made a comparison of the 2 treatments; it showed that participants in the ICBT reported significantly higher acceptability and satisfaction for the intervention at 2 time points.
4. Discussion
To be the best of our knowledge, this is the first comprehensive systematic review and meta-analysis in the novel research field of ICBT for children and adolescents with chronic pain. In spite of an extensive search and scrutiny of >1000 articles, only 4 trials were identified meeting the relatively broad inclusion criteria, indicating the field is still in its infancy.
The important finding of this study was that patients receiving ICBT experienced significantly great reductions of activity limitations, anxiety symptoms and parental protective behaviors, moderate to large effects of depressive symptoms and sleep quality, and small to moderate relief of pain, compared to pretreatment. However, only the result of parental protective behaviors in the ICBT group was favored over the control group, whereas the rest of the results did not indicate a preference of the ICBT. Therefore even though the efficacy of ICBT is unquestionable, we still cannot assert that it has a significant advantage over the control group. Until now, based on the limited evidence, we can only prove that the therapeutic effect of ICBT is not worse than the control group, maybe even better considering other aspects such as economy and convenience, etc.
It is worth noting that, we should interpret the statistical result of anxiety cautiously, it showed there was significant change in anxiety favoring the ICBT post-treatment; however, the results of 2 included trials were contradictory. One[36] demonstrated there was insufficient evidence supporting an effect in the ICBT group at post-treatment, and between-group differences were also not statistically significant different at the time point. The other[37] revealed adolescents receiving ICBT reported a significant reduction in anxiety relative to the control group immediately after treatment. Even though statistical data indicated that ICBT was beneficial to anxiety symptoms, the small effect value may have led to overestimated effects.
In addition, in terms of sleep quality, although the evidence showed a benefit of sleep quality after treatment immediately in the ICBT group, it is a remarkable fact that the results of 2 trials[32,36] demonstrated that treatments in 2 groups did not contribute to changes in sleep quality immediately post-treatment, only one[37] reported sleep quality improvement after receiving ICBT. And the CI crossed zero, so there was insufficient evidence of an effect in either direction at post-treatment. Moreover, we should also treat the statistically significant difference in parental protective behaviors between groups at post-treatment with caution. Similar to sleep quality, only Palermo et al[37] supported the superiority of ICBT from baseline to post-treatment, another 2 studies[33,36] did not favor that. Thus the data in the 2 measures may be false positive due to the biggest weight of the sample in the multicenter trial,[37] its effect size was small and may not be clinically meaningful. On the contrary, perhaps the assessment tools used were not stable enough over time to adequately measure so that the outcomes deviated from the right direction. We need more evidence to resolve these contradictions.
As for treatment acceptability and satisfaction, all the 3 trials[33,36,37] demonstrated that children and their parents weindicatre highly acceptable and satisfied with ICBT. What is more, Palermo et al[37] reported that the participants preferred ICBT to the Internet-delivered Education treatment.
These results indicate that ICBT has the potential to be widely disseminated , and could fill the gap in treatment delivery for those youth with chronic pain who would benefit from CBT but are unable to receive this care due to cost, distance, or other barriers. Based on the positive effectiveness of ICBT and child compliance, ICBT are more likely to be accepted and well used in clinical practice in the future. Therefore, we believe that ICBT can be more widely applied in routine clinical treatment, and stepped care approaches to pain management may use this program to provide low-cost access to cognitive and behavioral skills training. We also considered methods to enhance the clinical effectiveness of ICBT such as conducting more standardized treatment training for doctors and strengthening the intensity of the online coaching.
There are several limitations in the study. First, only 4 studies could be included due to the emerging nature of this field, although the overall quality of the studies was high, we are still not confident in yielding strong conclusions. Second, there was considerable heterogeneity (I2) in the effect estimates of pain intensity and activity limitations, the sensitivity analyses indicated that the Palermo et al[33] study was the source of statistical heterogeneity, probably because the sample size was too small to be representative or other uncertain bias. Third, because of the limitation of extracted data, follow-up results could not be pooled in meta-analyses. We need further research and more outcome studies to address these limitations.
Despite these limitations, this review provides the first systematic exploration of the use of ICBT for pain in youth; it highlights the importance of the development of pediatric ICBT field. We have 2 major strengthens, the availability of large evidence base for various outcome domains (including pain intensity, activity limitations, emotional functioning, sleep quality, parental protective behaviors, and treatment acceptability and satisfaction) and rigorous methodology in regard to quality and bias of included articles. In addition, we used a thorough systematic review methodology to explore the efficacy of ICBT, which included the physiological status, psychological condition, and other aspects of life of adolescents with chronic pain and their parents.
5. Conclusion
This review suggests that, even if the research is limited, ICBT for the treatment of pediatric chronic pain appears a promising development. Just over 10 years passed, the field of ICBT has developed very rapidly from being a nonexisting treatment to a well-established therapy for chronic pain; it is as effective as conventional treatments for pediatric chronic pain, maybe even better. In addition, ICBT is often presented as a more flexible and time- and cost-saving method compared with the control therapies, which could finally contribute to a wide dissemination of evidence-based psychological pain treatment. From a social perspective, ICBT could ease the pressure on the patients and medical care; from a methodological perspective, we need more high-quality trials in this field to demonstrate our conclusion and address controversial analyses.
Author contributions
W-XT wrote the main manuscript, L-FZ and Y-QA searched databases and extracted data, and Z-SL checked and modified the manuscript.
Conceptualization: Zhi-Song Li, Wen-Xin Tang.
Funding acquisition: Lu-Feng Zhang.
Investigation: Yan-Qiu Ai.
Footnotes
Abbreviations: CBT = cognitive behavioral therapy, CI = confidence interval, ICBT = Internet-delivered cognitive-behavioral therapy, MD = mean difference, RCT = randomized controlled trial, SD = standard deviation, SMD = standardized mean difference.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
We declare that we do not have any competing financial interests in relation to the work described.
The author(s) of this work have nothing to disclose.
References
- [1].Manworren RC, Stinson J. Pediatric pain measurement, assessment, and evaluation. Semin Pediatr Neurol 2016;23:189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Stanford EA, Chambers CT, Biesanz JC, et al. The frequency, trajectories and predictors of adolescent recurrent pain: a population-based approach. Pain 2008;138:11–21. [DOI] [PubMed] [Google Scholar]
- [3].King S, Chambers CT, Huguet A, et al. The epidemiology of chronic pain in children and adolescents revisited: a systematic review. Pain 2011;152:2729–38. [DOI] [PubMed] [Google Scholar]
- [4].Huguet A, Miro J. The severity of chronic pediatric pain: an epidemiological study. J Pain 2008;9:226–36. [DOI] [PubMed] [Google Scholar]
- [5].Palermo TM. Impact of recurrent and chronic pain on child and family daily functioning: a critical review of the literature. J Dev Behav Pediatr 2000;21:58–69. [DOI] [PubMed] [Google Scholar]
- [6].Turk DC, Fillingim RB, Ohrbach R, et al. Assessment of psychosocial and functional impact of chronic pain. J Pain 2016;17(9 suppl):T21–49. [DOI] [PubMed] [Google Scholar]
- [7].Forgeron PA, King S, Stinson JN, et al. Social functioning and peer relationships in children and adolescents with chronic pain: a systematic review. Pain Res Manag 2010;15:27–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Groenewald CB, Essner BS, Wright D, et al. The economic costs of chronic pain among a cohort of treatment-seeking adolescents in the United States. J Pain 2014;15:925–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Sleed M, Eccleston C, Beecham J, et al. The economic impact of chronic pain in adolescence: methodological considerations and a preliminary costs-of-illness study. Pain 2005;119:183–90. [DOI] [PubMed] [Google Scholar]
- [10].Ho IK. Healthcare utilization and indirect burden among families of pediatric patients with chronic pain. J Musculoskelet Pain 2008;3:155–64. [Google Scholar]
- [11].Luntamo T, Sourander A, Santalahti P, et al. Prevalence changes of pain, sleep problems and fatigue among 8-year-old children: years 1989, 1999, and 2005. J Pediatr Psychol 2012;37:307–18. [DOI] [PubMed] [Google Scholar]
- [12].Hermann C, Kim M, Blanchard EB. Behavioral and prophylactic pharmacological intervention studies of pediatric migraine: an exploratory meta-analysis. Pain 1995;60:239–55. [DOI] [PubMed] [Google Scholar]
- [13].Eccleston C, Morley S, Williams A, et al. Systematic review of randomised controlled trials of psychological therapy for chronic pain in children and adolescents, with a subset meta-analysis of pain relief. Pain 2002;99:157–65. [DOI] [PubMed] [Google Scholar]
- [14].Eccleston C, Malleson PN, Clinch J, et al. Chronic pain in adolescents: evaluation of a programme of interdisciplinary cognitive behaviour therapy. Arch Dis Child 2003;88:881–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Rudy TE, Kerns RD, Turk DC. Chronic pain and depression: toward a cognitive-behavioral mediation model. Pain 1988;2:129–40. [DOI] [PubMed] [Google Scholar]
- [16].Eccleston C, Yorke L, Morley S, et al. Psychological Therapies for the Management of Chronic and Recurrent Pain in Children and Adolescents. New Jersey: John Wiley & Sons, Ltd; 2003. [DOI] [PubMed] [Google Scholar]
- [17].Stallard P, Udwin O, Goddard M, et al. The availability of cognitive behaviour therapy within specialist Child and Adolescent Mental Health Services (CAMHS): a national survey. Behav Cogn Psychother 2007;35:501. [Google Scholar]
- [18].Peng P, Stinson JN, Choiniere M, et al. Dedicated multidisciplinary pain management centres for children in Canada: the current status. Can J Anaesth 2007;54:985–91. [DOI] [PubMed] [Google Scholar]
- [19].Palermo TM. Gebhart GF, Schmidt RF. Remote management of pediatric pain. Encyclopedia of Pain. Berlin, Heidelberg: Springer Berlin Heidelberg; 2013. 3389–93. [Google Scholar]
- [20].Palermo TM, Jamison RN. Innovative delivery of pain management interventions: current trends and future progress. Clin J Pain 2015;31:467–9. [DOI] [PubMed] [Google Scholar]
- [21].Marks IM, Cavanagh K, Gega L. Computer-aided psychotherapy: revolution or bubble? Br J Psychiatry 2007;191:471–3. [DOI] [PubMed] [Google Scholar]
- [22].McGrath PJ, Humphreys P, Keene D, et al. The efficacy and efficiency of a self-administered treatment for adolescent migraine. Pain 1992;49:321–4. [DOI] [PubMed] [Google Scholar]
- [23].Hicks CL, Von Baeyer CL, McGrath PJ. Online psychological treatment for pediatric recurrent pain: a randomized evaluation. J Pediatr Psychol 2006;31:724–36. [DOI] [PubMed] [Google Scholar]
- [24].Connelly M, Rapoff MA, Thompson N, et al. Headstrong: a pilot study of a CD-ROM intervention for recurrent pediatric headache. J Pediatr Psychol 2006;31:737–47. [DOI] [PubMed] [Google Scholar]
- [25].McClellan CB, Schatz JC, Puffer E, et al. Use of handheld wireless technology for a home-based sickle cell pain management protocol. J Pediatr Psychol 2009;34:564–73. [DOI] [PubMed] [Google Scholar]
- [26].Wade SL, Carey J, Wolfe CR. An online family intervention to reduce parental distress following pediatric brain injury. J Consult Clin Psychol 2006;74:445–54. [DOI] [PubMed] [Google Scholar]
- [27].Hedman E, Ljótsson B, Lindefors N. Cognitive behavior therapy via the internet: a systematic review of applications, clinical efficacy and cost-effectiveness. Expert Rev Pharmacoecon Outcomes Res 2012;12:745–64. [DOI] [PubMed] [Google Scholar]
- [28].Office for National Statistics. Internet access—households and individuals: 2016. Home internet and social media usage. Available at: https://www.ons.gov.uk/peoplepopulationandcommunity/householdcharacteristics/homeinternetandsocialmediausage/bulletins/internetaccesshouseholdsandindividuals/2016. Accessed June 22, 2017. [Google Scholar]
- [29].Australian Bureau of Statistics. Household Use of Information Technology, Australia, 2014–15. Households With Internet Access at Home. Available at: http://www.abs.gov.au/ausstats/abs@.nsf/mf/8146.8140. Accessed June 22, 2017. [Google Scholar]
- [30].Lenhart A. Teens, Social Media and Technology Overview 2015. Pew Reports. Available at: http://www.pewinternet.org/2015/2004/2009/teens-social-media-technology-2015/. Accessed June 22, 2017. [Google Scholar]
- [31].Horrigan JB. Home Broadband 2015. Pew Reports. Available at: http://www.pewinternet.org/2015/2012/2021/home-broadband-2015/. Accessed June 22, 2017. [Google Scholar]
- [32].Fales J, Palermo TM, Law EF, et al. Sleep outcomes in youth with chronic pain participating in a randomized controlled trial of online cognitive-behavioral therapy for pain management. Behav Sleep Med 2015;13:107–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Palermo TM, Wilson AC, Peters M, et al. Randomized controlled trial of an Internet-delivered family cognitive-behavioral therapy intervention for children and adolescents with chronic pain. Pain 2009;146:205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Han TW, Jan LY. Making antisense of pain. Nat Neurosci 2013;16:986–7. [DOI] [PubMed] [Google Scholar]
- [35].Egger M, Smith GD, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Law EF, Beals-Erickson SE, Noel M, et al. Pilot randomized controlled trial of internet-delivered cognitive-behavioral treatment for pediatric headache. Headache 2015;55:1410–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Palermo TM, Law EF, Fales J, et al. Internet-delivered cognitive-behavioral treatment for adolescents with chronic pain and their parents: a randomized controlled multicenter trial. Pain 2016;157:174–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Baeyer CLV. Numerical rating scale for self-report of pain intensity in children and adolescents: recent progress and further questions. Eur J Pain 2009;13:1005–7. [DOI] [PubMed] [Google Scholar]
- [39].Palermo TM, Witherspoon D, Valenzuela D, et al. Development and validation of the Child Activity Limitations Interview: a measure of pain-related functional impairment in school-age children and adolescents. J Pain 2004;109:461–70. [DOI] [PubMed] [Google Scholar]
- [40].Chorpita BF, Moffitt CE, Gray J. Psychometric properties of the Revised Child Anxiety and Depression Scale in a clinical sample. J Behav Res Ther 2005;43:309–22. [DOI] [PubMed] [Google Scholar]
- [41].Kovacs M. The Children's Depression Inventory (CDI) manual. Multi-Health Systems 1992. [Google Scholar]
- [42].Eccleston C, Jordan A, McCracken LM, et al. The Bath Adolescent Pain Questionnaire (BAPQ): development and preliminary psychometric evaluation of an instrument to assess the impact of chronic pain on adolescents. J Pain 2005;118:263–70. [DOI] [PubMed] [Google Scholar]
- [43].Reynolds C, Richmond B. Revised Children's Manifest Anxiety Scale, Second Edition (RCMAS-2): Manual. Western Psychological Services Torrance, CA 2008. [Google Scholar]
- [44].LeBourgeois MK, Giannotti F, Cortesi F, et al. The relationship between reported sleep quality and sleep hygiene in Italian and American adolescents. J Pediatrics 2005;115(1 suppl):257–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Van Slyke DA, Walker LS. Mothers’ responses to children's pain. Clin J Pain 2006;22:387–91. [DOI] [PubMed] [Google Scholar]
- [46].Kazdin AE. Acceptability of alternative treatments for deviant child behavior. J Appl Behav Anal 1980;13:259–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Kelley ML, Heffer RW, Gresham FM, et al. Development of a modified treatment evaluation inventory. J Psychopathol Behav Assess 1989;11:235–47. [Google Scholar]


