Abstract
Background
We hypothesized that immunomodulatory properties of mesenchymal stromal cells (MSC) may be considered for desensitization.
Methods
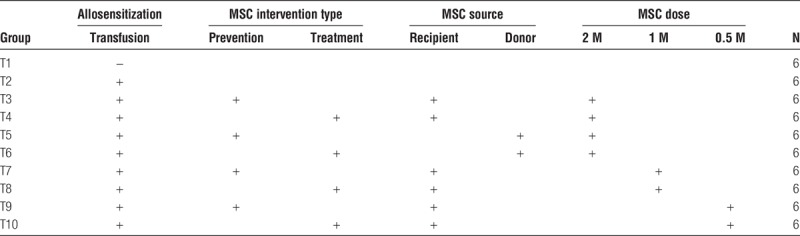
Autologous or allogeneic bone marrow derived MSC were infused via tail vein at 0.5 M (0.5 × 106), 1 M, or 2 M cells/dose on days −2, 3, 6, 9, 12 (prevention) or 14, 17, 20, 23, 26 (treatment) relative to transfusion in a Brown Norway to Lewis rat model (10 groups total, n = 6 per group).
Results
At 4 weeks, pooled analyses demonstrated that autologous and allogeneic MSC were equally effective in reducing IgG1 and IgG2a de novo donor-specific antibody (dnDSA, P < 0.001). Dose-response studies indicated that moderate-dose MSC (5 M total) was most effective in reducing IgG1, IgG2a, and IgG2c dnDSA (P ≤ 0.01). Time course studies determined that preventive and treatment strategies were equally effective in reducing IgG1 and IgG2a dnDSA (P ≤ 0.01). However, individual group analyses determined that moderate-dose (5 M) treatment with autologous MSC was most effective in reducing IgG1, IgG2a, and IgG2c dnDSA (P ≤ 0.01). In this group, dnDSA decreased after 1 week of treatment; regulatory B cells increased in the spleen and peripheral blood mononuclear cells; and transitional B cells increased in the spleen, peripheral blood mononuclear cells, and bone marrow (P < 0.05 for all).
Conclusions
Our findings indicate that autologous MSC prevent transfusion-elicited sensitization and upregulate transitional, and regulatory B cells. Additional studies are needed to determine the biological relevance of these changes after kidney transplantation.
Alloantibodies (anti-HLA antibodies) arise through previous transplants, blood transfusions, and pregnancy. Currently, 39% of patients on the active kidney transplant waitlist are sensitized, evidenced by a panel-reactive antibody (PRA) ≥ 1%.1 Of these, nearly 15 000 are highly sensitized, which means that they have a PRA ≥ 80%.1 Transplant rates vary by PRA, ranging from 143.0 per 100 active waitlist years for candidates with a PRA of less than 1% to only 6.9 for those with a PRA of 98% or higher.1 Median waiting time for kidney transplantation in highly sensitized patients approaches 12 years, which is more than 3 times than that for nonsensitized patients.1 As a result, a significant number of highly sensitized patients die before receiving a transplant, outlining the critical importance of desensitization strategies.
The 2 approaches for helping highly sensitized patients are: (1) to increase the chance of finding a crossmatch negative donor, or (2) to remove the preexisting antibodies using desensitization protocols.2-8 Emerging evidence suggests that strategies to improve transplant rates in highly sensitized patients enhance survival rates and the quality of life while reducing costs compared to chronic dialysis.9,10 Current desensitization protocols include Rituximab (anti-CD20 monoclonal antibody) to deplete B cells, plasmapheresis plus intravenous immunoglobulins (IVIG) to block or remove preformed donor-specific antibody (DSA),2-6 proteasome inhibitors to inhibit plasma cell activity,8 and IgG endopeptidase to cleave immunoglobulins.7 However, despite some success, these protocols are limited by their toxicity, inefficacy, and/or inability to desensitize 30% to 90% of patients.3,11,12 It is therefore important to define safe and effective strategies to reduce alloantibody in highly sensitized patients.
The immunomodulatory properties of bone marrow-derived mesenchymal stromal cells (MSC) have been recognized for a decade.13-21 Mesenchymal stromal cells suppress T-cell proliferation13,14,16,17,19,21-25 and dendritic cell differentiation,13,15-18,25,26 and modulate B-cell functions.13,17,19,22,27-29 In experimental models, MSC can improve skin,30 heart,18,21 and kidney transplant outcomes.14,16,31,32 Clinical trials of MSC therapy17,19,20,33-36 indicate that therapy can be used safely if administered prior to transplant and/or combined with adequate immunosuppression to avoid allosensitization. We hypothesized that the immunomodulatory properties of MSC may be considered for desensitization strategies. We tested this hypothesis in an experimental model of sensitization developed in our laboratory where Lewis rats (RT1l) are sensitized by blood transfusion from Brown Norway (BN) rats (RT1n).37,38 Autologous or allogeneic bone marrow derived MSC were infused at different doses in preventive or therapeutic strategies. Additional studies were conducted to assess DSA generation and B-cell responses to MSC infusion.
MATERIALS AND METHODS
Study Design and Intervention Groups
Adult (200-250 g) male Lewis and BN rats were purchased from Envigo and housed in the animal care facility at the University of Wisconsin in Madison, WI. All procedures were performed in accordance with the Animal Care and Use Policies at the University of Wisconsin as described previously.39-41 To create a clinically relevant sensitization model, Lewis rats received 500 μL of heparinized blood via the tail vein from BN rats on day 0 as described previously38 (groups T2-10, Figure 1, Table 1). To determine the effect of syngeneic versus allogeneic MSC infusions, Lewis or BN bone marrow derived MSC at passage 3 were delivered via the tail vein of Lewis rats. To determine the benefits of early versus late treatment we conducted time course studies using infusions on days −2,3,6,9,12 (prevention groups) or 14,17,20,23,26 (treatment groups) relative to transfusion (5 dose total). To understand the effect of MSC dose, we performed dose-response studies at 0.5, 1, or 2 × 106 cells/dose. There were a total of 10 groups total (n = 6 per group). Blood, spleen, and bone marrow were harvested 4 weeks after transfusion (Figure 1, Table 1). The 4-week timeframe was used based on our previously established sensitization model demonstrating peak DSA levels 3 to 4 weeks after transfusion.37,38
FIGURE 1.
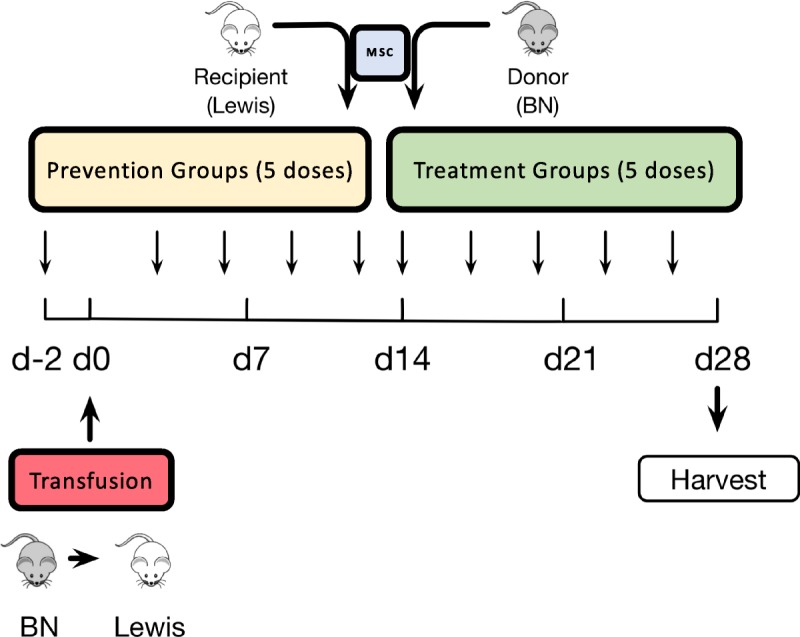
Study design. Sensitization experiments we conducted by transfusing 500 μL of heparinized blood from BN rats in Lewis recipients on day 0 (groups T2-T10). Two types of intervention were then defined based on whether syngeneic or allogeneic MSC were delivered to Lewis rats before (“prevention” groups) or after (“treatment” groups) sensitization. Lewis Rats in Prevention groups received tail vein injections of MSC from Lewis or BN at 2 M (T3, T5), 1 M (T7) or 0.5 M (T9) per injection on days −2, 3, 6, 9, 12 (5 total doses). Rats in Treatment groups (T4, T6, T8, T10) received the same treatment on days 14, 17, 20, 23, 26. Group T1 was composed of untreated Lewis rats (no transfusion, no MSC), while group T2 was composed of only sensitized Lewis rats. Animals were randomized into each treatment groups and remained in protocol for 4 weeks after transfusion. There were 10 groups total (n = 6 per group).
TABLE 1.
Experimental groups
MSC Isolation and Culture
Mesenchymal stromal cells were isolated from the bone marrow of Lewis or BN rats similar to the methods of Galipeau.42-45 Briefly, 6- to 8-week-old rats were euthanized by CO2, and the muscle was dissected from the femurs and tibiae immediately, leaving isolated bone. The tips of the bones were shaved off with a rongeur. Each bone was flushed with 10 ml of Dulbecco's modified eagle's medium +20% fetal bovine serum (FBS) (10-017; Corning, Manassas, VA; 35-016-CV, Corning). Then marrow cavity was flushed 10 times with an 18-gauge needle, and the isolated cells were filtered through a 40 μm nylon cell strainer (BD Falcon, Bedford, MA). The cell suspension was centrifuged for 5 minutes at 450g. Cells were counted and plated in T150 flask (Corning) at 106 cells/cm2 and incubated at 37°C 5% CO2 5% O2 in 25-mL StemXVivo media (CCM004; R&D Systems, Minneapolis, MN). After 24 hours, all media were removed, cells gently washed with 1× DPBS and refed with fresh media. Media were replaced every 72 hours for another 10 to 14 days. Cells were split by 0.05% trypsin ethylene diamine tetra acetic acid (SH30236.01; HyClone, Logan, UT), and seeded at 104 cells/cm2 for subsequent passages. A single large batch of cells were harvested after passage 2 and frozen in Cryostor CS5 (07933; Stem cell Technologies, Vancouver, Canada) in liquid nitrogen. Five days before injection into the tail vein, aliquots of cells were recovered and plated in flask with Dulbecco's modified eagle's medium +20% FBS and incubated at 37°C 5% CO2 5%O2. Media was changed 24 and 72 hours later. Cells at passage 3 were harvested using Trypsin on the day of injection, centrifuged for 5 minutes at 450g, counted, and washed twice with 1× DPBS. Then cells were suspended in 0.5 mL of DPBS, counted, and injected into the animal immediately. Mesenchymal stromal cells were phenotyped before the first and third infusions.
DSA Analysis
Rat DSA measurement was performed as described previously.38,46 Briefly, donor (BN) splenocytes were freshly isolated from spleen pushed through a 40-μm filter. After RBC lysis, cells were resuspended, counted and 500 000 cells were aliquoted into cluster tubes. Fifty microliters of a 1:16 dilution of plasma from the time point of interest were incubated on cells for 30 minutes at 37°C, then washed, and cells were stained. Flow cytometry was performed on a BD LSR II or BD Fortessa at the UWCCC Flow Cytometry Core, UW-Madison and data analyzed using FlowJo (TreeStar, Inc., Ashland, OR). Mean fluorescence intensity was determined for the singlet, lymphocyte-gated, CD3-positive cell population. Antibodies used were anti-IgG1 FITC (clone RG11/39.4; BD Biosciences, San Diego, CA), IgG2a FITC (clone RG7/1.30; BD Biosciences), IgG2b PE (clone RG7/11.1; BD Biosciences), IgG2c (biotinylated, clone A92-1; BD Biosciences) with Streptavidin Pacific Blue (S11222; Invitrogen, Waltham, MA), IgM PE (clone G53-238; BD Biosciences), CD3 A647 (clone 1F4; BioLegend, San Diego, CA), and CD45R (clone RA3-6B2; eBioscience, Waltham, MA).
Flow Cytometry Analysis for Cell Subsets
For MSC phenotype determination, passage 3 cells were split with 0.05% trypsin, centrifuged for 5 minutes 450g, washed twice with fluorescence-activated cell sorting buffer (1× PBS + 2% FBS + 1% w/v BSA), counted and suspended 500 000 cells in 100-μL fluorescence-activated cell sorting buffer. Surface antibodies used were CD45 APC (clone REA504; Miltenyi Biotec, Auburn, CA), CD11b A700 (clone M1/70; Biolegend, San Diego, CA), CD73 (clone 5F/B9; BD Bioscience) with antimouse IgG1 BV421 (clone RMG1-1; Biolegend), CD90 PE-Cy7 (clone OX-7; BD Bioscience), CD105 PE (clone MEM-226; Sysmex, Mundelein, IL). Cells were gated for singlets, lymphocytes, then CD45−, CD11b−, CD73+, CD90+, CD105+.
For lymphocyte analysis, splenocytes were isolated and aliquoted as above. For BM samples, the marrow cavities of femur and tibia were flushed 3 to 5 times with an 18-gauge needle with RPMI (15-040-CVR; Corning) + 10% FBS for bone marrow cells. For B-cell (except regulatory B [Breg] cell) stains, the antibodies used were CD45R PE-Cy7 (clone RA3-6B2; eBioscience), IgM FITC (clone G53-238, BD bioscience), IgD biotinylated (MCA190B; Bio-Rad, Hercules, CA) with Streptavidin PE-CF594 (562284, BD bioscience), CD24 APC (clone REA405; Miltenyi Biotec), CD27 BV605 (clone LG.3A10; BD Bioscience), CD38 PE (clone 14.27; BioLegend), CD138 PerCP (clone B-A38; Abcam, Cambridge, MA) and a live/dead discriminator (Ghost Dye 780; Tonbo bioscience, San Diego, CA). Regulatory B cell stains were CD24 PE (clone HIS50; BD Bioscience), CD27 BV605, CD45RA PE-Cy5 (clone OX-33; BD Bioscience), IL-10 A647 (clone A5-4; BD Bioscience), and live/dead discriminator. B-cell subpopulation were surface stained, and gated for singlets, lymphocytes, and live cells as above, then separated into subsets by different markers: follicular B cells (CD45R + IgM-IgD + CD38+), plasmablasts (IgM-CD45R-CD138+), and transitional B cells (IgD + CD45R + CD24 + CD38+). Regulatory B cells were incubated with 2-μL leukocyte activation cocktail (BD bioscience) in 1 ml RPMI +10% FBS for 4 hours and performed surface staining, fixation and permeabilization using FIX & PERM kit (GAS003; Invitrogen), then performed IL-10 staining at 4°C overnight protected from light. Regulatory B cells were gated as above for singles, lymphocytes and live cells, then gated by CD24+CD27+CD45RA+IL10+.
Statistical Analysis
One-way analysis of variance was used to compare between multiple groups simultaneously. Mann-Whitney or Student t tests were used to compare nonparametric and parametric continuous data between 2 groups when appropriate. P values of 0.05 or less were considered significant. The 2018 MedCalc Software was used for statistical analyses.
RESULTS
Characterization of Rat MSC
We used flow cytometry to ensure the characterization of MSC at passage 3 (Figure 2). Mesenchymal stromal cells were defined by the CD45− CD11b− CD73+ CD90+ CD105+ immunophenotype, consistent with previous investigations in mice and humans.47-49 Isotype control cells (Figure 2A) were negative for CD73, CD90, or CD105, whereas Lewis (Figure 2B) and BN (Figure 2C) derived MSC at passage 3 were positive for CD73, CD90, CD105, and negative for CD45, CD11b, consistent with the phenotype of bone marrow–derived MSC. We also determined that the MSC were able to develop into adipocytes, osteocytes, and chondrocytes, confirming their undifferentiated state (data not shown). Sufficient numbers of MSC were frozen back to use the same batch for the entire experiment.
FIGURE 2.
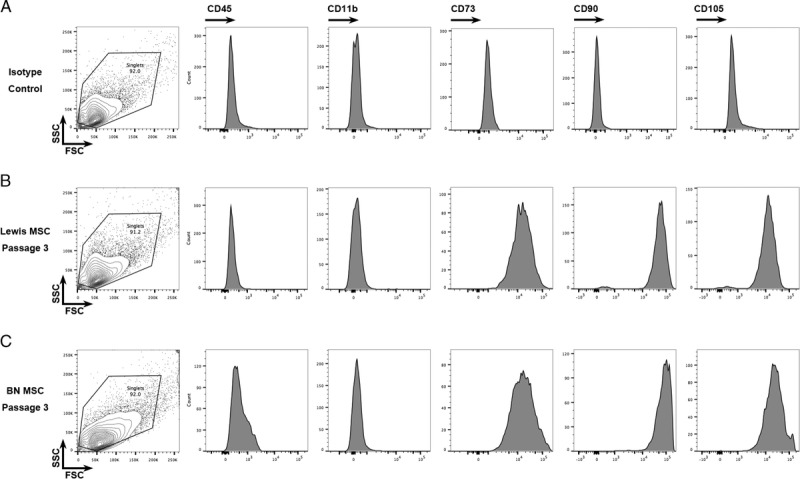
Isolated MSCs had the CD45-CD11b-CD73 + CD90 + CD105+ phenotype. MSC isolated from bone marrow of Lewis (B) or BN (C) rats were phenotyped by flow cytometry at passage 3. MSC were defined as CD45−, CD11b−, CD73+, CD90+, CD105+. MSC were purified >90% at passage 3.
Autologous and Allogeneic MSC Were Equally Effective in Reducing De Novo DSA
To determine the effect of autologous versus allogeneic MSC on de novo DSA (dnDSA), we compared IgG1, IgG2a, IgG2b, IgG2c, and IgM dnDSA levels in animals receiving Lewis-to-Lewis or BN-to-Lewis MSC (Figure 3). We first determined that all DSA isotypes increased significantly after blood transfusion (P < 0.001 for all). Notably, there was no difference in DSA levels based of the source of MSC in pooled analyses; infusion of either autologous or allogeneic MSC significantly reduced IgG1 and IgG2a dnDSA compared to sensitized animals (Figures 3A, B), whereas there was no significant effect on IgG2c or IgM dnDSA (Figures 3C, D). Mesenchymal stromal cells had no significant effect in IgG2b (data not shown). Together, these findings indicate that autologous and allogeneic MSC are equally effective in reducing dnDSA.
FIGURE 3.
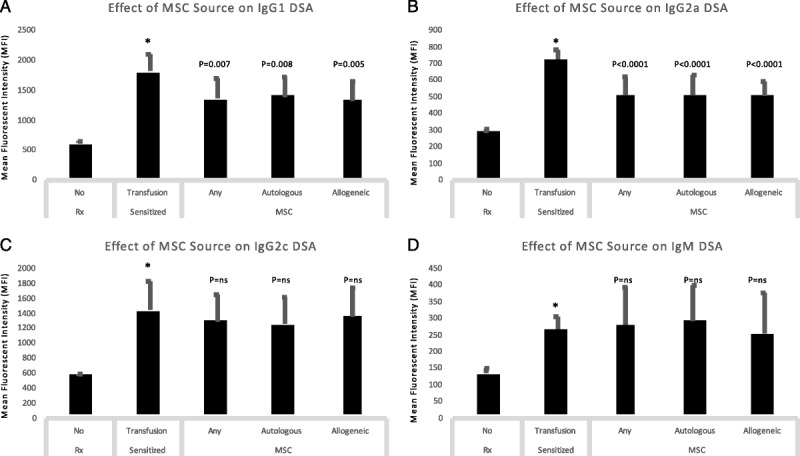
Autologous and allogeneic MSC were equally effective in reducing dnDSA. We compared IgG1, IgG2a, IgG2c, and IgM dnDSA levels in animals receiving Lewis-to-Lewis or BN-to-Lewis MSC. We first determined that all DSA isotypes increased significantly after blood transfusion (P < 0.001 for all). Notably, there was no difference in DSA levels based of the source of MSC in pooled analyses; infusion of either autologous or allogeneic MSC significantly reduced IgG1 and IgG2a dnDSA compared with sensitized animals (A, B), while there was no significant effect on IgG2c or IgM dnDSA (C, D).
Dose Response Studies: Moderate-Dose MSC (1 M Cells/Infusion) Was Most Effective in Reducing dnDSA
To determine which dose of MSC was most effective in reducing dnDSA, we conducted dose-response analyses comparing all animals receiving 0.5 M (0.5 × 106), 1 M (1 × 106) or 2 M (2 × 106) cells/infusion (Figure 4). We noted that compared to sensitized rats, the 1 M cells/dose (5 M cells total) significantly reduced IgG1, IgG2a and IgG2c dnDSA (Figures 4A, B, C). The 0.5 M dose significantly reduced IgG2a dnDSA, but no other isotypes (Figure 4B). The 2 M dose significantly reduced both IgG1 and IgG2a dnDSA but not the other isotypes (Figures 4A, B). IgM and IgG2b dnDSA were not significantly reduced by any of the 3 doses (Figure 4D, data not shown for IgG2b). In summary, moderate-dose MSC (5 M cells total) reduced dnDSA more effectively than either high-dose (10 M cells) or low-dose (2.5 M cells) MSC.
FIGURE 4.
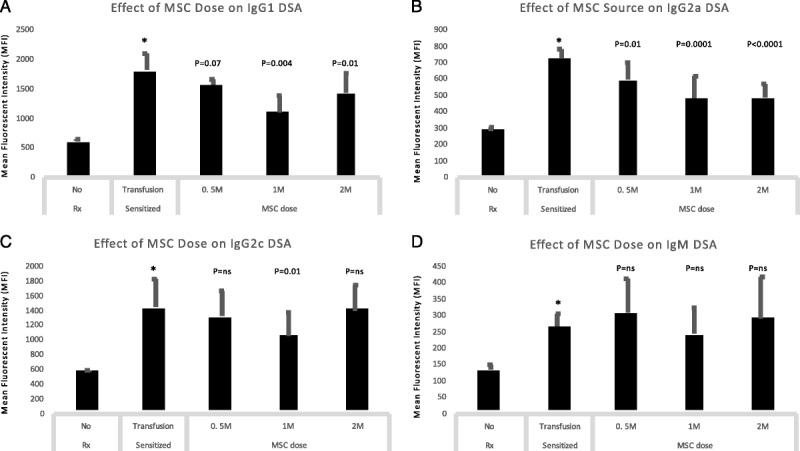
Dose-response studies: moderate-dose MSC (1 M cells/infusion) was most effective in reducing dnDSA. We conducted dose-response analyses comparing all animals receiving 0.5 M (0.5 × 106), 1 M (1 × 106) or 2 M (2 × 106) cells/infusion. We noted that compared to sensitized rats, the 1 M cells/dose (5 M cells total) significantly reduced IgG1, IgG2a and IgG2c dnDSA (A,B,C). The 0.5 M dose significantly reduced IgG2a dnDSA, but no other isotypes (B). The 2 M dose significantly reduced both IgG1 and IgG2a dnDSA but not the other isotypes (A,B). IgM dnDSA were not significantly reduced by any of the 3 doses (D).
Time Course Studies: MSC Prevention and Treatment Were Equally Effective in Reducing dnDSA
To determine the effect of early vs. late therapy we compared DSA levels between sensitized animals and those in Prevention and Treatment groups (Figure 5). We noted a significant decrease in IgG1 and IgG2a DSA with both early and late treatment strategies (Figures 5A, B) and no significant effect on either IgG2c, IgG2b, or IgM isotypes (Figures 5C, D, data not shown for IgG2b). These data suggest that in aggregate, prevention, and treatment strategies are equally effective in reducing dnDSA.
FIGURE 5.
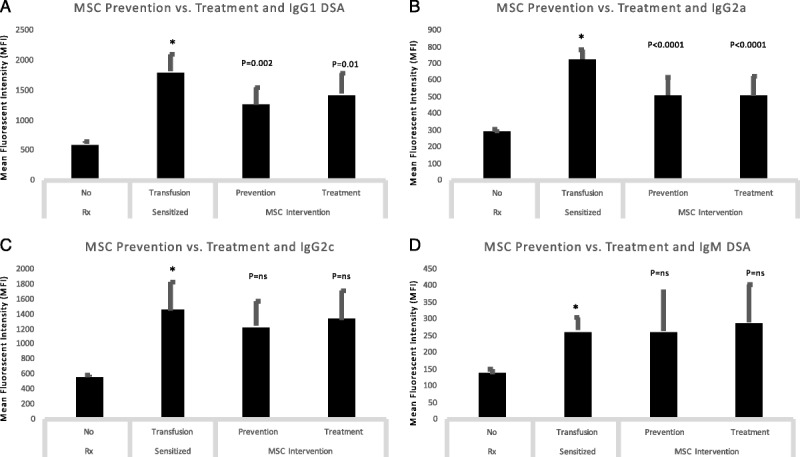
Time course studies: MSC prevention and treatment were equally effective in reducing dnDSA. We compared DSA levels between sensitized animals and those in Prevention and Treatment groups. We noted a significant decrease in IgG1 and IgG2a DSA with both early and late treatment strategies (A, B) and no significant effect on either IgG2c or IgM isotypes (C, D).
Individual Group Analyses: The Therapeutic Strategy With Moderate-dose Autologous MSC Decreased dnDSA Most Significantly
To determine the optimal specific approach to desensitization we considered individual treatment response in all separate groups (Figure 6). We observed that late therapeutic strategy with moderate-dose (5 M cells) autologous MSC was associated with the most significant decrease in DSA across 3 isotypes (group T8, Figure 6). IgG1, IgG2a, and IgG2c DSA were most strongly affected by MSC immunomodulation in this group (Figures 6A, B, C). No significant downregulation was seen for IgM DSA (Figure 6D). Although most strategies downregulated IgG2a DSA (Figure 3B), only T5 (10 M allogeneic MSC—preventive infusion schedule), T7 (5 M autologous MSC—preventive infusion schedule), and T8 (5 M autologous MSC—therapeutic infusion schedule) groups showed downregulation of IgG1 (Figure 3A), and T8 was associated with a decline in IgG2c isotype (Figure 3C). As a result, we selected T8 (5 M autologous MSC - therapeutic infusion schedule) as the most effective strategy and focused our mechanistic studies on this group.
FIGURE 6.
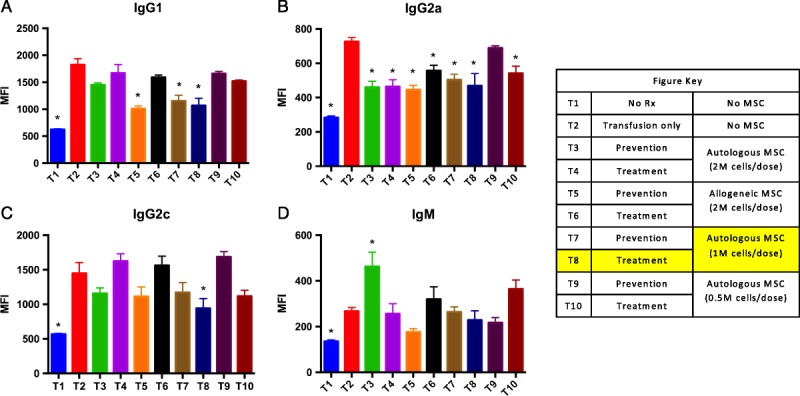
Individual group analyses: The therapeutic strategy with moderate-dose autologous MSC decreased dnDSA most significantly. We considered individual treatment response in all separate groups. We observed that late therapeutic strategy with moderate-dose (5 M cells) autologous MSC was associated with the most significant decrease in DSA across 3 isotypes (group T8). IgG1, IgG2a, and IgG2c DSA were most strongly affected by MSC immunomodulation in this group (A, B, C). No significant downregulation was seen for IgM DSA (D). Although most strategies downregulated IgG2a DSA (B), only T5 (10 M allogeneic MSC-preventive infusion schedule), T7 (5 M autologous MSC−preventive infusion schedule), and T8 (5 M autologous MSC−therapeutic infusion schedule) groups showed downregulation of IgG1 (A), and T8 was associated with a decline in IgG2c isotype (C).
MSC Treatment Resulted in Significant Decreases in DSA As Soon as 1 Week After Treatment Initiation
To assess the time course of treatment effect, we tested DSA levels at baseline, day 14, day 21, and day 28 in sensitized and treatment groups (T2 and T8, respectively, Figure 7). In the treatment group (T8), the first MSC infusion was delivered on day 14. One week later (day 21), a significant (P < 0.05) drop in IgG1 DSA was evident (Figure 7A). IgG2a and IgG2c isotypes had significant (P < 0.05) decreases by 2 weeks after initiation of MSC therapy (Figures 7B, C). These studies indicate that autologous MSC therapy has a relatively swift effect on DSA, starting at 1 week after the delivery of the first dose.
FIGURE 7.
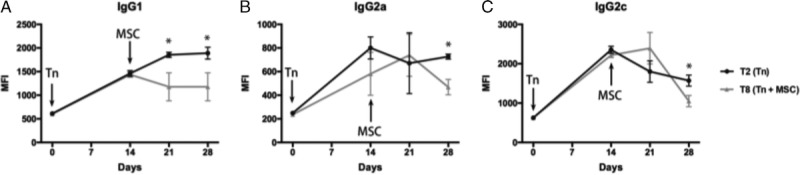
MSC treatment resulted in significant decreases in DSA as soon as 1 week after treatment initiation. We tested DSA levels at baseline, day 14, day 21, and day 28 in sensitized and treatment groups (T2 and T8, respectively). In the treatment group (T8), the first MSC infusion was delivered on day 14. One week later (day 21), a significant (P < 0.05) drop in IgG1 DSA was evident (A). IgG2a and IgG2c isotypes had significant (P < 0.05) decreases by 2 weeks after initiation of MSC therapy (B, C).
MSC Therapy Inhibited Splenic Follicular B Cells and Plasmablasts While Upregulating Transitional and Breg Cells
To better understand the pathogenesis of DSA generation in this model, we examined key B-cell phenotypes in the spleen, bone marrow, and PBMC on day 28 (Figure 8). We noted that follicular B cells (CD45R+IgM-IgD+CD38+) and plasmablasts (IgM-CD45R−CD138+) were significantly reduced in the spleen, while transitional B cells (IgD+CD45R+CD24+CD38+) and Breg cells (CD24+CD27+CD45RA+IL10+) were upregulated (Figure 8A). These changes were not significant in the bone marrow, except for transitional B cells (Figure 8B). Total B cell IL-10 expression followed changes consistent with Breg cells (Figures 8A-C).
FIGURE 8.
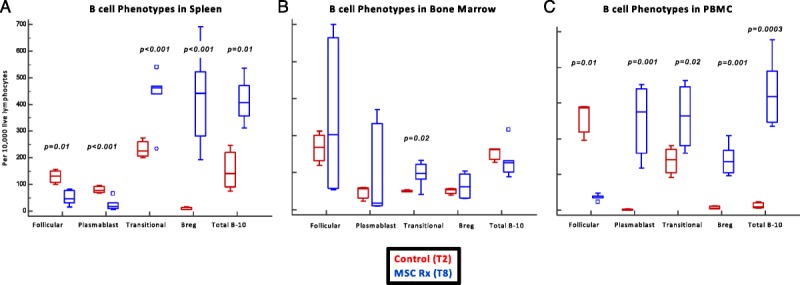
MSC therapy inhibited splenic follicular B cells and plasma cells, while upregulating transitional and regulatory B cells. We examined key B cell phenotypes in the spleen and bone marrow on day 28. We noted that follicular B cells (CD45R+IgM-IgD+CD38+) and plasma cells (IgM-CD45R-CD138+) were significantly reduced in the spleen, while transitional B cells (IgD+CD45R+CD24+CD38+) and Breg cell (CD24+CD27+CD45RA+IL10+) were upregulated (A). These changes were not significant in the bone marrow, except for transitional B cells (B).
DISCUSSION
Current desensitization strategies in kidney transplantation are limited by safety, efficacy, and cost.2-8 We hypothesized that immunomodulatory properties of MSC may be considered as part of desensitization strategies in high immunological risk kidney transplant candidates. We tested this hypothesis in a full major histocompatibility complex mismatch model where Lewis rats were sensitized by blood transfusion from BN donors. We conducted studies to determine the optimal source (autologous or allogeneic), dose, and timing of therapy. We determined that the treatment strategy with a total dose of 5 M autologous MSC was the most effective approach in reducing IgG1, IgG2a, and IgG2c dnDSA. Importantly, MSC significantly increased B regulatory cells as they inhibited follicular B cells and plasma cells in the spleen. In parallel, transitional B cells were increased in both the spleen and bone marrow. Taken together, our findings suggest that autologous MSC inhibit dnDSA generation in a transfusion elicited sensitization model. While the biological relevance of this approach remains unknown, we provide a proof of concept for safe and effective strategies to reduce DSA in sensitized kidney transplant candidates.
The interaction between MSC and B cells/plasma cells is extremely complicated and is orchestrated in time and space by different humoral and cellular factors including hematopoietic cells, T cells, and dendritic cells.50 This may explain the controversies noted in some studies examining the effect of MSC on B cells.51 Rasmusson et al52 demonstrated that under strong viral or LPS stimulation, BM-MSC reduced IgG production by B cells, while under low stimulation they increased IgG production. In these coculture studies, the effect on enriched B cells was cell contact-dependent.52 Similarly, Comoli et al53 observed that BM-MSC inhibit DSA generation in mixed lymphocyte culture (MLC) assays. Additional studies by Corcione et al54 and Tabera et al28 indicated that BM-MSC inhibit B cell proliferation in the G0/G1 phase, and prevent B cell to plasma cell differentiation, and IgG, IgA, and IgM production via soluble factors generated by MSC,54 and via inhibition of the ERK and p38 MAPK pathways.28 The different results among the groups might be explained by the different starting B cell population (source, purity, and isolation method), the stimuli used to trigger B-cell differentiation and proliferation, and MSC:B cell ratio. Considering this important observation, our findings are consistent with reports by Corcione et al and Tabera et al.28,54
In rats, IgG1 and IgG2a have the highest concentrations in the serum.55-57 IgG1 and IgM have the highest antigen avidity, while IgG2a and IgG2b have the greatest affinity for complement fixation.57 In our study, MSC therapy was associated with a significant decline in IgG1, IgG2a, and IgG2c isotypes, but not IgM and IgG2b. This might be connected to the timing of therapy related to transfusion-elicited sensitization and the effect of MSC on terminal B cell maturation. Follicular B cells residing in the spleen can mature in memory B cells and plasma cells via a T cell–dependent pathway58,59; they are involved in the adaptive immune response and can result in the generation of all IgG isotypes.58,59 Conversely, transitional B cells are associated with protection from acute rejection even in patients developing dnDSA.60 We hypothesize that the effect of MSC on transitional and Breg cells may explain the inhibition of DSA in our studies.
In clinical transplantation, MSC therapy has been used in tolerance induction or immunosuppression minimization strategies.17,19,20,33,34,36,61,62 In the largest clinical trial to date, 156 patients were enrolled in a single-site, prospective, open-label, randomized study in China.20 Patients received autologous MSC (1-2 M/kg) at reperfusion and 2 weeks later. Fifty-three patients received standard-dose and 52 patients received low-dose CNIs (80% of standard); 51 patients in the control group received anti-IL-2 receptor antibody plus standard-dose CNIs. In this study, autologous MSC compared with anti-IL-2 receptor antibody induction therapy resulted in lower incidence of acute rejection, decreased risk of opportunistic infection, and better estimated renal function at 1 year.20 In general, treatment with MSC has been safe; main complications include engraftment syndrome with AKI in patients receiving MSC in the immediate posttransplant period, opportunistic infections, and allosensitization in patients receiving allogeneic or third party MSC.17 Pioneering studies from the Bergamo team have demonstrated that these complications may be addressed by adjusting the timing of therapy and adjusting concomitant immunosuppression.34,36 From a mechanistic perspective, treatment with autologous MSC is associated with upregulation of CD4+ CD25+ FOXP3+ Tregs and a B cell signature consistent with spontaneous and induced immune tolerance.33,36,61
Our study has several limitations. First, the statistically significant inhibition of DSA by MSC therapy does not imply biological relevance. Kidney transplant studies following desensitization need to be performed to demonstrate practical potential. Next, no unifying phenotypic marker exists for Breg cells either in humans or in murine models. In the absence of comprehensive functional studies, the inferences on the effect of MSC on B-cell phenotypes remain hypothetical. Considering these limitations, we propose a proof of concept suggesting that MSC may be used in desensitization protocols. Although previous studies have addressed the use of MSC in low-risk kidney transplant recipients, preclinical and clinical studies are needed to assess this approach, alone or in combination with immunosuppressive therapy in desensitization protocols.
Footnotes
Published online 27 August 2018.
Z.Z. and N.A.W. contributed equally to this article.
The authors declare no conflicts of interest.
Z.Z. was supported by a grant from the National Natural Science Foundation of China, 81670679.
UW CCC Flow Cytometry Shared instrumentation core, including the Shared Instrumentation grant 1S00OD018202-01 Special BD LSR Fortessa.
Z.Z. participated in the design, writing, performance of research, and data analysis. N.A.W. participated in the design, writing, performance of research, and data analysis. R.C. participated in the writing, performance of research, and data analysis. S.E.P. participated in the writing and data analysis. R.R.R. participated in the writing and data analysis. S.R.R. participated in the writing, performance of research, and data analysis. J.G. participated in the writing and data analysis. A.D. participated in the design, writing, and data analysis.
REFERENCES
- 1.Hart A, Smith JM, Skeans MA, et al. Kidney. Am J Transplant. 2016;16(Suppl 2):11–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jordan SC, Pescovitz MD. Presensitization: the problem and its management. Clin J Am Soc Nephrol. 2006;1:421–432. [DOI] [PubMed] [Google Scholar]
- 3.Stegall MD, Gloor J, Winters JL, et al. A comparison of plasmapheresis versus high-dose IVIG desensitization in renal allograft recipients with high levels of donor specific alloantibody. Am J Transplant. 2006;6:346–351. [DOI] [PubMed] [Google Scholar]
- 4.Montgomery RA, Zachary AA. Transplanting patients with a positive donor-specific crossmatch: a single center's perspective. Pediatr Transplant. 2004;8:535–542. [DOI] [PubMed] [Google Scholar]
- 5.Thielke JJ, West-Thielke PM, Herren HL, et al. Living donor kidney transplantation across positive crossmatch: the University of Illinois at Chicago experience. Transplantation. 2009;87:268–273. [DOI] [PubMed] [Google Scholar]
- 6.Magee CC, Felgueiras J, Tinckam K, et al. Renal transplantation in patients with positive lymphocytotoxicity crossmatches: one center's experience. Transplantation. 2008;86:96–103. [DOI] [PubMed] [Google Scholar]
- 7.Jordan SC, Lorant T, Choi J, et al. IgG Endopeptidase in highly sensitized patients undergoing transplantation. N Engl J Med. 2017;377:442–453. [DOI] [PubMed] [Google Scholar]
- 8.Woodle ES, Shields AR, Ejaz NS, et al. Prospective iterative trial of proteasome inhibitor-based desensitization. Am J Transplant. 2015;15:101–118. [DOI] [PubMed] [Google Scholar]
- 9.Orandi BJ, Luo X, Massie AB, et al. Survival benefit with kidney transplants from HLA-incompatible live donors. N Engl J Med. 2016;374:940–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Montgomery RA, Lonze BE, King KE, et al. Desensitization in HLA-incompatible kidney recipients and survival. N Engl J Med. 2011;365:318–326. [DOI] [PubMed] [Google Scholar]
- 11.Trivedi HL, Terasaki PI, Feroz A, et al. Abrogation of anti-HLA antibodies via proteasome inhibition. Transplantation. 2009;87:1555–1561. [DOI] [PubMed] [Google Scholar]
- 12.Marfo K, Ling M, Bao Y, et al. Lack of effect in desensitization with intravenous immunoglobulin and rituximab in highly sensitized patients. Transplantation. 2012;94:345–351. [DOI] [PubMed] [Google Scholar]
- 13.Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110:3499–3506. [DOI] [PubMed] [Google Scholar]
- 14.De Martino M, Zonta S, Rampino T, et al. Mesenchymal stem cells infusion prevents acute cellular rejection in rat kidney transplantation. Transplant Proc. 2010;42:1331–1335. [DOI] [PubMed] [Google Scholar]
- 15.Eggenhofer E, Popp FC, Mendicino M, et al. Heart grafts tolerized through third-party multipotent adult progenitor cells can be retransplanted to secondary hosts with no immunosuppression. Stem Cells Transl Med. 2013;2:595–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Franquesa M, Herrero E, Torras J, et al. Mesenchymal stem cell therapy prevents interstitial fibrosis and tubular atrophy in a rat kidney allograft model. Stem Cells Dev. 2012;21:3125–3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perico N, Casiraghi F, Remuzzi G. Clinical translation of mesenchymal stromal cell therapies in nephrology. J Am Soc Nephrol. 2018;29:362–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Popp FC, Eggenhofer E, Renner P, et al. Mesenchymal stem cells can induce long-term acceptance of solid organ allografts in synergy with low-dose mycophenolate. Transpl Immunol. 2008;20:55–60. [DOI] [PubMed] [Google Scholar]
- 19.Reinders ME, de Fijter JW, Roelofs H, et al. Autologous bone marrow-derived mesenchymal stromal cells for the treatment of allograft rejection after renal transplantation: results of a phase I study. Stem Cells Transl Med. 2013;2:107–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tan J, Wu W, Xu X, et al. Induction therapy with autologous mesenchymal stem cells in living-related kidney transplants: a randomized controlled trial. JAMA. 2012;307:1169–1177. [DOI] [PubMed] [Google Scholar]
- 21.Zhou HP, Yi DH, Yu SQ, et al. Administration of donor-derived mesenchymal stem cells can prolong the survival of rat cardiac allograft. Transplant Proc. 2006;38:3046–3051. [DOI] [PubMed] [Google Scholar]
- 22.Rosado MM, Bernardo ME, Scarsella M, et al. Inhibition of B-cell proliferation and antibody production by mesenchymal stromal cells is mediated by T cells. Stem Cells Dev. 2015;24:93–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rasmusson I, Ringdén O, Sundberg B, et al. Mesenchymal stem cells inhibit lymphocyte proliferation by mitogens and alloantigens by different mechanisms. Exp Cell Res. 2005;305:33–41. [DOI] [PubMed] [Google Scholar]
- 24.Glennie S, Soeiro I, Dyson PJ, et al. Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood. 2005;105:2821–2827. [DOI] [PubMed] [Google Scholar]
- 25.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–1822. [DOI] [PubMed] [Google Scholar]
- 26.Beyth S, Borovsky Z, Mevorach D, et al. Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T-cell unresponsiveness. Blood. 2005;105:2214–2219. [DOI] [PubMed] [Google Scholar]
- 27.Asari S, Itakura S, Ferreri K, et al. Mesenchymal stem cells suppress B-cell terminal differentiation. Exp Hematol. 2009;37:604–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tabera S, Perez-Simon JA, Diez-Campelo M, et al. The effect of mesenchymal stem cells on the viability, proliferation and differentiation of B-lymphocytes. Haematologica. 2008;93:1301–1309. [DOI] [PubMed] [Google Scholar]
- 29.Jiang XX, Zhang Y, Liu B, et al. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood. 2005;105:4120–4126. [DOI] [PubMed] [Google Scholar]
- 30.Bartholomew A, Sturgeon C, Siatskas M, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30:42–48. [DOI] [PubMed] [Google Scholar]
- 31.Casiraghi F, Azzollini N, Todeschini M, et al. Localization of mesenchymal stromal cells dictates their immune or proinflammatory effects in kidney transplantation. Am J Transplant. 2012;12:2373–2383. [DOI] [PubMed] [Google Scholar]
- 32.Koch M, Lehnhardt A, Hu X, et al. Isogeneic MSC application in a rat model of acute renal allograft rejection modulates immune response but does not prolong allograft survival. Transpl Immunol. 2013;29:43–50. [DOI] [PubMed] [Google Scholar]
- 33.Mudrabettu C, Kumar V, Rakha A, et al. Safety and efficacy of autologous mesenchymal stromal cells transplantation in patients undergoing living donor kidney transplantation: a pilot study. Nephrology (Carlton). 2015;20:25–33. [DOI] [PubMed] [Google Scholar]
- 34.Perico N, Casiraghi F, Introna M, et al. Autologous mesenchymal stromal cells and kidney transplantation: a pilot study of safety and clinical feasibility. Clin J Am Soc Nephrol. 2011;6:412–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reinders ME, Dreyer GJ, Bank JR, et al. Safety of allogeneic bone marrow derived mesenchymal stromal cell therapy in renal transplant recipients: the Neptune study. J Transl Med. 2015;13:344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perico N, Casiraghi F, Gotti E, et al. Mesenchymal stromal cells and kidney transplantation: pretransplant infusion protects from graft dysfunction while fostering immunoregulation. Transpl Int. 2013;26:867–878. [DOI] [PubMed] [Google Scholar]
- 37.Reese SR, Wilson NA, Huang G, et al. Calcineurin inhibitor minimization with Ixazomib, an investigational proteasome inhibitor, for the prevention of antibody mediated rejection in a preclinical model. Transplantation. 2015;99:1785–1795. [DOI] [PubMed] [Google Scholar]
- 38.Huang G, Wilson NA, Reese SR, et al. Characterization of transfusion-elicited acute antibody-mediated rejection in a rat model of kidney transplantation. Am J Transplant. 2014;14:1061–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Djamali A, Reese S, Oberley T, et al. Heat shock protein 27 in chronic allograft nephropathy: a local stress response. Transplantation. 2005;79:1645–1657. [DOI] [PubMed] [Google Scholar]
- 40.Djamali A, Reese S, Yracheta J, et al. Epithelial-to-mesenchymal transition and oxidative stress in chronic allograft nephropathy. Am J Transplant. 2005;5:500–509. [DOI] [PubMed] [Google Scholar]
- 41.Djamali A, Vidyasagar A, Adulla M, et al. Nox-2 is a modulator of Fibrogenesis in kidney allografts. Am J Transplant. 2009;9:74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chinnadurai R, Copland IB, Patel SR, et al. IDO-independent suppression of T cell effector function by IFN-γ-licensed human mesenchymal stromal cells. J Immunol. 2014;192:1491–1501. [DOI] [PubMed] [Google Scholar]
- 43.Francois M, Romieu-Mourez R, Li M, et al. Human MSC suppression correlates with cytokine induction of indoleamine 2,3-dioxygenase and bystander M2 macrophage differentiation. Mol Ther. 2012;20:187–195. [DOI] [PubMed] [Google Scholar]
- 44.Lewis HC, Chinnadurai R, Bosinger SE, et al. The IDO inhibitor 1-methyl tryptophan activates the aryl hydrocarbon receptor response in mesenchymal stromal cells. Oncotarget. 2017;8:91914–91927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pennati A, Ng S, Wu Y, et al. Regulatory B cells induce formation of IL-10-expressing T cells in mice with autoimmune neuroinflammation. J Neurosci. 2016;36:12598–12610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sicard A, Phares TW, Yu H, et al. The spleen is the major source of antidonor antibody-secreting cells in murine heart allograft recipients. Am J Transplant. 2012;12:1708–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. [DOI] [PubMed] [Google Scholar]
- 48.Guess AJ, Daneault B, Wang R, et al. Safety profile of good manufacturing practice manufactured interferon γ-primed Mesenchymal stem/stromal cells for clinical trials. Stem Cells Transl Med. 2017;6:1868–1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wuchter P, Bieback K, Schrezenmeier H, et al. Standardization of good manufacturing practice-compliant production of bone marrow-derived human mesenchymal stromal cells for immunotherapeutic applications. Cytotherapy. 2015;17:128–139. [DOI] [PubMed] [Google Scholar]
- 50.Nutt SL, Hodgkin PD, Tarlinton DM, et al. The generation of antibody-secreting plasma cells. Nat Rev Immunol. 2015;15:160–171. [DOI] [PubMed] [Google Scholar]
- 51.Fan L, Hu C, Chen J, et al. Interaction between mesenchymal stem cells and B-cells. Int J Mol Sci. 2016;17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rasmusson I, Le Blanc K, Sundberg B, et al. Mesenchymal stem cells stimulate antibody secretion in human B cells. Scand J Immunol. 2007;65:336–343. [DOI] [PubMed] [Google Scholar]
- 53.Comoli P, Ginevri F, Maccario R, et al. Human mesenchymal stem cells inhibit antibody production induced in vitro by allostimulation. Nephrol Dial Transplant. 2008;23:1196–1202. [DOI] [PubMed] [Google Scholar]
- 54.Corcione A, Benvenuto F, Ferretti E, et al. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107:367–372. [DOI] [PubMed] [Google Scholar]
- 55.Bazin H, Beckers A, Querinjean P. Three classes and four (sub)classes of rat immunoglobulins: IgM, IgA, IgE and IgG1, IgG2a, IgG2b, IgG2c. Eur J Immunol. 1974;4:44–48. [DOI] [PubMed] [Google Scholar]
- 56.McGhee JR, Michalek SM, Ghanta VK. Rat immunoglobulins in serum and secretions: purification of rat IgM, IgA and IgG and their quantitation in serum, colostrum, milk and saliva. Immunochemistry. 1975;12:817–823. [DOI] [PubMed] [Google Scholar]
- 57.Medgyesi GA, Miklos K, Kulics J, et al. Classes and subclasses of rat antibodies: reaction with the antigen and interaction of the complex with the complement system. Immunology. 1981;43:171–176. [PMC free article] [PubMed] [Google Scholar]
- 58.Hoffman W, Lakkis FG, Chalasani G. B cells, antibodies, and more. Clin J Am Soc Nephrol. 2016;11:137–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lynch RJ, Silva IA, Chen BJ, et al. Cryptic B cell response to renal transplantation. Am J Transplant. 2013;13:1713–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shabir S, Girdlestone J, Briggs D, et al. Transitional B lymphocytes are associated with protection from kidney allograft rejection: a prospective study. Am J Transplant. 2015;15:1384–1391. [DOI] [PubMed] [Google Scholar]
- 61.Newell KA, Asare A, Sanz I, et al. Longitudinal studies of a B cell-derived signature of tolerance in renal transplant recipients. Am J Transplant. 2015;15:2908–2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pan GH, Chen Z, Xu L, et al. Low-dose tacrolimus combined with donor-derived mesenchymal stem cells after renal transplantation: a prospective, non-randomized study. Oncotarget. 2016;7:12089–12101. [DOI] [PMC free article] [PubMed] [Google Scholar]


