Abstract
Background:
The neutrophil-to-lymphocyte ratio (NLR) has been reported to possess significant prognostic value in multiple types of cancer. We conducted a meta-analysis to evaluate the prognostic value of pretreatment NLR in soft tissue sarcoma (STS).
Methods:
A systematic literature search through April 2018 was conducted to identify studies evaluating the prognostic value of the pretreatment NLR in STS patients. The end points were overall survival (OS), disease-free survival (DFS), progression-free survival (PFS), and clinicopathological parameters. All statistical analyses were conducted with Stata 13.0.
Results:
Fourteen cohorts with 2820 patients were analyzed. Elevated NLR was significantly correlated with worse OS [hazard ratio (HR): 1.59, 95% confidence interval (95% CI): 1.28–1.97, P < .001] and DFS/PFS (HR = 1.28; 95% CI = 1.12–1.47; P < .001). In addition, elevated NLR was highly correlated with age (≥ 65 years), tumor size (>5 cm), tumor depth (deep), Grade (G3), and TNM stage (III-IV).
Conclusion:
Overall, pretreatment NLR could be an adverse prognostic biomarker for STS.
Keywords: meta-analysis, neutrophil-to-lymphocyte ratio, prognosis, soft tissue sarcoma
1. Introduction
Soft tissue sarcomas (STSs) comprise a heterogeneous collective of rare tumors arising from almost any embryonic mesodermal tissue and accounting for approximately 1% of adult malignancies.[1,2] Surgical resection combined with radiation therapy is the standard of care for patients with STS.[3] Nevertheless, some 50% of all patients with adequate local control experience local recurrence and distant metastasis,[4] with 5-year survival rates of approximately 50%.[5,6] Therefore, it is necessary to find a suitable biomarker that can identify risk classification and even guide the treatment.
There is increasing evidence that cancer-related inflammation leads to worse prognosis. Increasing evidence shows that inflammation can largely influence several stages of tumorigenesis, from tumor initiation to promotion and metastatic progression.[7] Several indicators in peripheral blood often reflect the inflammatory response in the tumor microenvironment.[8] Inflammation-based prognostic indicators, such as the plasma fibrinogen, Glasgow prognostic score (GPS), and C-reactive protein (CRP), have been investigated in different type of cancers.[9,10] The pretreatment NLR has been demonstrated as significant predictors in patients with STS.[11–13] However, due to the inconsistent results, the prognostic role of NLR in STS remains controversial.[14–16] We therefore conducted a meta-analysis to quantify the prognostic effect of NLR and analyze the relationship between NLR and clinicopathological parameters in patients with STS.
2. Materials and methods
2.1. Search strategies
A systematic search of the association between NLR and survival was conducted up to April 2018 in this research. Essays were searched through EMBASE, PubMed, and the Cochrane library. Search terms included “sarcoma” and “NLR” or “neutrophil lymphocyte ratio” and “prognosis” or “survival” or “outcome.” The whole process of search was conducted by 2 reviewers, independently. All analyses were based on previous published studies. Thus, this study did not require the ethic approval and informed consent.
2.2. Selection criteria
The inclusion criteria for this study were as follows: all selected literatures investigated NLR and survival in STS; patients with STS were pathologically confirmed; hazard ratio (HR) with its 95% confidence intervals (95% CIs) was reported from the original paper or can be calculated by Kaplan–Meier curve; and reporting a cut-off value for NLR. Articles were excluded from the analyses if they were letters, reviews, or conference abstracts; studies with sample size less than 20; unable to extract relevant metrics data; and duplicate publication.
2.3. Data extraction and quality assessment
All eligible studies were reviewed and extracted independently by 2 reviewers. Data needed to be recorded was as follows: first author's name, publication year, area, ethnicity, number of patients, age, follow-up period, survival analysis methods, treatment, cut-off values, tumor size, tumor depth, tumor grade, TNM stage, and HR as well as corresponding 95% CI.
The quality of each study was assessed according to the Newcastle–Ottawa Scale (NOS).[17] We applied the NOS scale generally used for evaluating cohort studies. This scale consists of 3 primary domains: Selection, Comparability, and Outcome, which were scored separately. One star for each item can be given within the Selection and Outcome categories, while 2 stars for Comparability. Studies with a score of 6 or more were defined as high quality.
2.4. Statistical analysis
All statistical analyses were performed with Stata 13.0 statistical software (StataCorp, College Station, TX). Odds ratio (OR) and their 95% CI were used to assess the association between NLR and clinicopathological factors. If the statistical variables were not given in the study, we calculated them with Kaplan–Meier survival curves, which were read by Engauge Digitizer version 4.1 (free software downloaded from http://sourceforge.net) according to the methods described by and Parmar et al[18] and Tierney et al.[19] The between-study heterogeneity was evaluated with Chi-squared test and I2 statistics. A Chi-squared test of P < .10 or I2 > 50% showed the existence of heterogeneity. Subgroup analysis was furtherly performed to explore the source of existing heterogeneity. Sensitivity analysis to test the credibility of the result was performed by sequential omission of individual studies. Publication bias was estimated by Begg and Egger test. A P value less than .05 was considered to be statistically significant.
3. Results
3.1. Study selection and study characteristics
The search strategy identified 152 potentially relevant records, among which 38 were excluded, as they were duplicates. The remaining 114 manuscripts were subject to title and abstract screening. We further removed 85 publications because they were unrelated studies or studies without survival information. Hence, 29 articles were eligible for full-text review and data extraction. Finally, 16 articles were excluded due to letter, conference abstract, or duplicate data, and the remaining 13 studies were enrolled in the meta-analysis as it presents in Fig. 1. The major characteristics of the 14 eligible cohorts are listed in Table 1[11–16,20–26] (the study of Yanagisawa et al[25] was divided into 2 cohorts.) The sample size of the studies ranged from 25 to 818. Thirteen cohorts reported the outcomes of OS, and 11 cohorts presented DFS/PFS as primary outcome. HRs were reported directly in all included cohorts.
Figure 1.

Flow diagram of the study selection process.
Table 1.
Characteristics of the studies included in the meta-analysis.

3.2. Quality assessment
The quality of all eligible studies varied from 6 to 9, with average 7.5 according to NOS. Therefore, all studies were included subsequent analysis.
3.3. Meta-analysis
3.3.1. Correlation of NLR with clinicopathological features
The association between NLR and several clinicopathological parameters is illustrated in Table 2. The elevated NLR was highly correlated with age (≥ 65 vs <65; HR = 1.56, 95% CI: 1.10–2.21, P = .01), tumor size (> 5 vs <5 cm; HR = 2.02, 95% CI: 1.24–3.29, P = .005), tumor depth (deep vs superficial; HR = 2.26, 95% CI: 1.60–3.20, P < .001), Grade (G3 vs G2/G1; HR = 1.61, 95% CI: 1.16–2.25, P = .004), and TNM stage (III-IV vs I-II; HR = 3.16, 95% CI: 2.16–4.61, P < .001). However, elevated NLR was not related to gender (male vs female; HR = 1.02, 95% CI: 0.80–1.30, P = .85).
Table 2.
Meta-analysis of the association between NLR and clinicopathological features of STS.

3.4. Overall survival
The main results of this meta-analysis are listed in Fig. 2. As the studies evaluating OS were of obvious statistical heterogeneity (I2 = 75.8%, P < .001), we used a random-effects model to pool the HR. Meta-analysis of the 13 cohorts showed that elevated NLR was associated with poor OS (HR: 1.59, 95% CI: 1.28–1.97, P < .001). The correlation between NLR and OS was further assessed by subgroup analysis based on several related clinicopathological parameters (Table 3). The results demonstrated that elevated NLR was associated with worse OS both in Asian (HR = 1.59; 95% CI = 1.28–1.97; P = .001) and Caucasian populations (HR = 1.44; 95% CI = 1.03–2.00; P = .031). Pooled HRs for OS were stratified by disease stage, the negative effect of elevated NLR on OS was observed in patients with nonmetastatic (HR = 1.64; 95% CI = 1.06–2.56; P = .028), and mixed disease subgroups (HR = 1.53; 95% CI = 1.17–2.01; P = .002). Moreover, subgroup analyses showed that elevated NLR predicted worse OS in patient with STS, regardless of the treatment (surgery and mixed), analysis method (univariate and multivariate), and the cut-off value for NLR (≥3.0 and <3.0).
Figure 2.

Forest plots for the association between NLR and OS.
Table 3.
Pooled hazard ratios (HRs) for DFS/PFS according to subgroup analyses.

3.5. Disease-free survival/progression-free survival
Seven cohorts explored the association between elevated NLR and DFS/PFS. Elevated NLR was significantly associated with poor DFS/PFS (HR = 1.28; 95% CI = 1.12–1.47; P < .001; Fig. 3) and significant heterogeneity was observed (I2 = 60.4%; P = .005).
Figure 3.

Forest plots for the association between NLR and DFS/PFS.
3.6. Sensitivity analysis and publication bias
We found that the result was not obviously impacted by any single study, therefore indicating that our results were statistically robust (Fig. 4). Significant publication bias was observed in OS (P = .951 for Begg test and P = .001 for Egger test, Fig. 5) and DFS/PFS (P = .533 for Begg test and P = .003 for Egger test, Fig. 6).
Figure 4.
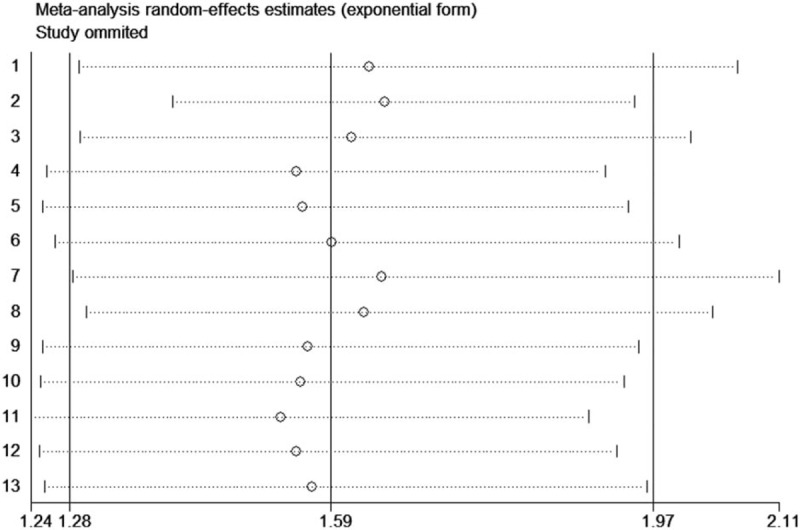
Sensitivity analysis of NLR on OS in STS patients.
Figure 5.

Begg funnel plot of publication bias test for OS in STS.
Figure 6.

Begg funnel plot of publication bias test for DFS/PFS in STS.
4. Discussion
To date, the relationship between NLR and the outcome of STS remains inconclusive. Our current study chiefly assessed the prognostic role of pretreatment NLR and the relationship between NLR and clinical features in patients with STS. Pooled results from 13 studies with 2820 patients showed that elevated NLR was significantly associated with poor OS and DFS/PFS. In addition, subgroup analyses indicated that elevated NLR was associated poor OS in patient with STS, regardless of the ethnicity, treatment, stage, analysis method, and the cut-off value for NLR. In addition, elevated NLR was highly correlated with age (≥ 65 years), tumor size (> 5 cm), tumor depth (deep), Grade (G3), and TNM stage (III-IV).
The actual mechanisms of the prognostic impact of the NLR for a patient with a STS are unclear. Cancer-related inflammation is an emerging hallmark of cancer.[27] Accumulating evidence suggested a strong link between inflammation and tumor development.[7,28,29] High densities of tumor tissue infiltrating neutrophil provides a favorable tumor environment for cancer progression by secreting many inflammation mediators such as tumor necrosis factor α (TNF-α), vascular endothelia growth factor (VEGF), interleukin-2 (IL-2), interleukin-6 (IL-6), and interleukin-10 (IL-10).[30–32] Moreover, infiltration of neutrophils can suppress the immune activity of lymphocytes and natural killer cells by producing some chemokines and cytokines.[30,33] Lymphocytes play critical roles in the host immune response. They can inhibit the proliferative and metastatic ability of cancer cells via inducing cytotoxic cell death and cytokine production.[34] Tumor-infiltrating lymphocytes (TILs) are involved in several stages of tumor progression.[35,36] A growing body of evidence has reported tumor-infiltrating CD4+ and CD8+ T lymphocytes may be a prognostic biomarker in many types of cancer.[37–39] A low lymphocyte count might result in an inadequate immune response in the control of tumor.[40,41] Thus, NLR may represent a balance between the tumor promotion reaction and antitumor immune function.
There were several limitations of our study. First, the heterogeneity between studies was statistically significant. However, interestingly, subgroup analyses indicated that the heterogeneity diminished in Asian populations, in patients who received surgery and in studies with cut-off ≥ 3. Second, due to the lack of a unified standard, different cut-off values were applied in various studies, which may affect the outcomes of the value that NLR plays as a biomarker in STS prognosis. Third, all included studies were retrospective.
In summary, our findings demonstrated that the pretreatment NLR is associated with unfavorable outcomes in conjunction with advanced clinicopathological features in patients with STS, suggesting that NLR could serve as a predicative biomarker for STS patients.
Acknowledgment
We gratefully acknowledge the statistical assistance of Professor Wei Sun from Department of Medical Statistics, Wuhan University.
Author contributions
Conceived and designed the experiments: GL, LCK, SRS. Performed the experiments: GL, LCK, SRS. Analyzed the data: GL, LCK, SRS. Contributed reagents/materials/analysis tools: GL, LCK, SRS. Wrote the paper: all authors.
Conceptualization: Gang Liu, Li-chi Ke, Sheng-rong Sun.
Data curation: Gang Liu, Li-chi Ke, Sheng-rong Sun.
Formal analysis: Gang Liu, Li-chi Ke, Sheng-rong Sun.
Funding acquisition: Gang Liu, Li-chi Ke, Sheng-rong Sun.
Investigation: Gang Liu, Li-chi Ke, Sheng-rong Sun.
Methodology: Gang Liu, Li-chi Ke, Sheng-rong Sun.
Project administration: Gang Liu, Li-chi Ke, Sheng-rong Sun.
Resources: Gang Liu, Li-chi Ke, Sheng-rong Sun.
Software: Gang Liu, Li-chi Ke, Sheng-rong Sun.
Supervision: Gang Liu, Li-chi Ke, Sheng-rong Sun.
Validation: Gang Liu, Li-chi Ke, Sheng-rong Sun.
Visualization: Gang Liu, Li-chi Ke, Sheng-rong Sun.
Writing – original draft: Gang Liu, Li-chi Ke, Sheng-rong Sun.
Writing – review & editing: Gang Liu, Li-chi Ke, Sheng-rong Sun.
Footnotes
Abbreviations: CI = confidence interval, CRP = C-reactive protein, DFS = disease-free survival, GPS = Glasgow Prognostic Score, HR = hazard ratio, IL-2 = interleukin-2, NLR = neutrophil-to-lymphocyte ratio, NOS = Newcastle–Ottawa Scale, OR = odds ratio, OS = overall survival, PFS = progression-free survival, STS = soft tissue sarcoma, TILs = tumor-infiltrating lymphocytes, TNF-α = tumor necrosis factor α, VEGF = vascular endothelia growth factor.
GL and L-CK contributed equally to this work.
The authors report no conflicts of interest in this work.
References
- [1].van der Graaf WT, Orbach D, Judson IR, et al. Soft tissue sarcomas in adolescents and young adults: a comparison with their paediatric and adult counterparts. Lancet Oncol 2017;18:e166–75. [DOI] [PubMed] [Google Scholar]
- [2].Zambo I, Vesely K. WHO classification of tumours of soft tissue and bone 2013: the main changes compared to the 3rd edition. Cesk Patol 2014;50:64–70. [PubMed] [Google Scholar]
- [3].Group ESESNW. Soft tissue and visceral sarcomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2014;25(suppl 3):iii102–12. [DOI] [PubMed] [Google Scholar]
- [4].Zagars GK, Ballo MT, Pisters PW, et al. Prognostic factors for patients with localized soft-tissue sarcoma treated with conservation surgery and radiation therapy: an analysis of 1225 patients. Cancer 2003;97:2530–43. [DOI] [PubMed] [Google Scholar]
- [5].Linch M, Miah AB, Thway K, et al. Systemic treatment of soft-tissue sarcoma-gold standard and novel therapies. Nat Rev Clin Oncol 2014;11:187–202. [DOI] [PubMed] [Google Scholar]
- [6].Cormier JN, Pollock RE. Soft tissue sarcomas. CA Cancer J Clin 2004;54:94–109. [DOI] [PubMed] [Google Scholar]
- [7].Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell 2010;140:883–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ohtani H. Focus on TILs: prognostic significance of tumor infiltrating lymphocytes in human colorectal cancer. Cancer Immun 2007;7:4. [PMC free article] [PubMed] [Google Scholar]
- [9].Yu X, Wen Y, Lin Y, et al. The value of preoperative Glasgow Prognostic Score and the C-reactive protein to albumin ratio as prognostic factors for long-term survival in pathological T1N0 esophageal squamous cell carcinoma. J Cancer 2018;9:807–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kuo YH, Wang JH, Hung CH, et al. Albumin-Bilirubin grade predicts prognosis of HCC patients with sorafenib use. J Gastroenterol Hepatol 2017;32:1975–81. [DOI] [PubMed] [Google Scholar]
- [11].Li Y, Yang X, Zhang W, et al. Clinical implications of six inflammatory biomarkers as prognostic indicators in Ewing sarcoma. Cancer Manag Res 2017;9:443–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Liu B, Huang Y, Sun Y, et al. Prognostic value of inflammation-based scores in patients with osteosarcoma. Sci Rep 2016;6:39862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Jiang L, Jiang S, Situ D, et al. Prognostic value of monocyte and neutrophils to lymphocytes ratio in patients with metastatic soft tissue sarcoma. Oncotarget 2015;6:9542–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Nakamura T, Matsumine A, Matsubara T, et al. Infiltrative tumor growth patterns on magnetic resonance imaging associated with systemic inflammation and oncological outcome in patients with high-grade soft-tissue sarcoma. PLoS One 2017;12:e0181787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kobayashi H, Okuma T, Oka H, et al. Neutrophil-to-lymphocyte ratio after pazopanib treatment predicts response in patients with advanced soft-tissue sarcoma. Int J Clin Oncol 2018;23:368–74. [DOI] [PubMed] [Google Scholar]
- [16].Que Y, Qiu H, Li Y, et al. Preoperative platelet-lymphocyte ratio is superior to neutrophil-lymphocyte ratio as a prognostic factor for soft-tissue sarcoma. BMC Cancer 2015;15:648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- [18].Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 1998;17:2815–34. [DOI] [PubMed] [Google Scholar]
- [19].Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Idowu OK, Ding Q, Taktak AF, et al. Clinical implication of pretreatment neutrophil to lymphocyte ratio in soft tissue sarcoma. Biomarkers 2012;17:539–44. [DOI] [PubMed] [Google Scholar]
- [21].Szkandera J, Gerger A, Liegl-Atzwanger B, et al. The derived neutrophil/lymphocyte ratio predicts poor clinical outcome in soft tissue sarcoma patients. Am J Surg 2015;210:111–6. [DOI] [PubMed] [Google Scholar]
- [22].Choi ES, Kim HS, Han I. Elevated preoperative systemic inflammatory markers predict poor outcome in localized soft tissue sarcoma. Ann Surg Oncol 2014;21:778–85. [DOI] [PubMed] [Google Scholar]
- [23].Xia WK, Liu ZL, Shen D, et al. Prognostic performance of pre-treatment NLR and PLR in patients suffering from osteosarcoma. World J Surg Oncol 2016;14:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Liang Y, Xiao W, Guan YX, et al. Prognostic value of the C-reactive protein/Albumin Ratio (CAR) in patients with operable soft tissue sarcoma. Oncotarget 2017;8:98135–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Yanagisawa M, Gingrich AA, Judge S, et al. Serum C-reactive protein and neutrophil/lymphocyte ratio after neoadjuvant radiotherapy in soft tissue sarcoma. Anticancer Res 2018;38:1491–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Maretty-Kongstad K, Aggerholm-Pedersen N, Keller J, et al. A validated prognostic biomarker score for adult patients with nonmetastatic soft tissue sarcomas of the trunk and extremities. Transl Oncol 2017;10:942–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646–74. [DOI] [PubMed] [Google Scholar]
- [28].Palumbo JS, Degen JL. Mechanisms coupling the hemostatic system to colitis-associated cancer. Thromb Res 2010;125(suppl 2):S39–43. [DOI] [PubMed] [Google Scholar]
- [29].Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature 2008;454:436–44. [DOI] [PubMed] [Google Scholar]
- [30].el-Hag A, Clark RA. Immunosuppression by activated human neutrophils. Dependence on the myeloperoxidase system. J Immunol 1987;139:2406–13. [PubMed] [Google Scholar]
- [31].Jablonska E, Kiluk M, Markiewicz W, et al. TNF-alpha, IL-6 and their soluble receptor serum levels and secretion by neutrophils in cancer patients. Arch Immunol Ther Exp (Warsz) 2001;49:63–9. [PubMed] [Google Scholar]
- [32].Schaider H, Oka M, Bogenrieder T, et al. Differential response of primary and metastatic melanomas to neutrophils attracted by IL-8. Int J Cancer 2003;103:335–43. [DOI] [PubMed] [Google Scholar]
- [33].Petrie HT, Klassen LW, Kay HD. Inhibition of human cytotoxic T lymphocyte activity in vitro by autologous peripheral blood granulocytes. J Immunol 1985;134:230–4. [PubMed] [Google Scholar]
- [34].Terzic J, Grivennikov S, Karin E, et al. Inflammation and colon cancer. Gastroenterology 2010;138:2101–14.e5. [DOI] [PubMed] [Google Scholar]
- [35].Chen KJ, Zhou L, Xie HY, et al. Intratumoral regulatory T cells alone or in combination with cytotoxic T cells predict prognosis of hepatocellular carcinoma after resection. Med Oncol 2012;29:1817–26. [DOI] [PubMed] [Google Scholar]
- [36].Zhou J, Ding T, Pan W, et al. Increased intratumoral regulatory T cells are related to intratumoral macrophages and poor prognosis in hepatocellular carcinoma patients. Int J Cancer 2009;125:1640–8. [DOI] [PubMed] [Google Scholar]
- [37].Nguyen N, Bellile E, Thomas D, et al. Tumor infiltrating lymphocytes and survival in patients with head and neck squamous cell carcinoma. Head Neck 2016;38:1074–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Mao Y, Qu Q, Chen X, et al. The prognostic value of tumor-infiltrating lymphocytes in breast cancer: a systematic review and meta-analysis. PLoS One 2016;11:e0152500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Sznurkowski JJ, Zawrocki A, Emerich J, et al. Prognostic significance of CD4+ and CD8+ T cell infiltration within cancer cell nests in vulvar squamous cell carcinoma. Int J Gynecol Cancer 2011;21:717–21. [DOI] [PubMed] [Google Scholar]
- [40].Hoffmann TK, Dworacki G, Tsukihiro T, et al. Spontaneous apoptosis of circulating T lymphocytes in patients with head and neck cancer and its clinical importance. Clin Cancer Res 2002;8:2553–62. [PubMed] [Google Scholar]
- [41].Vayrynen JP, Tuomisto A, Klintrup K, et al. Detailed analysis of inflammatory cell infiltration in colorectal cancer. Br J Cancer 2013;109:1839–47. [DOI] [PMC free article] [PubMed] [Google Scholar]


