Abstract
Stem cell therapy is considered as the most promising treatment for chronic wounds. Extracellular matrix/stromal vascular fraction gel (ECM/SVF gel), an adipose-derived stem cell-based cytotherapy, has shown healing potential in experimental wounds in animal models. However, the effects of ECM/SVF gel on human chronic wounds have not been investigated. The aim of the present study is to investigate the therapeutic effect of ECM/SVF gel on human chronic wounds.
Autologous ECM/SVF gel was prepared and used to treat patients with chronic wounds in clinics, with negative pressure wound therapy as the positive control. Wound healing rate per week and histological changes were performed.
The average wound healing rate per week in the ECM/SVF gel group was 34.55 ± 11.18% compared with 10.16 ± 2.67% in the negative pressure wound therapy group (P < .001). Histological analysis with hematoxylin and eosin, Masson's trichrome staining, and CD31 immunohistochemistry showed less lymphocyte infiltration, more collagen accumulation, and more newly formed vessels in the ECM/SVF gel group treated skins compared to the control.
ECM/SVF gel is an effective therapeutic option for chronic wound healing in clinics.
Keywords: adipose-derived stem cell, extracellular matrix, negative pressure wound therapy, stromal vascular fraction, wound healing
1. Introduction
Chronic wounds are those that have not proceeded through a systematic and timely repair process to produce anatomic and functional integrity after 3 months.[1] Currently, chronic wounds present a noteworthy social and economic burden due to the increasingly aging population and the prevalence of cardiovascular and metabolic diseases worldwide. It has been estimated that approximately 1% to 2% of individuals at any given time could be affected by chronic wounds.[2,3] It has been reported that chronic wounds affect around 6.5 million patients.[4] Moreover, the costs for the treatment of venous leg ulcers are 1% of the total annual health care budget, and health care costs for chronic venous diseases are spiraling.[5,6] Therefore, it is very important, although difficult, to manage and monitor chronic wounds.
Currently, the therapies for chronic wounds may be roughly divided into 4 categories based on trauma to the patients and clinical efficacy: conventional, novel, plastic surgery, and cell based.[7–9] Conventional therapies include active dressings through topical application of growth factors, and various biological dressings such as silver and alginate, and hyperbaric oxygen, among others.[10] These therapies are noninvasive and especially suitable for outpatients under nursing supervision, while the clinical efficacy is moderate. Novel therapies include the use of platelet-rich plasma, negative pressure wound therapy (NPWT), and artificial dermis, among others.[11–13] These require surgical debridement and are minimally invasive with much better healing efficacy than conventional therapies. Plastic operations, such as skin and flap grafting, are invasive and require the sacrifice of healthy skin tissue, which has strict indications.
Adipose-derived stem cell-based therapy is one of the most promising therapeutic strategies for wound healing based on precise physiologic requirements, such as re-epithelization, angiogenesis, and immunomodulation.[14–16] However, several drawbacks still need to be resolved. Application of collagenase in the adipose-derived stem cells (ASCs) harvesting increases the risk of biological contamination. Additionally, culture and purification of ASCs still require specific laboratory equipment and experience. Moreover, in most studies, stromal vascular fraction (SVF) cells and ASC suspensions are used in isolation without the protection of the extracellular matrix (ECM) components, leaving the cells at the recipient site vulnerable to the immune system. Rapid elimination of SVF cells or ASCs by the immune system causes inadequate cell retention after injection, thereby failing therapeutically.[17–20] All these factors limit further application of ASC/SVF cell therapies.
We previously introduced an injectable adipose tissue-derived product, extracellular matrix/stromal vascular fraction gel (ECM/SVF-gel), which is rich in ASCs and adipose native ECM and can be rapidly fabricated by pure mechanical force.[21] With the support of native ECM, ECM/SVF-gel maintains the optimal cell (i.e., SVF cells) retention. It has been reported that the superior healing effect of ECM/SVF gel on wound healing than SVF suspension was observed.[22] However, the effects of ECM/SVF gel on human chronic wounds have not been investigated. The aim of the present study is to investigate the therapeutic effect of ECM/SVF gel on human chronic wounds and NPWT treatment served as positive control.
2. Materials and methods
2.1. Subjects
The study was approved by the Ethical Committee of Southern Medical University and Affiliated Hospital of Zunyi Medical College and investigations were conducted per the Declaration of Helsinki. All subjects provided written informed consent. Subjects were >18 and < 80 years of age and had wounds of duration >3 months. Subjects were excluded if they were in critical condition, such as shock, resulting from various disorders, multiple organ dysfunction, or serious infections; had any hematological diseases or psychiatric conditions; had severe undernourishment or low body weight; were pregnant; or were participating in another clinical trials.
2.2. Fat harvesting and ECM/SVF gel procedure
The ECM/SVF gel was prepared according to previously reported methods.[21] Briefly, under spinal anesthesia, liposuction was performed in the medial thigh with a 3-mm multiport cannula that contained several sharp side-holes of 1-mm in diameter (Tulip Medical Products, San Diego, CA), at –0.75 atm of suction pressure. After sedimentation, the fat was centrifuged at 1200g for 3 minutes, the liquid portion was discarded, and the oil layer collected and saved for further use. The Coleman fat was then mechanically emulsified by shifting between the two 10-mL syringes connected by a female-to-female Luer-Lok connector, at a stable speed (10 mL/s) for 1 minute. The resulting emulsion was filtered using a Nano Transfer filter (Tulip Medical Products, San Diego, CA) to remove connective tissue remnants. Oil (0.5 mL) was added and mixed gently by shifting between syringes 3–5 times until a flocculate was observed within the emulsion. The mixture was then centrifuged at 2000g for 3 minutes resulting in 2 different layers: an oily layer and the ECM/SVF gel layer (Fig. 1).
Figure 1.
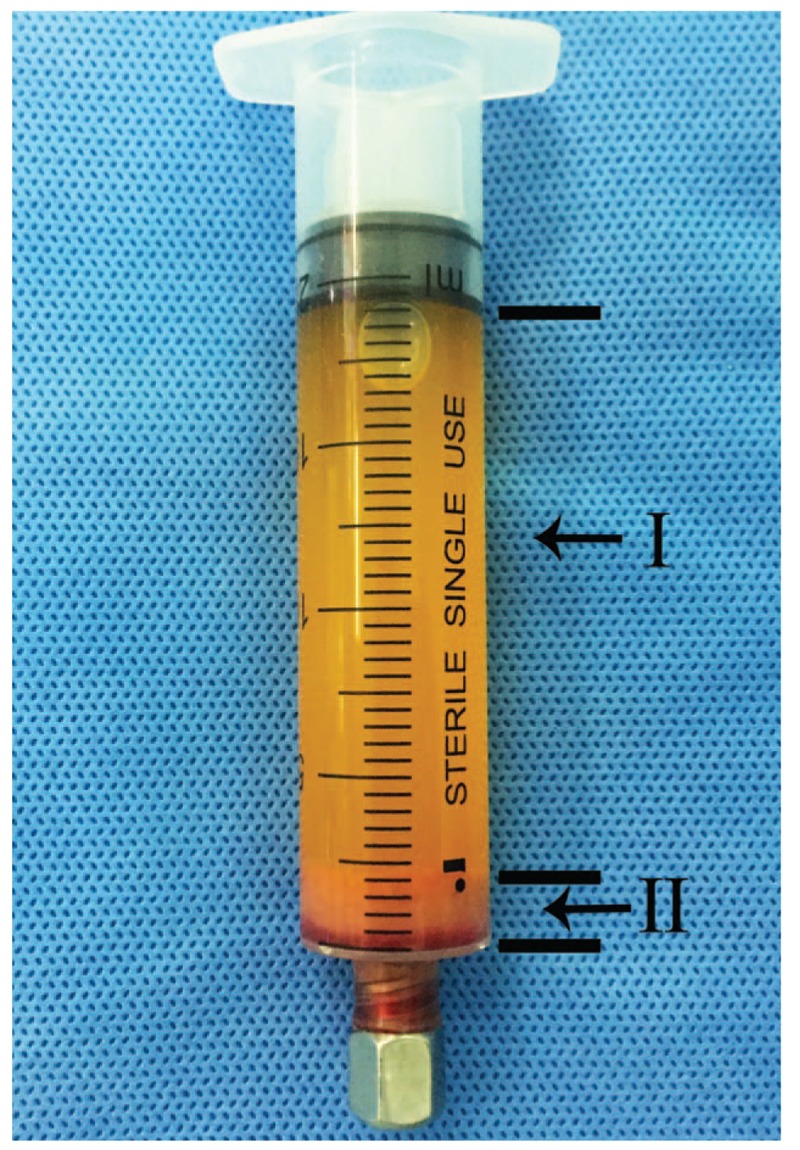
The continuous mechanical dissociation procedure results in 2 different layers: I, oily layer; II, ECM/SVF gel layer. ECM = extracellular matrix, SVF = stromal vascular fraction gel.
2.3. Wound intervention
ECM/SVF group: Conventional wound debridement was performed and the ECM/SVF gel was applied. The volume used was based on the area of the wound, and the ratio was approximately 0.25 mL/cm2. Approximately three-fourths of the prepared ECM/SVF gel was injected directly into the base and edges of the wound and the remaining gel covered the wound as a dressing, which was then covered with physiological saline gauze. After 3 days, the first dressing change was performed, and the gauze was reapplied to retain moisture. The dressings were changed at 2- to 3-day intervals. After discharge, the subjects returned weekly so the wound could be monitored; the remaining wound area was measured and evaluated for complications.
Control group: Conventional wound debridement was performed and NPWT was applied. At 2 weeks postsurgery, the negative pressure drainage equipment was removed, and the wound condition was examined and recorded.
For all subjects, before and at 2 weeks after surgery, digital photographs of the wounds were taken, and peri-wound tissues were harvested by punch biopsy and fixed in 10% paraformaldehyde for histological analysis. All subjects received the same conventional treatments during this study, including pressure off-load of the wound, blood pressure management, blood glucose monitoring, and antibiotics.
2.4. Observational indices
2.4.1. Wound healing rate
Two weeks after surgery, the residual wound area was calculated using a pixel/area ratio program (Adobe Photoshop CS6.0). The healing rate was evaluated as follows: (Original wound area—residual wound area)/original wound area × 100%.
2.4.2. Histological analysis
Peri-wound tissues were fixed in 10% paraformaldehyde, then embedded in paraffin and cut into approximately 4-mm sections that were processed and stained with hematoxylin and eosin (H&E). To observe collagen deposition, Masson's trichrome staining was performed, and relative collagen expression was calculated by Image J. Evaluation of neovascularization was performed using CD31 immunostaining and the number of new blood vessels per 5 × high magnification were counted.
2.4.3. Statistical analysis
Results are expressed as mean ± standard deviation. Data were analyzed using a t test of independent samples by SPSS ver. 19.0 (Armonk, NY). P < .05 was considered statistically significant.
3. Results
3.1. Wound healing rate
Twenty patients who were admitted to the affiliated Hospital of Zunyi Medical College between March of 2016 and September of 2017 were enrolled and evaluated (14 men and 6 women, 40–74 years of age). Wound types included 9 venous stasis ulcers, 5 traumatic infections, 3 diabetic ulcers, 2 scar ulcers, and 1 sarcoidosis. All wounds had remained unhealed for more than 3 months and were unresponsive to standard wound care or skin grafting. Ten subjects were assigned to each treatment group. All patients treated with autologous ECM/SVF gel had a good final outcome. The average wound healing rate was 34.55 ± 11.18%. In the NPWT group, the rate was 10.16 ± 2.67%. The difference was statistically significant (P < .001) (Table 1). There were no donor site complications in the patients who had fat harvested from their thighs (Figs. 2 and 3).
Table 1.
Summary of patient characteristics (mean ± standard deviation).

Figure 2.
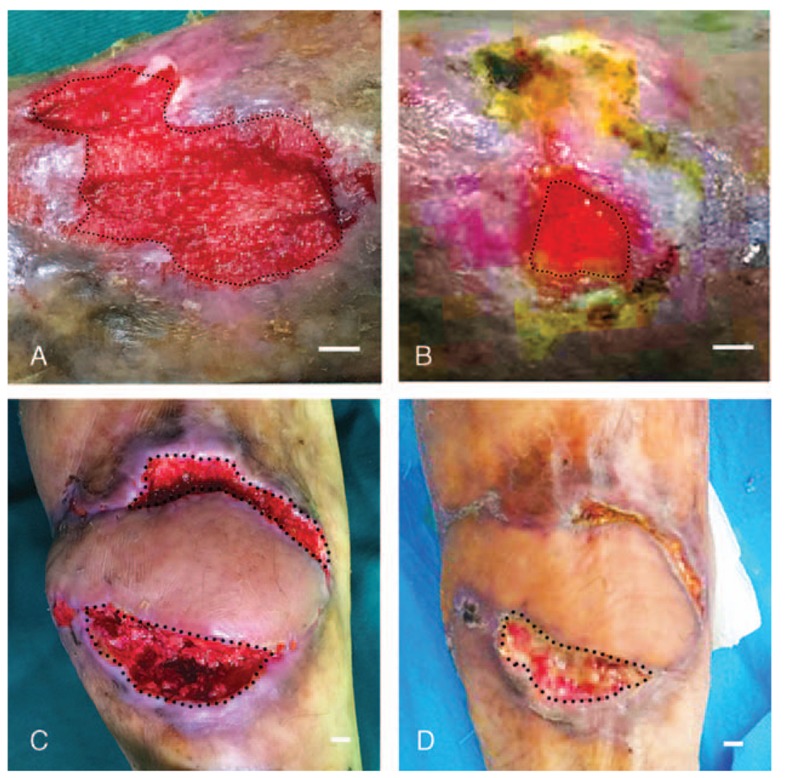
Changes in wounds after ECM/SVF gel grafting. (A) A chronic left foot wound with an original area of 5.70 cm2. (B) Residual area 14 days post-treatment was 1.04 cm2. (C) A chronic left leg wound with an original area of 11.03 cm2. (D) Residual area 14 days post-treatment with ECM/SVF gel grafting was 5.14 cm2. Scale bar = 1 cm. ECM = extracellular matrix, SVF = stromal vascular fraction gel.
Figure 3.
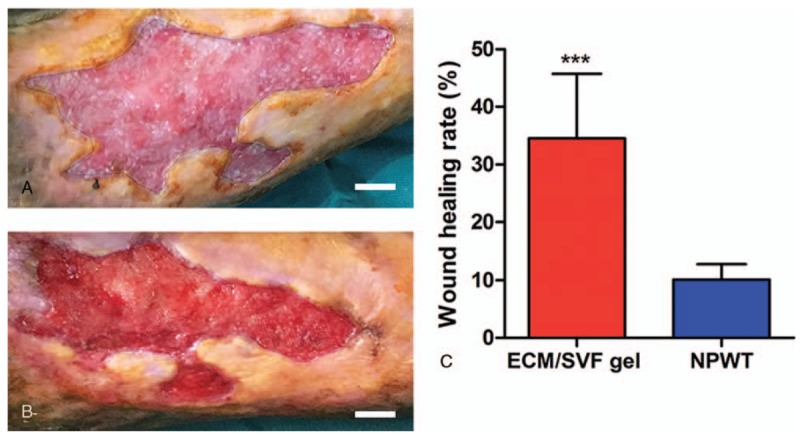
Changes in wounds after treatment with negative pressure wound therapy. (A) A chronic right leg wound with an original area of 19.65 cm2. (B) Residual area 14 days post-treatment with negative pressure wound therapy was 17.34 cm2. (C) Quantification of the wound area of the 2 treatment groups. ∗∗∗P < .001. Scale bar = 1 cm.
3.2. Histological analysis
H&E staining revealed decreased lymphocyte infiltration into the deep dermis layers following ECM/SVF gel administration on day 14 (Fig. 4). Masson's trichrome staining indicated a higher level of collagen accumulation in the ECM/SVF gel group compared with NPWT (P < .001); the collagen was also thicker in the ECM/SVF specimens (Fig. 5). CD31 staining indicated more newly formed vessels in the ECM/SVF specimens than for NPWT (P < .001) (Fig. 6).
Figure 4.
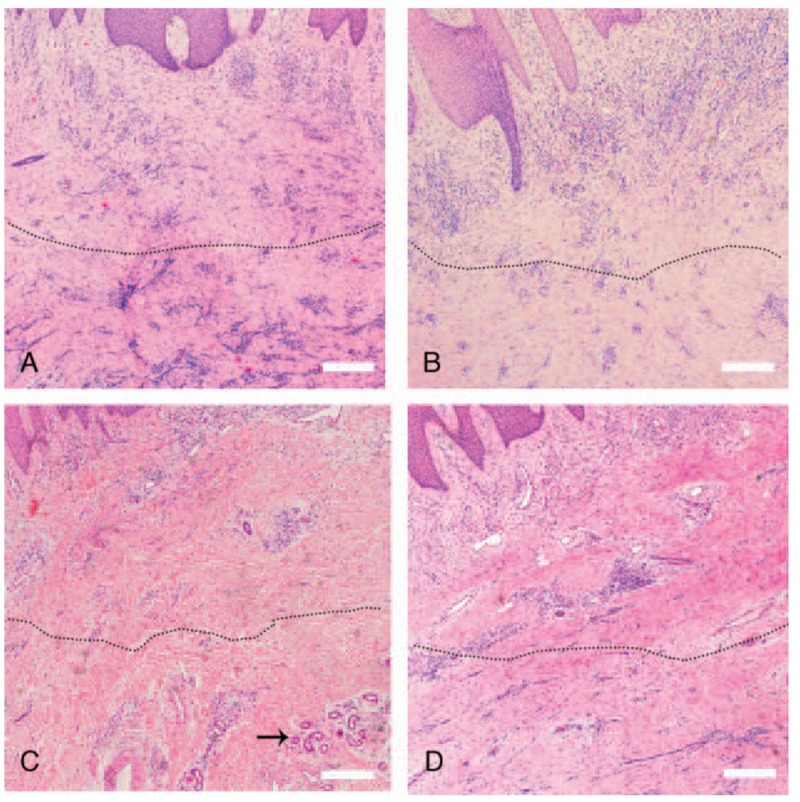
Hematoxylin and eosin staining of tissue from the wound area. (A) Numerous inflammatory cells infiltrate the deep dermal layers (below dotted line) before treatment with ECM/SVF gel grafting. (B) Numerous inflammatory cells infiltrate the deep dermal layers (below dotted line) before negative pressure wound therapy. (C) Decreased inflammatory cell infiltration in the dermis deep layer (below dotted line) and new vascular structures (black arrow) after ECM/SVF gel grafting. (D) The inflammatory cell infiltration had not significantly changed (below dotted line) after negative pressure wound therapy. Scale bar=20 μm. ECM = extracellular matrix, SVF = stromal vascular fraction gel.
Figure 5.
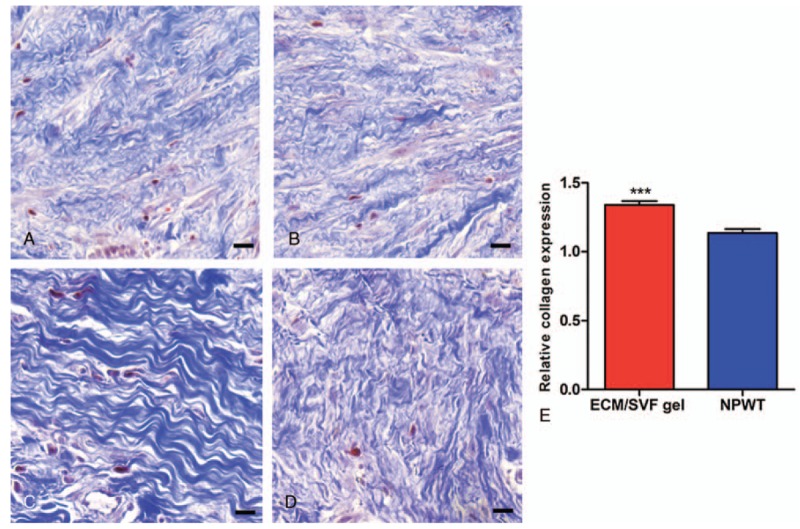
Masson's trichrome staining of tissue from the wound area. (A) Thin collagen layer before ECM/SVF gel grafting. (B) Thin collagen layer before negative pressure wound therapy. (C) The thickest collagen layer was seen after ECM/SVF gel grafting. (D) The collagen layer is thicker after negative pressure wound therapy. (E) Relative quantification of collagen density. ∗∗∗P < .001. Scale bar=20 μm. ECM = extracellular matrix, SVF = stromal vascular fraction gel.
Figure 6.
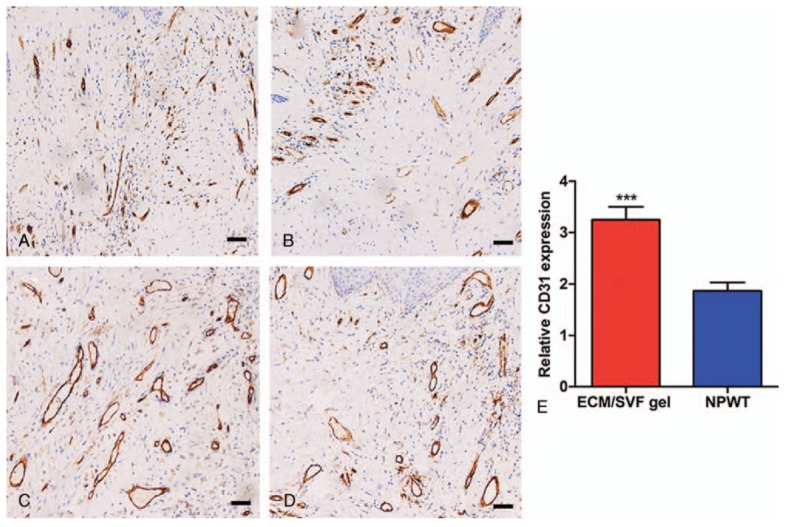
Immunohistochemistry with CD31 of tissue from the wound area. (A) Scant neovascularization before ECM/SVF gel grafting. (B) Scant neovascularization before negative pressure wound therapy. (C) The greatest extent of neovascularization was seen after ECM/SVF gel grafting. (D) Neovascularization increased after negative pressure wound therapy. (E) Relative quantification of neovascularization. ∗∗∗P < .001. Scale bar=20 μm. ECM = extracellular matrix, SVF = stromal vascular fraction gel.
4. Discussion
Although bone marrow-derived mesenchymal stem cells (MSCs) were the first stem cells used to treat wounds,[23,24] adipose-derived stem cells (ASCs) may be a superior alternative. ASCs can be easily harvested from abundant adipose tissue, and have advantages in terms of proangiogenic properties and immunomodulatory effects.[25,26] Numerous animal and clinical works have confirmed the healing effects of ASCs or stromal vascular fraction (SVF), which are heterogeneous cells containing ASCs, on wound healing.[27–32] Our study demonstrates that adipose ECM/SVF gel can support healing of human chronic wounds; this therapy promoted the neoangiogenesis, synthesis of collagen tissue, and induce a favorable immunomodulatory effect on chronic wounds.
The reason of this superior therapeutic effect may be attributed to the concentrated SVF cells and adipose-derived ECM in ECM/SVF gel. According to our previous study, the density of ASCs and endothelial cells (ECs) is approximately 1.9 ± 0.2 × 105 cells/mL and 7.7 ± 2.4 × 104 cells/mL, respectively, in ECM/SVF gel.[21] Therefore, the first factor for accelerating wound healing may be the condensed SVF cells (e.g., ASCs, ECs, pericytes, etc.). There are many studies reported that ASCs have the potential to promote re-epithelization, angiogenesis, and immunomodulatory effects by secretion of many growth factors such as vascular endothelial growth factor (VEGF), hepatocyte growth factor (HGF), epidermal growth factor (EGF), and fibroblast growth factor (FGF), among others.[33–35] Additionally, the differentiation pluripotency of ASC, such as differentiating to epidermal cells and ECs, is also important for re-epithelization and angiogenesis in chronic wounds.[36] It has been reported that ECs directly form capillary cells and are important in angiogenesis.[37]
Another factor for accelerating wound healing is ECM that contains collagen, elastin, mucopolysaccharides, and fibronectin. It has been reported that ECM facilitates migration and morphogenesis of angiogenesis,[38] while collagen and fibronectin are important components during the wound healing process.[39] Moreover, the synergistic interaction among SVF cells, ECM, and the growth factors in the ECM/SVF gel may be the unique factor for accelerating wound healing. For example, the combined application of ECs and pericytes has been proven to augment angiogenesis compared with their individual use.[40] It has already been reported that adipose native ECM may provide a favorable cellular microenvironment for SVF cells adhesion and survival, leading to an accelerated wound healing.[41]
The potential mechanism for accelerating wound healing from ECM/SVF gel was further verified by histology analysis. H&E staining revealed that lymphocyte infiltration into the dermis layers was obviously decreasing after ECM/SVF gel injection, which confirms immunomodulation of ASCs in the lymphocyte-mediated chronic inflammatory response. It has been reported that collagen synthesis and neovascularization are essential for wound healing. Collagen is essential for wound healing as it provides a biological scaffold for the migration of various repair cells and the generation of new vessels.[42] Additionally, increasing the number of type I collagens in the early stages of wound healing can promote healing and reduce scarring.[43] In our study, Masson's trichrome and CD31 staining demonstrated an increased collagen accumulation and newly formed vessels in the ECM/SVF gel group compared with NPWT group.
Recently, Lafosse et al[44] reported the implantation of ASCs seeded a biological dressing (human acellular collagen matrix) in 3 patients with chronic wounds. The results revealed that the matrix supports the cellular adhesion and spreading and improves local ASCs delivery without a significant local or systemic prolonged inflammatory reaction. Although the therapy of this biological dressing is promising, the time-consuming manufacturing remains to be improved. The advantages of using ECM/SVF gel as a cytotherapy tool in treating chronic nonhealing wounds can be summarized as follow. First, ECM/SVF gel therapy is a minimally invasive procedure. The surgical incision is only 3 to 5 mm on the ipsilateral or contralateral thigh for liposuction. Unlike skin grafting or skin flaps, ECM/SVF gel is useful for treating intractable skin ulcers on the lower leg in the elderly who are unable to tolerate general anesthesia without the need to sacrifice healthy skin tissue. Second, there are no ethical issues associated with the clinical applications of ECM/SVF gel. Without collagenase digestion and cultured-in vitro, special permits are not required, which is especially suitable for countries with strict regulation in the use of stem cell therapy. Third, the preparation and injection of ECM/SVF gel is simple and time-saving without much finical burden. The total operation time could be < 2 hours, and the patients can be discharged several days postsurgery and return to the hospital weekly to monitor the wound.
However, a standard ECM/SVF gel-based chronic wound treatment scheme is required. For example, the ratio between transplanted ECM/SVF gel volume and wound bed area should be developed. In addition, more clinical cases and clinical trials are needed to support efficacy, side effect profiles, and long term outcomes.
5. Conclusions
ECM/SVF gel can exert a therapeutic effect on human chronic wounds, which is likely attributed to a favorable immunomodulatory effect, increased collagen accumulation, and improved neovascularization. This therapy is simple, safe, and minimally invasive but should be further improved in terms of the establishment of a standard treatment scheme.
Acknowledgments
This manuscript has been thoroughly edited by a native English speaker from an editing company.
Author contributions
Formal analysis: Jingwei Feng.
Funding acquisition: Feng Lu.
Investigation: Chengliang Deng.
Methodology: Jingwei Feng, Feng Lu.
Project administration: Chengliang Deng, Feng Lu.
Resources: Feng Lu.
Supervision: Feng Lu.
Validation: Liangyue Wang, Feng Lu.
Writing – original draft: Chengliang Deng.
Writing – review & editing: Liangyue Wang.
Footnotes
Abbreviations: ASCs = adipose-derived stem cells, ECM = extracellular matrix, EGF = epidermal growth factor, FGF = fibroblast growth factor, HGF = hepatocyte growth factor, MSCs = mesenchymal stem cells, NPWT = negative pressure wound therapy, SVF = stromal vascular fraction, VEGF = vascular endothelial growth factor.
CD and LW have contributed equally to this work.
This work is supported by National Nature Science Foundation of China (81471881, 81660323).
The authors have no funding and no conflicts of interest to disclose.
References
- [1].Werdin F, Tenenhaus M, Rennekampff HO. Chronic wound care. Lancet (London, England) 2008;372:1860–2. [DOI] [PubMed] [Google Scholar]
- [2].Crovetti G, Martinelli G, Issi M, et al. Platelet gel for healing cutaneous chronic wounds. Transfus Apheresis Sci 2004;30:145–51. [DOI] [PubMed] [Google Scholar]
- [3].Simka M, Majewski E. The social and economic burden of venous leg ulcers: focus on the role of micronized purified flavonoid fraction adjuvant therapy. Am J Clin Dermatol 2003;4:573–81. [DOI] [PubMed] [Google Scholar]
- [4].Boyce ST, Warden GD. Principles and practices for treatment of cutaneous wounds with cultured skin substitutes. Am J Surg 2002;183:445–56. [DOI] [PubMed] [Google Scholar]
- [5].Purwins S, Herberger K, Debus ES, et al. Cost-of-illness of chronic leg ulcers in Germany. Int Wound J 2010;7:97–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Rice JB, Desai U, Cummings AK, et al. Burden of venous leg ulcers in the United States. J Med Econ 2014;17:347–56. [DOI] [PubMed] [Google Scholar]
- [7].Shankaran V, Brooks M, Mostow E. Advanced therapies for chronic wounds: NPWT, engineered skin, growth factors, extracellular matrices. Dermatol Ther 2013;26:215–21. [DOI] [PubMed] [Google Scholar]
- [8].Han G, Ceilley R. Chronic wound healing: a review of current management and treatments. Adv Ther 2017;34:599–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Nicholas MN, Yeung J. Current status and future of skin substitutes for chronic wound healing. J Cutan Med Surg 2017;21:23–30. [DOI] [PubMed] [Google Scholar]
- [10].Raul SK, Joseph SC. With regards to optimal closure method of five-millimeter trocar sites. Am J Surg 2004;187:24–7. Am J Surg 2006;191:566–7. [DOI] [PubMed] [Google Scholar]
- [11].Menke MN, Menke NB, Boardman CH, et al. Biologic therapeutics and molecular profiling to optimize wound healing. Gynecol Oncol 2008;111(2 suppl):S87–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Martinez-Zapata MJ, Marti-Carvajal AJ, Sola I, et al. Autologous platelet-rich plasma for treating chronic wounds. Cochrane Database Syst Rev 2016;CD006899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Langer V, Bhandari PS, Rajagopalan S, et al. Negative pressure wound therapy as an adjunct in healing of chronic wounds. Int Wound J 2015;12:436–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wu Y, Chen L, Scott PG, et al. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells (Dayton, Ohio) 2007;25:2648–59. [DOI] [PubMed] [Google Scholar]
- [15].Kuo YR, Wang CT, Cheng JT, et al. Bone marrow-derived mesenchymal stem cells enhanced diabetic wound healing through recruitment of tissue regeneration in a rat model of streptozotocin-induced diabetes. Plast Reconstr Surg 2011;128:872–80. [DOI] [PubMed] [Google Scholar]
- [16].Sorice S, Rustad KC, Li AY, et al. The role of stem cell therapeutics in wound healing: current understanding and future directions. Plast Reconstr Surg 2016;138(3 suppl):S31–41. [DOI] [PubMed] [Google Scholar]
- [17].Park IS, Chung PS, Ahn JC. Enhanced angiogenic effect of adipose-derived stromal cell spheroid with low-level light therapy in hind limb ischemia mice. Biomaterials 2014;35:9280–9. [DOI] [PubMed] [Google Scholar]
- [18].Cheng NC, Wang S, Young TH. The influence of spheroid formation of human adipose-derived stem cells on chitosan films on stemness and differentiation capabilities. Biomaterials 2012;33:1748–58. [DOI] [PubMed] [Google Scholar]
- [19].Yoshimura K, Aoi N, Suga H, et al. Ectopic fibrogenesis induced by transplantation of adipose-derived progenitor cell suspension immediately after lipoinjection. Transplantation 2008;85:1868–9. [DOI] [PubMed] [Google Scholar]
- [20].Arana M, Gavira JJ, Pena E, et al. Epicardial delivery of collagen patches with adipose-derived stem cells in rat and minipig models of chronic myocardial infarction. Biomaterials 2014;35:143–51. [DOI] [PubMed] [Google Scholar]
- [21].Yao Y, Dong Z, Liao Y, et al. Adipose extracellular matrix/stromal vascular fraction gel: a novel adipose tissue-derived injectable for stem cell therapy. Plast Reconstr Surg 2017;139:867–79. [DOI] [PubMed] [Google Scholar]
- [22].Sun M, He Y, Zhou T, et al. Adipose extracellular matrix/stromal vascular fraction gel secretes angiogenic factors and enhances skin wound healing in a murine model. BioMed Res Int 2017;2017:3105780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Chen L, Tredget EE, Wu PY, et al. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One 2008;3:e1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Rogers LC, Bevilacqua NJ, Armstrong DG. The use of marrow-derived stem cells to accelerate healing in chronic wounds. Int Wound J 2008;5:20–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Schubert T, Xhema D, Veriter S, et al. The enhanced performance of bone allografts using osteogenic-differentiated adipose-derived mesenchymal stem cells. Biomaterials 2011;32:8880–91. [DOI] [PubMed] [Google Scholar]
- [26].Veriter S, Aouassar N, Adnet PY, et al. The impact of hyperglycemia and the presence of encapsulated islets on oxygenation within a bioartificial pancreas in the presence of mesenchymal stem cells in a diabetic Wistar rat model. Biomaterials 2011;32:5945–56. [DOI] [PubMed] [Google Scholar]
- [27].Zonari A, Martins TM, Paula AC, et al. Polyhydroxybutyrate-co-hydroxyvalerate structures loaded with adipose stem cells promote skin healing with reduced scarring. Acta Biomaterial 2015;17:170–81. [DOI] [PubMed] [Google Scholar]
- [28].Nie C, Yang D, Xu J, et al. Locally administered adipose-derived stem cells accelerate wound healing through differentiation and vasculogenesis. Cell Transplant 2011;20:205–16. [DOI] [PubMed] [Google Scholar]
- [29].Marino G, Moraci M, Armenia E, et al. Therapy with autologous adipose-derived regenerative cells for the care of chronic ulcer of lower limbs in patients with peripheral arterial disease. J Surg Res 2013;185:36–44. [DOI] [PubMed] [Google Scholar]
- [30].Rigotti G, Marchi A, Galie M, et al. Clinical treatment of radiotherapy tissue damage by lipoaspirate transplant: a healing process mediated by adipose-derived adult stem cells. Plast Reconstr Surg 2007;119:1409–22. discussion 1423-1404. [DOI] [PubMed] [Google Scholar]
- [31].Atalay S, Coruh A, Deniz K. Stromal vascular fraction improves deep partial thickness burn wound healing. Burns 2014;40:1375–83. [DOI] [PubMed] [Google Scholar]
- [32].Han SK, Kim HR, Kim WK. The treatment of diabetic foot ulcers with uncultured, processed lipoaspirate cells: a pilot study. Wound Repair Reg 2010;18:342–8. [DOI] [PubMed] [Google Scholar]
- [33].Shingyochi Y, Orbay H, Mizuno H. Adipose-derived stem cells for wound repair and regeneration. Exp Opin Biol Ther 2015;15:1285–92. [DOI] [PubMed] [Google Scholar]
- [34].Hassan WU, Greiser U, Wang W. Role of adipose-derived stem cells in wound healing. Wound Repair Regen 2014;22:313–25. [DOI] [PubMed] [Google Scholar]
- [35].Kuo YR, Wang CT, Cheng JT, et al. Adipose-derived stem cells accelerate diabetic wound healing through the induction of autocrine and paracrine effects. Cell Transplant 2016;25:71–81. [DOI] [PubMed] [Google Scholar]
- [36].Rehman J, Traktuev D, Li J, et al. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation 2004;109:1292–8. [DOI] [PubMed] [Google Scholar]
- [37].Chavakis E, Dimmeler S. Regulation of endothelial cell survival and apoptosis during angiogenesis. Arteriosclerosis Thrombosis Vasc Biol 2002;22:887–93. [DOI] [PubMed] [Google Scholar]
- [38].Bauer AL, Jackson TL, Jiang Y. Topography of extracellular matrix mediates vascular morphogenesis and migration speeds in angiogenesis. PLoS Comput Biol 2009;5:e1000445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Midwood KS, Williams LV, Schwarzbauer JE. Tissue repair and the dynamics of the extracellular matrix. Int J Biochem Cell Biol 2004;36:1031–7. [DOI] [PubMed] [Google Scholar]
- [40].Traktuev DO, Prater DN, Merfeld-Clauss S, et al. Robust functional vascular network formation in vivo by cooperation of adipose progenitor and endothelial cells. Circ Res 2009;104:1410–20. [DOI] [PubMed] [Google Scholar]
- [41].van Dongen JA, Stevens HP, Parvizi M, et al. The fractionation of adipose tissue procedure to obtain stromal vascular fractions for regenerative purposes. Wound Repair Regen 2016;24:994–1003. [DOI] [PubMed] [Google Scholar]
- [42].Finnerty CC, Jeschke MG, Branski LK, et al. Hypertrophic scarring: the greatest unmet challenge after burn injury. Lancet (London, England) 2016;388:1427–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Slemp AE, Kirschner RE. Keloids and scars: a review of keloids and scars, their pathogenesis, risk factors, and management. Curr Opin Pediatr 2006;18:396–402. [DOI] [PubMed] [Google Scholar]
- [44].Lafosse A, Desmet C, Aouassar N, et al. Autologous adipose stromal cells seeded onto a human collagen matrix for dermal regeneration in chronic wounds: clinical proof of concept. Plastic Reconstruct Surg 2015;136:279–95. [DOI] [PubMed] [Google Scholar]


