Abstract
Background:
The association between serum lipids and diabetic retinopathy (DR) was controversial. Therefore, we performed a meta-analysis to evaluate the relationship between triglycerides (TG), serum total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C), and DR.
Methods:
A systematic review and meta-analysis of observational studies was carried out to explore the association between serum lipids and DR. Studies related were initially indentified by searching PubMed, Cochrane Library, and Elsevier databases through June, 2017. Then a manual retrieval was also performed. RevMan 5.3 software was used to calculate the pooled mean differences (MDs) and related 95% confidence intervals (CIs). To test the stability of the final results, a sensitivity analysis was also performed.
Results:
A total of 7 studies were included in this meta-analysis. When compared with the controls, the DR cases did not show significantly higher TG levels (MD 9.18 mg/dL, 95%CI –4.14 to 22.49, P = .18), higher TC levels (MD 3.77 mg/dL, 95%CI: –2.45 to 9.98, P = .24), as well as lower HDL-C levels (MD –1.14 mg/dL, 95%CI: –2.43 to 0.15, P = .08). But slightly higher LDL-C levels were observed (MD 3.74 mg/dL, 95%CI: 0.13–7.35, P = .04). In addition, whether serum lipids involved in the progression of DR were relatively unexplored, but fenofibrate was confirmed to benefit the DR cases.
Conclusions:
Based on recent published data, we did not find obvious differences in TG, TC, and HDL-C levels between patients with DR and without DR. However, slightly higher LDL-C levels were observed in the DR cases.
Keywords: cohort studies, diabetic retinopathy, meta-analysis, serum lipids
1. Introduction
Diabetic retinopathy (DR), one of the most common microvascular complications of diabetes mellitus (DM), was the foremost cause of blindness in the working age group.[1] Except for the damaging effects on vision, presence of DR also increased the risk of cardiovascular diseases[2,3] with the increasing morbidity of DM, worldwide prevalence of DR was expected to increase to 5.4% by 2025. Although hyperglycemia was a classical risk factor for DR, better glucose control was reported to have an unsatisfactory effect on preventing the development of DR.[4] In addition, the Diabetes Control and Complications Trial[5] reported that only ∼11% of the total risk of DR could be explained by glycemic exposure, and the remaining 89% might be generated by other potential factors. Therefore, to explore the potential risk factors and the possible treatments for DR would be imperative.
Early in 1952, Keiding et al[6] firstly had reported the involvement of serum lipids in the progression of DR. Since then, increasing studies investigating the relationship between serum lipids and DR emerged. However, the results remained controversial. In Early Treatment Diabetic Retinopathy Study (ETDRS),[7] elevated serum lipids levels were reported to be associated with retinal hard exudates. As well, Chennai Urban Rural Epidemiology Study[8] demonstrated higher serum lipids levels in DR cases. However, the Australian Diabetes, Obesity and Lifestyle study,[9] involving 11,247 adults from 42 areas of Australia, did not show significant association between serum lipids and DR. Similarly, another study[10] found no obvious differences in DR prevalence among patients with different total cholesterol (TC) levels. Unexpectedly, higher TC levels were reported to have a protective effect on DR in the Singapore Malay Eye Study.[11] Since most of the previous studies were in a cross-sectional design, a causal relationship could not be confirmed. We, therefore, carried out a meta-analysis of cohort studies to explore the association between triglycerides (TG), TC, high-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C), and the occurrence of DR.
2. Methods
In performing this meta-analysis, we adhered to the Meta-Analysis of Observational Studies in Epidemiology (MOOSE)[12] and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).
2.1. Study design
This was a meta-analysis of observational studies, and thus no ethical approval was warranted.
2.2. Literature search
We systematically searched the studies investigating the association between dyslipidemia and DR from PubMed, Cochrane Library, and Elsevier databases through June, 2017. No language restrictions were used in the process. Key words about the serum lipids (“dyslipidemia” or “cholesterol” or “hypercholesterolemia” or “lipids” or “lipoprotein” or “triglyceride” or “hyperlipidemia”) and DR (“diabetic retinopathy”) were combined to identify the relevant studies. A manual retrieval of the reference lists was also applied to refine the search.
2.3. Eligibility criteria
The studies were included if they fulfilled the following: evaluating the effects of dyslipidemia on the occurrence of DR; being cohort studies or nested case-control studies; serum lipids levels were presented as mean values and corresponding standard deviations (SDs). The following were defined as the exclusion criteria: the endpoint was the DR progression rather than the new-onset DR; investigating the association between apolipoprotein and DR; certain publication types (e.g., reviews, letters, case reports, comments); the cross-sectional studies and case-control studies.
2.4. Data extraction
ZY, WCY, and SK extracted the following data independently: the first author, publication year, geographic regions of original studies, sample size, age, exposures, follow-up years, outcomes, mean values, and SDs of serum lipids concentrations. Different units adopted in different studies were converted to a same unit. For TC/HDL-C/LDL-C, 1 mmol/L equaled to 38.67. While 1 mmol/L TG was converted to 88.545 mg/dL. Newcastle–Ottawa scale (NOS)[13] was used to assess the quality of the included studies from 3 aspects, namely selection, comparability, and outcome. We defined studies with a NOS of ≥6 stars as moderate-to-high quality studies. A senior (YXL) helped us to solve any disagreements if necessary.
2.5. Statistical analysis
Statistical software Revman Manager 5.3 (Nordic Cochrane Center, Rigshospitalet, Copenhagen, Denmark; http://ims.cochrane.org/revman) was used to deal with the entire statistical process. The final statistical results were presented as mean differences (MDs) and relevant 95%CIs. We selected a random-effects model with inverse variance weighting to calculate the pooled results, since it was more conservative and could provide better estimates with wider CIs.[14] Besides, a fixed-effects model was also calculated for comparison. I2 statistic was used to detect any heterogeneity between studies, where I2 values of 25%, 50%, and 75% were defined as cut-off points for low, moderate, and high heterogeneity.[15] To examine the influence of each study included, a sensitivity analysis was performed by removing each study.
3. Results
3.1. Study selection
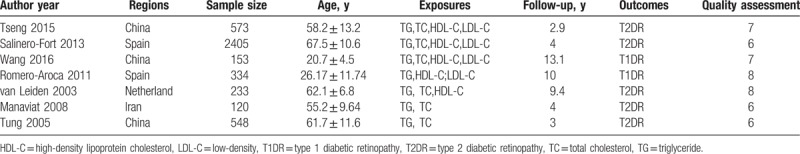
As shown in Fig. 1, a total of 2575 articles were initially indentified from the databases, of which 2528 articles were removed after screening the titles and the abstracts. Then the remaining potential 47 relevant articles were thoroughly assessed for eligibility, and 40 of them were excluded for different reasons. Thirty-two studies used a cross-sectional/case-control design. Two studies[7,16] described the association between serum lipids and hard exudates instead of the occurrence of DR. One cohort study[17] calculated the effects of serum lipids with increments of 10 units. Three studies[18–20] estimated the effects of serum lipids on PDR based on different exposure levels. The endpoints of 2 studies[21,22] were DR progression rather than new-onset DR. Finally, a total of 7 studies[22–28] were included. Among them, the majority were from China (n = 3), Spain (n = 2), the Netherlands (n = 1), and Iran (n = 1) with durations of follow-up ranging from 2.9 to 13.1 years. All the characteristics and the NOS scores of included studies were shown in Table 1.
Figure 1.
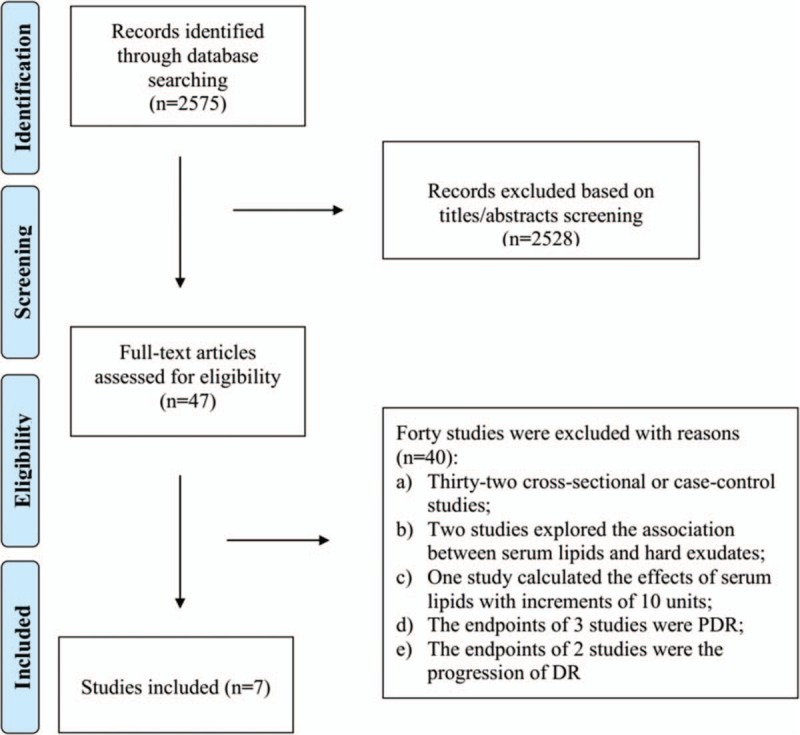
Study search diagram.
Table 1.
Characteristics of the 7 included studies in this meta-analysis.
3.2. Meta-analysis
In this meta-analysis, we explored the relationship between serum lipids (TG, TC, HDL, and LDL) and DR via comparing the serum lipid concentrations. All of the 7 studies included compared the serum lipid concentrations between individuals with and without DR. Among them, 2 studies also calculated the ORs for every 1 mg/dL increment of serum lipids. Besides, in the remaining 5 studies, they classified the patients into normal or risk levels according to the serum lipid concentrations and calculated the ORs for each risk level. Regretfully, because of the limited number of studies and the different cut-off points of serum lipid concentration for each risk level, the pooled ORs could not be finally calculated.
TG and DR as shown in Fig. 2, 7 studies evaluated the differences in TG levels between patients with DR present and DR absent (607 DR patients and 3759 controls). The present meta-analysis showed no significantly higher levels in DR cases (MD 9.18 mg/dL, 95%CI –4.14 to 22.49, P = .18, I2 = 65%, P-heterogeneity = .009) in the random-effects model. Results from the fixed-effects model were similar (MD 3.22 mg/dL, 95%CI –2.08 to 8.52, P = .23, I2 = 65%, P-heterogeneity = .009). The pooled results were stable through a sensitivity analysis by omitting one study in each turn. TC and DR as shown in Fig. 3, 6 studies evaluated the differences in TC levels between patients with DR present and DR absent (487 DR patients and 3545 controls). The present meta-analysis showed no significantly higher levels in DR cases (MD 3.77 mg/dL, 95%CI: –2.45 to 9.98, P = .24, I2 = 41%, P-heterogeneity = .13) in the random-effects model. Results from the fixed-effects model were similar (MD 2.61 mg/dL, 95%CI: –1.52 to 6.74, P = .22, I2 = 41%, P-heterogeneity = .13). The pooled results were stable through a sensitivity analysis by omitting 1 study in each turn.
Figure 2.
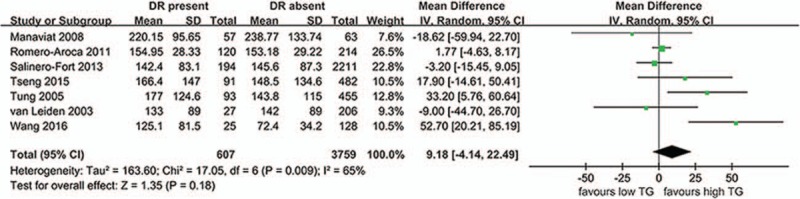
The differences in TG levels between patients with DR and without DR. CI = confidence interval, DR = diabetic retinopathy, SD = standard deviation.
Figure 3.
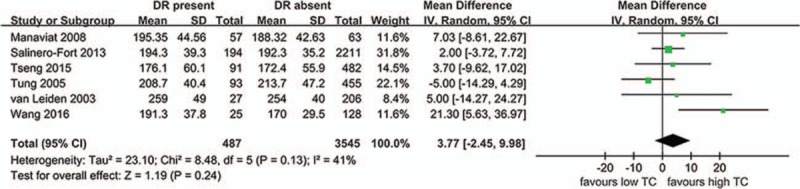
The differences in TC levels between patients with DR and without DR. CI = confidence interval, DR = diabetic retinopathy, SD = standard deviation.
HDL-C and DR as shown in Fig. 4, 5 studies evaluated the differences in HDL-C levels between patients with DR present and DR absent (457 DR patients and 3241controls). The present meta-analysis showed no significantly lower levels in DR cases (MD –1.14 mg/dL, 95%CI: –2.43 to 0.15, P = .08, I2 = 0, P-heterogeneity = .84) in the random-effects model. The fixed-effects model showed an identical results since no heterogeneity between studies was observed. The pooled results were stable through a sensitivity analysis by omitting one study in each turn.
Figure 4.
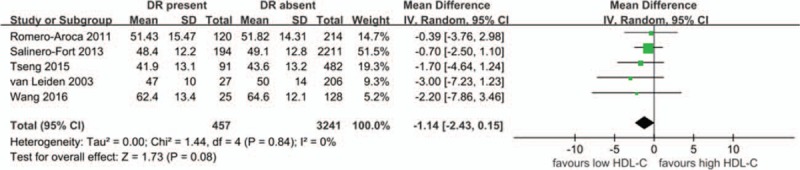
The differences in HDL-C levels between patients with DR and without DR. CI = confidence interval, DR = diabetic retinopathy, HDL-C = high-density lipoprotein cholesterol, SD = standard deviation.
LDL-C and DR as shown in Fig. 5, only 4 studies evaluated the differences in LDL-C levels between patients with DR present and DR absent (430 DR patients and 3035 controls). The present meta-analysis showed slightly higher levels in DR cases (MD 3.74 mg/dL, 95%CI: 0.13–7.35, P = .04, I2 = 20, P-heterogeneity = .29) in the random-effects model. Results from the fixed-effects model were similar (MD 3.55 mg/dL, 95%CI: 0.45–6.66, P = .02, I2 = 20%, P-heterogeneity = .29). However, a sensitivity analysis showed unstable results after omitting the study by Wang et al[23] (MD 2.92 mg/dL, 95%CI: –0.25 to 6.09, P = .07, I2 = 0%, P-heterogeneity = .97).
Figure 5.
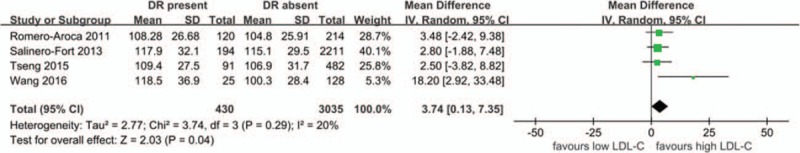
The differences in LDL-C levels between patients with DR and without DR. CI = confidence interval, DR = diabetic retinopathy, LDL-C = low-density lipoprotein cholesterol, SD = standard deviation.
4. Discussion
Decades ago, the effects of dyslipidemia on DR had been reported, but the results were controversial. To our knowledge, this was the first meta-analysis of cohort studies to exam the association between serum lipids and DR. Both a random-effects model and a fixed-effects model showed comparable results. First, the concentrations of TG were not significantly different between patients with DR present and DR absent, but a statistically significant heterogeneity was observed. However, because of the limited number of studies, we failed to carry out a meta-regression or a subgroup analysis to explore the source of heterogeneity. Thus, the results we got should be interpreted cautiously. Besides, in patients with DR, TC, and HDL-C levels were not significantly different from the levels in controls. In addition, slightly higher but unstable LDL-C levels were found in the DR patients. We thought the small number of studies included in this part should be partly responsible for the unstable results. Hence, more studies would be warranted to confirm the relationship between them. Generally, our results were consistent with the previous large-scale studies. The Wisconsin Epidemiologic Study of Diabetic Retinopathy,[18] conducted in 1979 to 2014, involving 903 diabetic patients, finally found little effect of TG or HDL-C on the prevalence of proliferative DR. Another global case–control study,[29] conducted in 24 sites in 13 countries, including 1202 DR patients, demonstrated a slightly higher risk of DR in patients with higher TG or lower HDL-C levels. However, after adjustment for hypertension and glycated hemoglobin (HbA1c), no significant results were presented. May be the effects of serum lipids in many studies were exaggerated by other confounders, such as hypertension and HbA1c.
Nevertheless, our results were somewhat different from a previous meta-analysis,[30] in which higher TG, TC, and LDL-C levels were found in patients with diabetic macular edema (DME). Although DME was always an indicator of server stages of DR, we thought the role of hyperlipidemia involved in DR and DME might be different to some extent. As previously demonstrated, for DME, it was the ischaemic or inflammatory process[31] that leading to breakdown of the blood retinal barrier and consequently leakage of serum lipids[32] into the intercellular spaces. While for DR, it was the lipid-induced arteriosclerotic changes[33] that dominantly accounting for the pathology.
Till now, the role of dyslipidemia in DR progression has been relatively unexplored. Two prospective studies[34,35] with more than 5-year follow-up recorded no significant differences in serum lipids levels between DR progressors and the controls. Recently, another longitudinal follow-up study,[36] named Sankara Nethralaya Diabetic Retinopathy Epidemiology and Molecular Genetic Study (SN-DREAMS II), involving 890 diabetic patients, observed a 3 times higher risk of progressing to PDR in patients with higher TG levels. Maybe the following 3 reasons could partly account for the diverse results. First, different definitions of DR progression in articles might confound the results. For example, the earlier 2 studies[34,35] defined the progression as an increase of DR severity regardless of DR present or absent at baseline. Thus, the progressors also included the new-onset ones. In contrast, the SN-DREAMS II defined those who were diagnosed with pre-existing DR at baseline and deteriorated on follow-up as progressors. Besides, the sample size of some studies was too small to achieve statistical significance. In addition, treatment of the dysplidemia during the follow-up could also affect the final results. Thus, more large-scale prospective studies with uniform definition would be warranted in the future.
Although the relationship between serum lipids and DR progression remained inconclusive, the fenofibrate was confirmed to benefit the DR cases[37,38] the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study[38] demonstrated that fenofibrate could prevent the progression of DR without significantly changing the serum lipids levels, and the authors pointed out that intraretinal lipid transport rather than serum lipids concentrations might be more important for DR progression. As another randomized trial, the Action to Control Cardiac Risk in Diabetes (ACCORD) Eye study[37] observed comparable effects of controlling DR progression between fenofibrate treatment and the intensive treatment of glycemia. Thus, the fenofibrate treatment should be strongly recommended for the DR cases, and the exact mechanisms of how fenofibrate functioned should be further investigated as well.
4.1. Study strengths and limitations
For decades, multiple studies had been performed to explore the association between serum lipids and DR, but no consistent results were finally achieved. To the best of our knowledge, this was the first meta-analysis of cohort studies to investigate the association between them. However, there were some limitations could not be ignored. First, only 4 studies were pooled to calculate the association between LDL-C and DR, leading to the relatively less credible results. Second, because of the limited number of studies, we failed to calculate the effects of serum lipids on different stages of DR. Third, we did not differentiate the type 1 DR or type 2 DR. In fact, their response to dyslipidemia might be something different.
5. Conclusions
In conclusion, we did not find obvious differences in TG, TC, and HDL-C levels between patients with DR and without DR. However, a little higher levels of LDL-C with borderline statistical significance were observed in patients with DR. In addition, whether any association existed between serum lipids and DR progression was unknown, but the fenofibrate treatment should be recommended since it could prevent the DR progression through lipid-modulating independent pathways. In the future, more prospective large-scale studies would be needed to further investigate the association between serum lipids and DR. As well, the mechanisms of fenofibrate involved in the control of DR progression needed further investigation.
Author contributions
Data curation: Yue Zhou, Changyun Wang, Ke Shi.
Formal analysis: Changyun Wang, Ke Shi.
Methodology: Xiaolong Yin.
Project administration: Xiaolong Yin.
Resources: Xiaolong Yin.
Software: Changyun Wang.
Writing – original draft: Yue Zhou.
Footnotes
Abbreviations: ACCORD = Action to Control Cardiac Risk in Diabetes, CIs = confidence intervals, DM = diabetes mellitus, DME = diabetic macular edema, DR = diabetic retinopathy, ETDRS = Early Treatment Diabetic Retinopathy Study, FIELD = Fenofibrate Intervention and Event Lowering in Diabetes, HbA1c = glycated hemoglobin, HDL-C = high-density lipoprotein cholesterol, LDL-C = low-density lipoprotein cholesterol, MDs = mean differences, MOOSE = Meta-Analysis of Observational Studies in Epidemiology, NOS = Newcastle-Ottawa scale, PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses, SDs = standard deviations, SN-DREAMS II = Sankara Nethralaya Diabetic Retinopathy Epidemiology and Molecular Genetic Study, TC = serum total cholesterol, TG = triglycerides.
Contributors: ZY, WCY, and SK took part in the whole work with the help of YXL.
Funding: None.
Ethical approval: No ethical approval is warranted as a meta-analysis of the articles available.
No conflicts of interest exist.
References
- [1].Yamada M, Hiratsuka Y, Roberts CB, et al. Prevalence of visual impairment in the adult Japanese population by cause and severity and future projections. Ophthalmic Epidemiol 2010;17:50–7. [DOI] [PubMed] [Google Scholar]
- [2].Cheung N, Wang JJ, Klein R, et al. Diabetic retinopathy and the risk of coronary heart disease: the Atherosclerosis Risk in Communities Study. Diabetes Care 2007;30:1742–6. [DOI] [PubMed] [Google Scholar]
- [3].Cheung N, Rogers S, Couper DJ, et al. Is diabetic retinopathy an independent risk factor for ischemic stroke? Stroke 2007;38:398–401. [DOI] [PubMed] [Google Scholar]
- [4].Ismail-Beigi F, Craven T, Banerji MA, et al. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet 2010;376:419–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Lachin JM, Genuth S, Nathan DM, et al. Effect of glycemic exposure on the risk of microvascular complications in the diabetes control and complications trial--revisited. Diabetes 2008;57:995–1001. [DOI] [PubMed] [Google Scholar]
- [6].Keiding NR, Mann GV, Root HF, et al. Serum lipoproteins and cholesterol levels in normal subjects and in young patients with diabetes in relation to vascular complications. Diabetes 1952;1:434–40. [DOI] [PubMed] [Google Scholar]
- [7].Chew EY, Klein ML, Ferris FR, et al. Association of elevated serum lipid levels with retinal hard exudate in diabetic retinopathy. Early Treatment Diabetic Retinopathy Study (ETDRS) Report 22. Arch Ophthalmol 1996;114:1079–84. [DOI] [PubMed] [Google Scholar]
- [8].Rema M, Srivastava BK, Anitha B, et al. Association of serum lipids with diabetic retinopathy in urban South Indians--the Chennai Urban Rural Epidemiology Study (CURES) Eye Study--2. Diabet Med 2006;23:1029–36. [DOI] [PubMed] [Google Scholar]
- [9].Tapp RJ, Shaw JE, Harper CA, et al. The prevalence of and factors associated with diabetic retinopathy in the Australian population. Diabetes Care 2003;26:1731–7. [DOI] [PubMed] [Google Scholar]
- [10].Wong TY, Klein R, Islam FM, et al. Diabetic retinopathy in a multi-ethnic cohort in the United States. Am J Ophthalmol 2006;141:446–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wong TY, Cheung N, Tay WT, et al. Prevalence and risk factors for diabetic retinopathy: the Singapore Malay Eye Study. Ophthalmology 2008;115:1869–75. [DOI] [PubMed] [Google Scholar]
- [12].Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008–12. [DOI] [PubMed] [Google Scholar]
- [13].Wells G. SBOC, The Newcastle Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses; 2014. [Google Scholar]
- [14].Kontopantelis E, Reeves D. Performance of statistical methods for meta-analysis when true study effects are non-normally distributed: a simulation study. Stat Methods Med Res 2012;21:409–26. [DOI] [PubMed] [Google Scholar]
- [15].Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–58. [DOI] [PubMed] [Google Scholar]
- [16].Papavasileiou E, Davoudi S, Roohipoor R, et al. Association of serum lipid levels with retinal hard exudate area in African Americans with type 2 diabetes. Graefes Arch Clin Exp Ophthalmol 2017;255:509–17. [DOI] [PubMed] [Google Scholar]
- [17].Forga L, Goni MJ, Ibanez B, et al. Influence of age at diagnosis and time-dependent risk factors on the development of diabetic retinopathy in patients with Type 1 diabetes. J Diabetes Res 2016;2016:9898309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Klein BE, Myers CE, Howard KP, et al. Serum lipids and proliferative diabetic retinopathy and macular edema in persons with long-term Type 1 diabetes mellitus: the Wisconsin Epidemiologic Study of Diabetic Retinopathy. JAMA Ophthalmol 2015;133:503–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Nittala MG, Keane PA, Zhang K, et al. Risk factors for proliferative diabetic retinopathy in a Latino American population. Retina 2014;34:1594–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Aldebasi YH, Mohieldein AH, Almansour YS, et al. Dyslipidemia and lipid peroxidation of Saudi type 2 diabetics with proliferative retinopathy. Saudi Med J 2013;34:616–22. [PubMed] [Google Scholar]
- [21].Popescu T, Mota M. Dyslipidemia and hypertension in patients with type 2 diabetes and retinopathy. Rom J Intern Med 2009;47:235–41. [PubMed] [Google Scholar]
- [22].Manaviat MR, Rashidi M, Afkhami-Ardekani M. Four years incidence of diabetic retinopathy and effective factors on its progression in type II diabetes. Eur J Ophthalmol 2008;18:572–7. [DOI] [PubMed] [Google Scholar]
- [23].Wang NK, Lai CC, Wang JP, et al. Risk factors associated with the development of retinopathy 10 yr after the diagnosis of juvenile-onset type 1 diabetes in Taiwan: a cohort study from the CGJDES. Pediatr Diabetes 2016;17:407–16. [DOI] [PubMed] [Google Scholar]
- [24].Tseng ST, Chou ST, Low BH, et al. Risk factors associated with diabetic retinopathy onset and progression in diabetes patients: a Taiwanese cohort study. Int J Clin Exp Med 2015;8:21507–15. [PMC free article] [PubMed] [Google Scholar]
- [25].Salinero-Fort MA, San AF, de Burgos-Lunar C, et al. Four-year incidence of diabetic retinopathy in a Spanish cohort: the MADIABETES study. PLoS One 2013;8:e76417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Romero-Aroca P, Baget-Bernaldiz M, Fernandez-Ballart J, et al. Ten-year incidence of diabetic retinopathy and macular edema. Risk factors in a sample of people with type 1 diabetes. Diabetes Res Clin Pract 2011;94:126–32. [DOI] [PubMed] [Google Scholar]
- [27].Tung TH, Chen SJ, Liu JH, et al. A community-based follow-up study on diabetic retinopathy among type 2 diabetics in Kinmen. Eur J Epidemiol 2005;20:317–23. [DOI] [PubMed] [Google Scholar]
- [28].van Leiden HA, Dekker JM, Moll AC, et al. Risk factors for incident retinopathy in a diabetic and nondiabetic population: the Hoorn study. Arch Ophthalmol 2003;121:245–51. [DOI] [PubMed] [Google Scholar]
- [29].Sacks FM, Hermans MP, Fioretto P, et al. Association between plasma triglycerides and high-density lipoprotein cholesterol and microvascular kidney disease and retinopathy in type 2 diabetes mellitus: a global case-control study in 13 countries. Circulation 2014;129:999–1008. [DOI] [PubMed] [Google Scholar]
- [30].Das R, Kerr R, Chakravarthy U, et al. Dyslipidemia and diabetic macular edema: a systematic review and meta-analysis. Ophthalmology 2015;122:1820–7. [DOI] [PubMed] [Google Scholar]
- [31].Joussen AM, Smyth N, Niessen C. Pathophysiology of diabetic macular edema. Dev Ophthalmol 2007;39:1–2. [DOI] [PubMed] [Google Scholar]
- [32].Landmesser U, Hornig B, Drexler H. Endothelial dysfunction in hypercholesterolemia: mechanisms, pathophysiological importance, and therapeutic interventions. Semin Thromb Hemost 2000;26:529–37. [DOI] [PubMed] [Google Scholar]
- [33].Klein R, Sharrett AR, Klein BE, et al. The association of atherosclerosis, vascular risk factors, and retinopathy in adults with diabetes: the atherosclerosis risk in communities study. Ophthalmology 2002;109:1225–34. [DOI] [PubMed] [Google Scholar]
- [34].Cikamatana L, Mitchell P, Rochtchina E, et al. Five-year incidence and progression of diabetic retinopathy in a defined older population: the Blue Mountains Eye Study. Eye (Lond) 2007;21:465–71. [DOI] [PubMed] [Google Scholar]
- [35].Morisaki N, Watanabe S, Kobayashi J, et al. Diabetic control and progression of retinopathy in elderly patients: five-year follow-up study. J Am Geriatr Soc 1994;42:142–5. [DOI] [PubMed] [Google Scholar]
- [36].Srinivasan S, Raman R, Kulothungan V, et al. Influence of serum lipids on the incidence and progression of diabetic retinopathy and macular oedema: Sankara Nethralaya Diabetic Retinopathy Epidemiology And Molecular genetics Study-II. Clin Exp Ophthalmol 2017;45:894–900. [DOI] [PubMed] [Google Scholar]
- [37].Chew EY, Davis MD, Danis RP, et al. The effects of medical management on the progression of diabetic retinopathy in persons with type 2 diabetes: the Action to Control Cardiovascular Risk in Diabetes (ACCORD) Eye Study. Ophthalmology 2014;121:2443–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Keech AC, Mitchell P, Summanen PA, et al. Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD study): a randomised controlled trial. Lancet 2007;370:1687–97. [DOI] [PubMed] [Google Scholar]


