Abstract
Background:
Immunotherapy is emerging as a new treatment strategy for gastric cancer(GC). However, the efficacy and safety of this technique remain unclear. This meta-analysis aimed to assess the effect of cytokine-induced killer cell (CIK)/dendritic cell–cytokine-induced killer cell (DC-CIK) treatment for GC after surgery.
Methods:
Hazard ratio (HR), overall survival (OS) rates, and disease-free survival (DFS) rates were calculated using a Mantel-Haenszel (M-H) fixed-effects model (FEM), and results were displayed using forest plots. Publication bias was assessed by Begg test, and data were presented using funnel plots. Date robustness was assessed by the trim and fill method. Descriptive analysis was performed on T lymphocytes and adverse effects.
Results:
In total, 9 trials, including 1216 patients, were eligible for inclusion in this meta-analysis. Compared with the control group, the HR for OS was 0.712 (95% confidence interval [CI] 0.594–0.854) and 0.66 (95% CI 0.546–0.797) for overall (DFS). The risk ratio (RR) of the 3 and 5-year OS rate was 1.29 (95% CI 1.15–1.46) and 1.73 (95% CI 1.36–2.19), respectively. The RR for the 3 and 5-year DFS rate 1.40 (95% CI 1.19–1.65) and 2.10 (95% CI1.53–2.87), respectively. The proportion of patients who were CD3+, CD4+, and CD4+/CD8+ increased in the cellular therapy groups. No fatal adverse reactions were noted.
Conclusion:
Chemotherapy combined with CIK/DC-CIK therapy after surgery resulted in low HR, and significantly increasing OS rates, DFS rates, and T-lymphocyte responses in patients with GC.
Keywords: cytokine-induced killer, dendritic cell, gastric cancer, meta-analysis
1. Introduction
Gastric cancer (GC) is 1 of the most common malignant tumors in the world, and Japan, South Korea, and China are associated with the high incidence of GC. In 2015, China's cancer statistics highlighted that the incidence of male GC was 0.477%, and that the mortality rate was 0.339%—second only to lung cancer. The incidence of GC in women was 0.201%, whereas the mortality rate was 0.158%—second only to breast cancer. Surgical treatment is considered to an effective strategy for GC.[1] However, the use of chemotherapy after surgery can further improve patient survival. However, the effective rate of chemotherapy for GC varies from 10% to 60%, and the survival rate of patients with GC remains unsatisfactory.[2]
The present study aimed to highlight ways in which the survival time of patients could be increased by chemotherapy after surgery. In April 2010, the US Food and Drug Administration (FDA) approved the first autologous cellular immunotherapy drug, Sipuleucel-T,[3] indicating that immunotherapy has great potential for cancer treatment. In recent years, cancer immunotherapy has developed rapidly, including tumor-infiltrating lymphocytes (TILs), natural killer (NK) cells, and cytokine-induced killer (CIK) cells; of these, dendritic cells (DCs), combined with CIKs (DC-CIK), was the focus of our current research.[4,5] CIK cells can be cultured from peripheral blood lymphocytes using anti-CD3 monoclonal antibodies, interferon (IFN)-gamma and interleukin (IL)-2 in vitro, and have specific cytotoxic effects on tumor cells.[6] CIK cells can induce apoptosis in tumor cells or lead to cell death directly, and secreted cytokines such as IL-2 and IFN-gamma can regulate the immune system.[7] DCs are the most effective antigen-presenting cells in the body, presenting tumor antigens to T lymphocytes and inducing antitumor immune responses.[8] DCs also play a key role in tumor immunity. The combination of DCs and CIKs leads to a remarkable increase in cytotoxic activity.[9]
Some clinical trials had suggested that immunotherapy can be effective in the treatment of GC. However, there is still no valid evidence to prove the accuracy of such treatment. Thus, we performed a systematic review and meta-analysis of existing clinical trials to assess the efficacy and tolerability of immunotherapy for patients with GC.
2. Materials and methods
2.1. Search strategy and selection criteria
The trials analyzed in this study were identified by an electronic search of the Cochrane Library, PubMed, and Embase. The search was limited to articles published between January, 2000 and August, 2017.The search terms were “dendritic cells,” “Cytokine-Induced Killer Cells,” or “DC-CIK” combined with “gastric cancer.” The detailed search strategy for PubMed is shown in Fig. 1.
Figure 1.

Search strategy.
2.2. Data collection and quality assessment
This meta-analysis included articles comparing chemotherapy combining CIK/DC-CIK therapy and conventional chemotherapy for patients with GC after surgery. The inclusion criteria were as follows: English-language studies published in PubMed, the Cochrane Library, and Embase; articles comparing CIK cell therapy and conventional chemotherapy for patients with GC after surgery; clinical trials in humans approved by a local ethical committee in which patients provided informed written consent before entry into the study.
Books, letters, expert opinions, case reports, editorials, and studies in animals and cell lines were excluded. In the second round of selection, we also excluded articles which were not written in English and not aimed at investigating the association between CIK cell therapy and GC.
2.3. Data collection and quality assessment
Data extraction was independently conducted by 2 investigators (XW and ST), according to a predefined protocol. Disagreement was adjudicated by another reviewer (JY) after referring back to the original publications.
The Newcastle-Ottawa Scale(NOS) (www.ohri.ca/programs/clinical_epidemiology/oxford.asp) was used to evaluate all selected literature. Two investigators graded literature based on the NOS, and disputes were settled by discussion.
2.4. Evaluation of curative effect
The primary clinical endpoints in trials for cancer therapies were the hazard ratios (HRs) of overall survival (OS) and disease-free survival (DFS). The secondary endpoints were the 3 and 5-year survival rates, and the 3 and 5-year DFS rates.
Overall survival was defined as the time from the initiation of treatment until death from any cause. DFS was defined as the length of time from the initiation of treatment to the first evidence of recurrence or death. The HR represented the hazard in 1 group as a constant proportion of the hazard to the other group. Descriptive analysis was performed on T lymphocytes and adverse effects. Lymphocyte subsets determined before and after combination therapy were used to assess immunization status, including CD3+, CD4+, CD8+, CD4+/CD8+, and NK cell ratios.
2.5. Bias analysis
The I2 index was first calculated to assess heterogeneity. If the I2 was greater than 50, then a Mantel-Haenszel (M-H) fixed-effects model (FEM) was used for data without statistically significant heterogeneity, whereas a DerSimonian and Laird (D-L) random-effects model (REM) was used for data with statistically significant heterogeneity.
Publication bias was assessed by Begg tests using STATA 12.0 software (Stata Statistical Software: Release 12. College Station, TX: StataCorp LP) or Review Manager 5.3, and data were presented with funnel plots. The robustness of our results were assessed by the trim and fill method.
3. Results
3.1. Search results
A total of 2905 articles were identified during the initial search. After careful review of the title and abstract, 2777 articles were excluded. In total, 58 studies were selected as potentially relevant. After referring to the full texts, 49 articles were removed (45 due to insufficient data and 4 because they were reviews or meta-analyses). Finally, 9 trials including 1216 patients were eligible for inclusion in the present meta-analysis. The reasons for exclusion are illustrated in Fig. 2.
Figure 2.
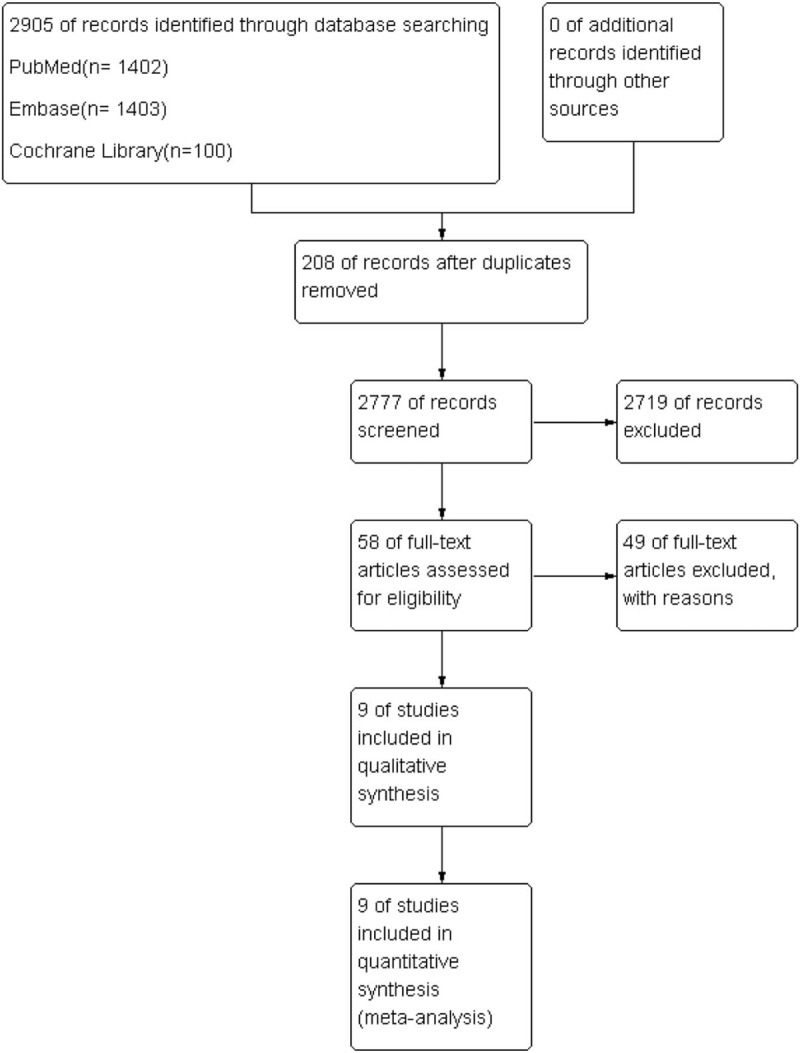
Selection procedure used to identify clinical trials.
3.2. Characteristics of the patients and clinical trials and quality assessment
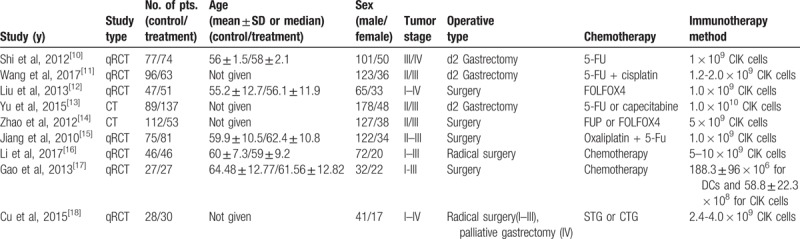
The characteristics of all trials involved in this study are summarized in Table 1; in all, 1159 patients of stage I to IV who underwent different surgical treatments for GC were included in the present analysis. In total, 562 patients were treated using CIK/DC-CIK combined with chemotherapy, whereas 597 patients were treatment using chemotherapy alone. The major adoptive cellular treatments utilized in all trials contained CIK cells, expanded activated autologous lymphocytes (EAALs), and tumor-associated lymphocytes (TALs).The number of adoptive cells transfused into patients in these studies exceeded 1.0 × 109.
Table 1.
Summary of characteristics.
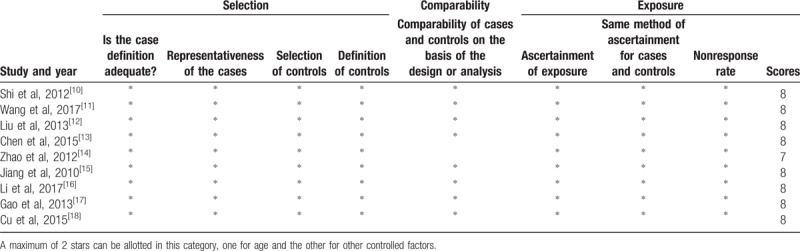
The NOS was used to assess the quality of studies in this meta-analysis. Eight studies were scored at 8 points and 1 study was scored at 7 points (Table 2).
Table 2.
Newcastle-Ottawa Scale for assessing the quality of studies in meta-analysis.
3.3. Efficacy assessments
The M-H FEM was applied for our meta-analysis for 562 patients who received CIK/DC-CIK therapy in combination with conventional chemotherapy, and for 597 patients who received conventional therapy alone. HR is represented as HR with 95% CIs. A small HR value indicates a better therapeutic effect and a HR <1 indicates lower risk. In the overall analysis, the survival status in the treatment group was significantly better than that in the control group with an HR of 0.712 (95% CI 0.594–0.854) for OS (Fig. 3A) and a HR of 0.66 (95% CI 0.546–0.797) for overall DFS (Fig. 3B). In the subgroup analysis, compared with the control group, patients in the treatment group exhibited better survival status in the 3 and 5-year OS rate with corresponding risk ratios (RRs) of 1.29 (95% CI 1.15–1.48) and 1.73 (95% CI 1.36–2.19) (Fig. 4). In addition, the DFS of patients in the treatment group was significantly improved compared with that in the control groups for the 3 and 5-year DFS rate with corresponding RRs of 1.40 (95% CI 1.19–1.65) and 2.10 (95% CI 1.53–2.87), respectively (Fig. 4).
Figure 3.
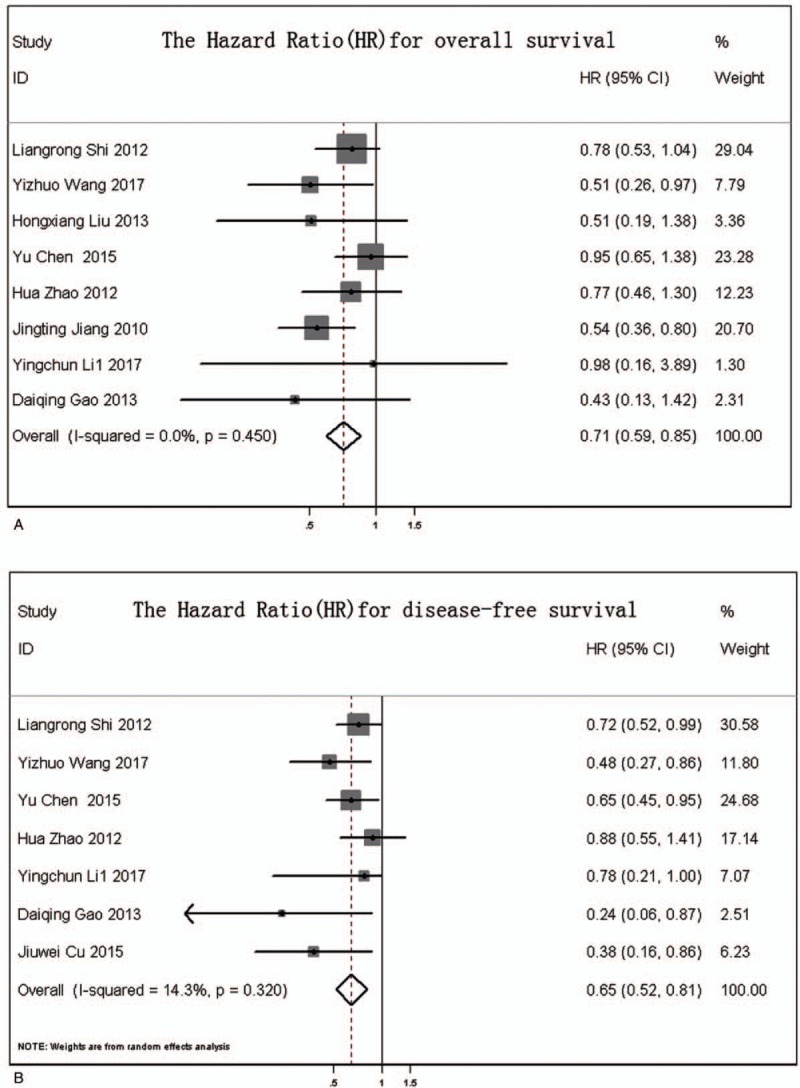
(A) Forest plot of hazard ratio (HR) for overall survival; (B) forest plot of HR for overall disease-free survival.
Figure 4.
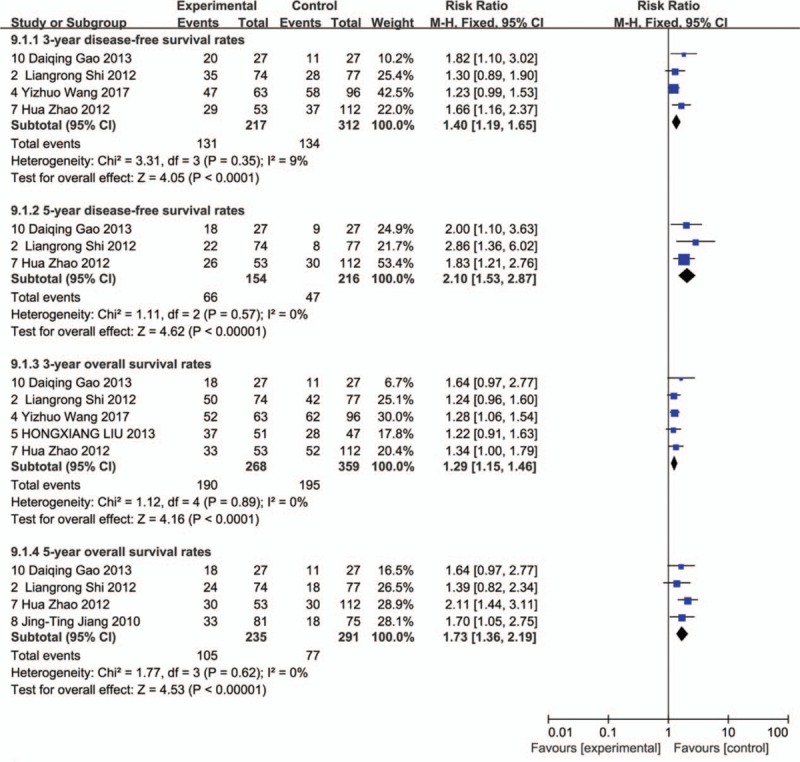
Forest plot of overall survival and disease-free survival for treatment and control groups over 3 and 5-year periods.
3.4. T lymphocytes and subsets
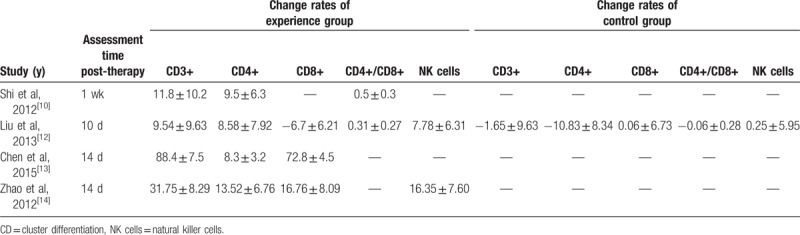
The effects of immune function were evaluated by comparing changes in T lymphocytes before and after treatment across a range of different studies. After 1 to 2 weeks of CIK/DC-CIK treatment, the number of CD3+, CD4+, CD4+/CD8+, and NK cells in patients of the treatment group were increased significantly. The results of the study by Liu et al[12] showed that the proportion of CD8+ decreased after CIK/DC-CIK cells immunotherapy and increased slightly in the control group, which was also increased in the treatment group of other studies (Table 3).
Table 3.
Phenotypic analysis of T lymphocytes and subsets of the control and experience groups.
3.5. Toxicity and side effects
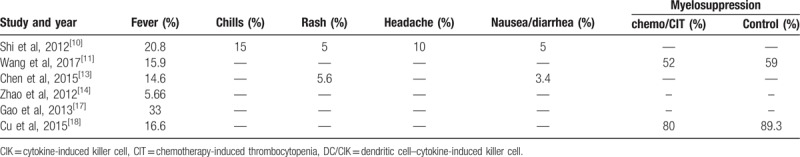
Adverse reactions during treatment are shown in Table 4. The most common adverse event was fever. Chills, rashes, headaches, and nausea were also reported, but not common. There were no severe instances of diarrhea, shock, abnormalities in routine blood tests, hepatic dysfunction, or renal dysfunction. In addition, the incidence of myelosuppression in the immunotherapy group was lower than in the control group.
Table 4.
Side effects of CIK/DC-CIK treatment and myelosuppression.
3.6. Response rate
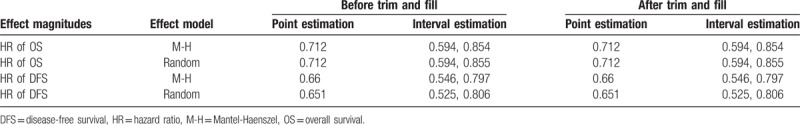
The I2 was less than 20% for both groups. To determine whether there was publication bias for the included studies, we performed Begg test and presented data in the form of Funnel plots. As shown in Fig. 5, there was poor symmetry in our meta-analysis of HR post-CIK and conventional treatments. Trim and fill analysis was then used to evaluate potential bias in the results (Table 5). There was no change before and after data processing.
Figure 5.
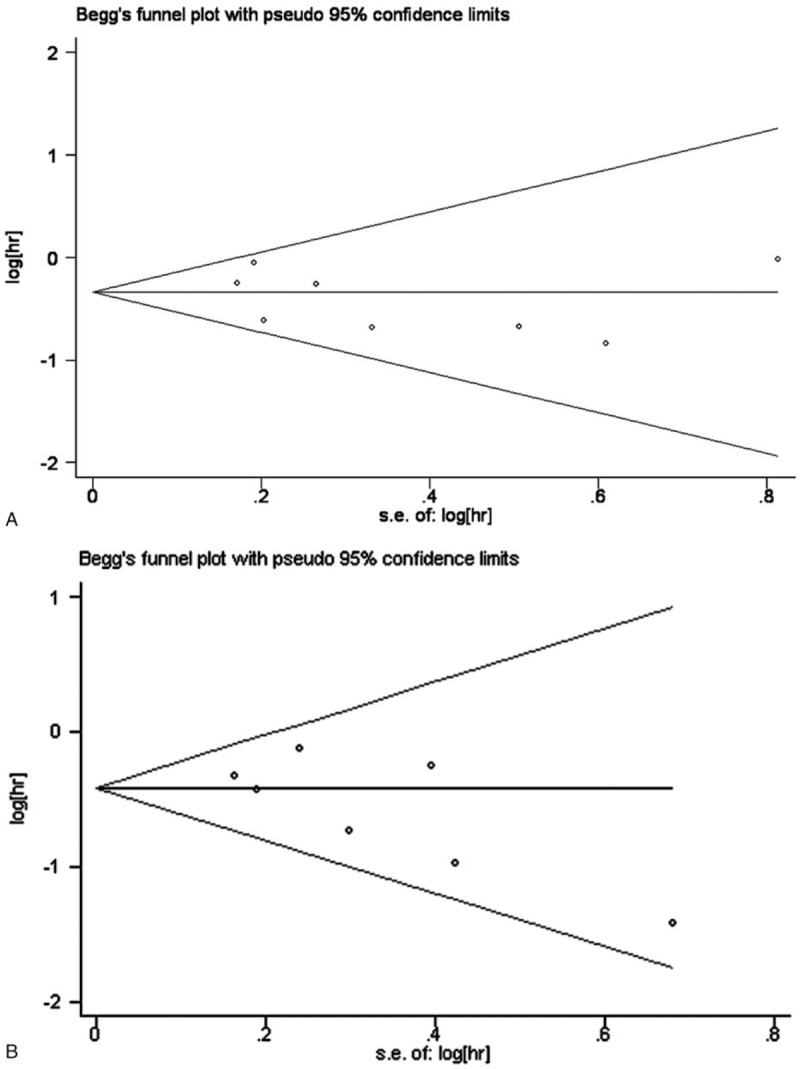
(A) Funnel plot of HR for the overall survival; (B) funnel plot of HR for overall disease-free survival.
Table 5.
Results of sensitivity analysis.
4. Discussion
With excellent performance of immunotherapy as an antitumor therapy, a number of clinical trials for immunotherapy have been carried out in a wide range of centers. DCs represent an important cell type for immunotherapy. For example, Ishigami et al[19] found that GC patients with high levels of DC cell invasion were less likely to develop lymph node metastasis and the 5-year survival rate improved significantly (approximately 78%). DCs are often used as an active immunotherapy for antitumor therapy as a key cell for antitumor immunity. Furthermore, CIK cells have shown strong cytotoxicity against tumor cell lines and freshly isolated tumor samples, such as liver cancer,[20] lung cancer,[21] glioma,[22] and GC.[23] In comparison with other immune cells, CIK cells showed higher proliferation rates and more resistance to tumor activity across a broader spectrum of tumors.[24] Therefore, cellular immunotherapy, based on CIK/DC-CIK technology, presents a promising method for treating malignant tumors.
Chemotherapy has been considered to impair immune mechanisms. In fact, chemotherapy may increase the antitumor response by increasing the release of tumor antigens.[25] By eliminating the activity of Treg cells and myeloid-derived suppressor cells, chemotherapy can enhance antitumor T-cell function and may lead to a more effective antitumor immune response.[26] The study of chemotherapy, combined with immunotherapy for GC, has attracted extensive attention. However, the precise effects of chemotherapy combining immunotherapy in GC after gastrectomy are still unclear. In this meta-analysis, we studied 9 clinical trials including 594 GC patients who received chemotherapy combining CIK/DC-CIK therapy after gastrectomy. The efficacy and safety of GC patients who underwent gastrectomy and then received chemotherapy combining CIK/DC-CIK therapy were evaluated.
Efficacy was a key factor for clinical therapy. Our analysis showed that chemotherapy, combined with CIK/DC-CIK, after gastrectomy, increased the efficacy of GC treatment. The HR of OS was 0.712 (95% CI 0.594–0.854), compared with the control group, showing a lower risk of death in group receiving chemotherapy combined with CIK/DC-CIK. We also analyzed the 3 and 5-year survival rate. RR was used as an indicator of intervention and was 1.29 (95% CI 1.15–1.46) for the 3-year OS and 1.73 (95% CI 1.36–2.19) for the 5-year OS. These results indicate that the 3 and 5-year survival rate for the group receiving CIK/DC-CIK increased by 29% and 73%, respectively, compared with the control group.
Disease-free survival was used to assess the quality of living. Compared with the control group, the HR of DFS was 0.66 (95% CI 0.546–0.797). The RR of 3 and 5-year DFS rates were 1.40 (95% CI 1.19–0.1.65) and 2.1 (95% CI 1.53–2.87), respectively. We found a significant improvement in the quality of life of the patients, in which the 3-year DFS rate increased by 40% and 3-year DFS rate increased by 110%.
The I2 was less than 20% for both groups, indicating no evidence of heterogeneity among the individual studies. The result of Begg test for the included studies showed that the P value was greater than 0.05 and not very symmetrical. This meant that the results may have been biased. Thus, we used the trim and fill method to specifically assess bias. There were no changes in the 95% CIs before and after the trim and fill method; thus, the analysis was stable. Bias analysis showed that the results of our meta-analysis were reliable with no obvious heterogeneity.
Typically, the numerical changes in T lymphocytes can be used to reflect changes in immune function. The articles included in our study only showed the changes of immune cells before and after treatment. Only 1 article showed a change in the control group. Therefore, we aimed to describe changes in the number of immune cells. Numerous cycles of chemotherapy may lead to a reduction in the immune function of patients with GC, with reduced proportions of T lymphocytes.[27] The measurement of T-lymphocyte subsets has been reported to be a useful clinical indicator of immunosuppression in a number of disease states.[28] CD3+ is predominantly a phenotypic marker of T cells, and CD8+ is predominantly a phenotypic marker of cytotoxic T lymphocytes (CTLs). CD4+T is predominantly a marker of helper T cell, which promotes the activation of CD8+ T cells and enhances the differentiation of CTLs. By comparing changes in T lymphocytes, and its subsets, for 1 to 2 weeks, several studies[10,12,13] have shown that the number of CD3+, CD4+, CD8+, and NK cells were significantly increased, after immunotherapy with CIK/DC-CIK cells. These results indicate that CIK/DC-CIK therapy can improve the immune status of patients, and the reason may be associated with increased T lymphocyte and cytotoxic T lymphocytes. However, these studies only showed short-term changes in T-lymphocyte numbers after treatment, and there was no evidence for long-term results.
Toxicity and adverse reactions are important indicators of immunotherapy. Our analysis revealed that fever was the most common adverse event in CIK/DC-CIK treatment, which could represent an immunological response to the presence of effective cells in cancer patients. Other effects (such as nausea and headache) could be relieved without medication or by simple treatment. No fatal adverse reactions were reported in the internalized study. In addition, CIK/DC-CIK therapy reduced bone marrow suppression caused by chemotherapy. Thus, adoptive cell immunotherapy combined with adjuvant therapy was considered to be a feasible and safe method for the treatment of GC.
Although the results were clear, this study has several limitations. Firstly, the difference between the number of patients involved in each study may have led to partial differences. Secondly, there were differences in the use of immune cells across different studies. The immune responses induced by different immune cells were different and may have had different effects on the development of the disease. Furthermore, different surgical procedures may have led to different outcomes, thus creating a study bias; patients in stages I to III underwent radical surgery, whereas patients in stage IV underwent palliative surgery.
In this meta-analysis, we demonstrate that immunotherapy was significantly effective for patients when combined with chemotherapy after surgery. We expect more multicenter randomized trials to be conducted to verify the efficacy of this technique in the near future. This therapy is a potentially effective strategy for the treatment of GC. Although preclinical studies showed that immunotherapy has a significant effect upon GC, many problems need to be solved urgently, for example, is use of immunotherapy combined with chemotherapy more effective? What is the cycle and duration of maintenance of immunotherapy? The prospect of immunotherapy for GC is promising, but more research and a standardized treatment regimen are still required.
Acknowledgments
The authors are grateful to the authors of all eligible studies included in this meta-analysis.
Author contributions
Xiang Wang contributed to concept, design, date, acquisition, analysis, data interpretation and drafted the manuscript. Song Tang helped with data extraction, statistical input and interpretation. Xiang Cui was involved in search strategy and literature searches. Jinwei Yang assisted with screening, abstraction, and assessing the risk of bias. Chunyu Geng was involved in screening and data abstraction. Cong Chen took part in the initial study designand edited drafts of the manuscript. Ning Zhou was involved in initial study design and edited drafts of the manuscript. Yumin Li took part in supervision, final editing/critical revisions, interpretation and important intellectual content.
Conceptualization: Xiang Wang, Yumin Li.
Data curation: Xiang Wang, Song Tang, Xiang Cui, Chunyu Geng, Cong Chen.
Formal analysis: Xiang Wang, Song Tang, Xiang Cui, Chunyu Geng, Cong Chen.
Funding acquisition: Yumin Li.
Software: Jinwei Yang.
Validation: Jinwei Yang.
Writing – original draft: Xiang Wang.
Writing – review & editing: Cong Chen, Ning Zhou, Yumin Li.
Footnotes
Abbreviations: CD = cluster differentiation, CIK = cytokine-induced killer cell, CTLs = cytotoxic T lymphocytes, DC = dendritic cell, DC-CIK = dendritic cell–cytokine-induced killer cell, FDA = Food and Drug Administration, FEM = fixed-effects model, GC = gastric cancer, HR = hazard ratio, IFN = interferon, IL-2 = interleukin-2, M-H = Mantel-Haenszel, NK cells = natural killer cells, NOS = Newcastle-Ottawa Scale, OS = overall survival, DFS= disease-free survival, TILs = tumor-infiltrating lymphocytes.
XW and ST contributed equally.
Funding: This study was supported by the National Natural Science Foundation of China (grant no. 31270532) and National International Scientific and Technological Cooperation Program, China (grant no. 2015DFA31650).
The authors declare that they have no competing financial interests.
References
- [1].Biondi A, Persiani R, Cananzi F, et al. R0 resection in the treatment of gastric cancer: room for improvement. World J Gastroenterol 2010;16:3358–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Sastre J, Garcia-Saenz JA, Diaz-Rubio E. Chemotherapy for gastric cancer. World J Gastroenterol 2006;12:204–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].DeFrancesco L. Landmark approval for Dendreon's cancer vaccine. Nat Biotechnol 2010;28:531–2. [DOI] [PubMed] [Google Scholar]
- [4].Chen B, Liu L, Xu H, et al. Effectiveness of immune therapy combined with chemotherapy on the immune function and recurrence rate of cervical cancer. Exp Ther Med 2015;9:1063–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Zhu XP, Xu YH, Zhou J, et al. A clinical study evaluating dendritic and cytokine-induced killer cells combined with concurrent radiochemotherapy for stage IIIB non-small cell lung cancer. Genet Mol Res 2015;14:10228–35. [DOI] [PubMed] [Google Scholar]
- [6].Zhong GC, Yan B, Sun Y, et al. [Clinical efficacy of immunotherapy of dendritic cell and cytokine-induced killer cell combined with chemotherapy for treatment of multiple myeloma]. Zhonghua xue ye xue za zhi 2012;33:1000–3. [PubMed] [Google Scholar]
- [7].Li GX, Zhao SS, Zhang XG, et al. Comparison of the proliferation, cytotoxic activity and cytokine secretion function of cascade primed immune cells and cytokine-induced killer cells in vitro. Mol Med Rep 2015;12:2629–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer 2012;12:265–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Mao Q, Li L, Zhang C, et al. Clinical effects of immunotherapy of DC-CIK combined with chemotherapy in treating patients with metastatic breast cancer. Pak J Pharm Sci 2015;28(3 suppl):1055–8. [PubMed] [Google Scholar]
- [10].Shi L, Zhou Q, Wu J, et al. Efficacy of adjuvant immunotherapy with cytokine-induced killer cells in patients with locally advanced gastric cancer. Cancer Immunol Immunother 2012;61:2251–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wang Y, Wang C, Xiao H, et al. Adjuvant treatment combining cellular immunotherapy with chemotherapy improves the clinical outcome of patients with stage II/III gastric cancer. Cancer Med 2017;6:45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Liu H, Song J, Yang Z, et al. Effects of cytokine-induced killer cell treatment combined with FOLFOX4 on the recurrence and survival rates for gastric cancer following surgery. Exp Ther Med 2013;6:953–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Chen Y, Guo ZQ, Shi CM, et al. Efficacy of adjuvant chemotherapy combined with immunotherapy with cytokine-induced killer cells for gastric cancer after d2 gastrectomy. Int J Clin Exp Med 2015;8:7728–36. [PMC free article] [PubMed] [Google Scholar]
- [14].Zhao H, Fan Y, Li H, et al. Immunotherapy with cytokine-induced killer cells as an adjuvant treatment for advanced gastric carcinoma: a retrospective study of 165 patients. Cancer Biother Radiopharm 2013;28:303–9. [DOI] [PubMed] [Google Scholar]
- [15].Jiang JT, Shen YP, Wu CP, et al. Increasing the frequency of CIK cells adoptive immunotherapy may decrease risk of death in gastric cancer patients. World J Gastroenterol 2010;16:6155–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Li Y, Wang C, Xu M, et al. Preoperative NLR for predicting survival rate after radical resection combined with adjuvant immunotherapy with CIK and postoperative chemotherapy in gastric cancer. J Cancer Res Clin Oncol 2017;143:861–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Gao D, Li C, Xie X, et al. Autologous tumor lysate-pulsed dendritic cell immunotherapy with cytokine-induced killer cells improves survival in gastric and colorectal cancer patients. PLoS One 2014;9:e93886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Cui J, Li L, Wang C, et al. Combined cellular immunotherapy and chemotherapy improves clinical outcome in patients with gastric carcinoma. Cytotherapy 2015;17:979–88. [DOI] [PubMed] [Google Scholar]
- [19].Ishigami S, Natsugoe S, Tokuda K, et al. Prognostic value of intratumoral natural killer cells in gastric carcinoma. Cancer 2000;88:577–83. [PubMed] [Google Scholar]
- [20].Wang FS, Liu MX, Zhang B, et al. Antitumor activities of human autologous cytokine-induced killer (CIK) cells against hepatocellular carcinoma cells in vitro and in vivo. World J Gastroenterol 2002;8:464–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kim HM, Lim J, Park SK, et al. Antitumor activity of cytokine-induced killer cells against human lung cancer. Int Immunopharmacol 2007;7:1802–7. [DOI] [PubMed] [Google Scholar]
- [22].Wang P, Yu JP, Gao SY, et al. Experimental study on the treatment of intracerebral glioma xenograft with human cytokine-induced killer cells. Cell Immunol 2008;253:59–65. [DOI] [PubMed] [Google Scholar]
- [23].Sun S, Li XM, Li XD, et al. Studies on inducing apoptosis effects and mechanism of CIK cells for MGC-803 gastric cancer cell lines. Cancer Biother Radiopharm 2005;20:173–80. [DOI] [PubMed] [Google Scholar]
- [24].Lin T, Song C, Chuo DY, et al. Clinical effects of autologous dendritic cells combined with cytokine-induced killer cells followed by chemotherapy in treating patients with advanced colorectal cancer: a prospective study. Tumour Biol 2016;37:4367–72. [DOI] [PubMed] [Google Scholar]
- [25].Moschella F, Proietti E, Capone I, et al. Combination strategies for enhancing the efficacy of immunotherapy in cancer patients. Ann N Y Acad Sci 2010;1194:169–78. [DOI] [PubMed] [Google Scholar]
- [26].Mahoney KM, Rennert PD, Freeman GJ. Combination cancer immunotherapy and new immunomodulatory targets. Nat Rev Drug Discov 2015;14:561–84. [DOI] [PubMed] [Google Scholar]
- [27].Zhou QM, Wu PH, Zhao M, et al. [Short-term curative efficacy of cytokine-induced killer cells combined micro-invasive treatments on hepatocellular carcinoma]. Ai Zheng 2006;25:1414–8. [PubMed] [Google Scholar]
- [28].Robinson E, Segal R, Struminger L, et al. Lymphocyte subpopulations in patients with multiple primary tumors. Cancer 1999;85:2073–6. [DOI] [PubMed] [Google Scholar]


