Abstract
Hand, foot, and mouth disease (HFMD), caused by enteroviruses, is an acute contagious disease in children. Some severe infections caused by human enterovirus 71 (HEV71) lead to rapid death in children with acute heart failure (HF). N-terminal probrain natriuretic peptide (NT-proBNP) is an important indicator of HF; however, its normal reference values in children and role in HFMD remain unclear.
This study aimed to investigate the correlation between NT-proBNP and heart function and establish normal reference values of NT-proBNP in children with HFMD aged 0 to 18 years.
In this study, 95% normal reference values were established in 1031 healthy children aged 0 to 18 years. The correlation between NT-proBNP and left ventricular ejection (LVEF) was analyzed in 392 children with HFMD using Spearman correlation and receiver operating characteristic analysis.
NT-proBNP levels were negatively correlated with LVEF in 392 children with HFMD. The median NT-proBNP level was 921 pg/mL in the early cardiorespiratory failure group, but only 55 pg/mL in the nervous system involvement group. Serum NT-proBNP levels were negatively correlated with age. The normal reference value in the neonatal period (0 to <1 month) and adolescence (13–18 years) was 250.0 to 3987.0 pg/mL and 20.0 to 145.0 pg/mL, respectively.
NT-proBNP levels can reflect the severity of HFMD and discriminate the second stage from the third stage of HFMD effectively. NT-proBNP is a useful biomarker to predict the early stage of severe HFMD in children with HF. Different ages fit with different normal reference values of NT-proBNP in children.
Keywords: children; hand, foot, and mouth disease; heart failure; N-terminal probrain natriuretic peptide
1. Introduction
Hand, foot, and mouth disease (HFMD) is an acute infection caused by enteroviruses. Severe HFMD is associated with human enterovirus 71 (HEV71).[1] In 2010, the largest recorded outbreak of HEV71-associated HFMD occurred in China, comprising more than 1.7 million cases (27,000 patients exhibited severe neurological complications and 905 patients died).[2] Hunan province in the central-south region of China was one of the hardest-hit regions. According to the data from the Hunan Provincial Center for Disease Control (CDC), there were 189,382 HFMD cases in Hunan in 2012, including 98 deaths while the population is 66,390,000, and the mortality was ranked third among a total of 33 provinces around the country.[3] HFMD is still prevalent in recent years. Severe HFMD develops quickly in the HEV71-invaded brain stem, causing neuronal heart failure (HF) and pulmonary edema, and the patients die in 1 to 2 days. Therefore, it is critical to screen out severe HFMD in children and intervene as early as possible. This is also key to reduce HFMD-associated morbidity. Patients with severe HFMD are usually aged <3 years. It may be related to immature and poor immune system of infants.
NT-proBNP is a neurohormone secreted by the ventricles predominantly. Patients with congestive or acute HF exhibit high plasma concentrations of NT-proBNP.[4–6] It is synthesized in the heart due to the activation of cardiac wall distension and stretching and neurohormonal activation. Cardiomyocytes synthesize a pre-propeptide preproBNP with 134 amino acids, which is split into a signal peptide and a propeptide (proBNP with 108 amino acids). During secretion from the cardiomyocytes, proBNP is split in a ratio of 1:1 into a physiologically active C-terminal fragment BNP (with 32 amino acids) and a biologically inactive N-terminal fragment NT-proBNP (with 76 amino acids).[7] NT-proBNP has a longer half-life than BNP in the blood, can be detected in the plasma and serum, and is stable at room temperature.[8] It was advocated as a beneficial biomarker for evaluating HF by the American Association for Clinical Chemistry in 2007.[9]
However, reference values of NT-proBNP in Chinese children are not available. A few studies have reported the use of serum NT-proBNP for diagnosing severe HFMD with the risk of HF. Therefore, this study aimed to establish the reference range of NT-proBNP in Chinese healthy children aged 0 to 18 years. It investigated the diagnostic standard of serum NT-proBNP in patients with severe HFMD by comparing the levels of cardiac troponin I (cTnI), creatine kinase-MB (CKMB), and NT-proBNP and the results of bedside echocardiogram in 392 children with HFMD. The purpose of the study was to improve the early diagnostic and prognostic rates of severe HFMD and decrease the mortality.
2. Methods
2.1. Healthy children
A total of 1031 healthy children were randomly selected at the Children Health Care Institute of Hunan Children’ Hospital from August 2013 to September 2015. Medical examinations and liver function, renal function, heart function (myocardial enzymes), and routine blood tests were normal for all children. The children were divided into 7 groups: neonatal period (0 to <1 month), infant period (1 month to <1 year), toddler period (1 year to <4 years), preschool age (4 years to <7 years), early school-age (7 years to <10 years), middle school-age (10 years to <13 years), and adolescence (13 years to <18 years). All the participants were explained about the process, risks, and benefits of the study, and they signed an informed consent form. This study was approved by the ethics committee of Hunan Children's hospital.
2.2. Children with HFMD
A total of 392 children with HFMD were enrolled in this study, who visited and were hospitalized at the Department of Infectious Diseases and Intensive Care Unit of Hunan Children’ Hospital from August 2013 to October 2015. Children with HFMD having congenital heart disease, kidney disease, or cardiopulmonary function damage caused by the infection of Epstein–Barr virus, influenza virus, and respiratory syncytial virus were excluded from the study. All the patients were diagnosed as serologically HEV71 positive. Age ranged from 8 months to 5 years. Five stages of HFMD were confirmed according to the Expert Consensus on Clinical Treatment in Severe Enterovirus 71 Infection (2011 edition) issued by the China Ministry of Health. The 5 groups were as follows: 100 cases of rash period (first stage), 120 cases of nervous system involvement (second stage), 100 cases of early cardiorespiratory failure (third stage), 72 cases of cardiorespiratory failure (fourth stage), and 103 cases of recovery (fifth stage, 58 cases from the third stage and 45 cases from the fourth stage). A total of 200 healthy children in the control group came from 1031 healthy children in the community (age, 8 months to 5 years). No significant difference was observed in age and sex between the control and HFMD groups. All the children and their families were informed about the research contents, risks, and benefits, and they signed an informed consent form.
2.3. Measures
The recovery group underwent the serum assay of NT-proBNP, cTnI, and CK-MB and echocardiogram examination again in the recovery stage. Further, 42 cases in the third stage and 15 cases in the fourth stage did not undergo the examinations because their parents did not allow for the same. Twelve death cases (6 boys and 6 girls in the fourth stage) were reported due to treatment failure. The second stage is a severe stage of HFMD, and most cases in this stage can be cured. High risks of death occur in the third and fourth stages of HFMD.
2.4. Serum assay of NT-proBNP, cTnI, and CK-MB
Venous blood (2 mL) was collected from each participant of the control and HFMD groups in separation gel coagulation promoting tubes made in China.
The levels of serum NT-proBNP and cTnI were detected using a VIDAS fluorescence immunoassay analyzer (bioMérieux). NT-proBNP and cTnI reagents were also produced by the same company. The quality control of NT-proBNP was performed every day. It took about 25 minutes to finish the whole test process from adding serum to achieving outcomes; the tests were strictly performed according to the established protocols. The analytical measurement range of serum NT-proBNP and cTnI was 20 to 25,000 pg/mL and 0.01 to 32 μg/mL, respectively. The measurement range of serum CK-MB was 2 to 500 U/L (Siemens Bayer 2400 biochemical analyzer); all the results were obtained in 3 hour.
2.5. Cardiac function examination
Echocardiography (ECG) examinations were performed immediately when the patients with HFMD reached the hospital. The US GE Vividi color ultrasonic diagnostic instrument with an S3 RS probe and a frequency of 1.7 to 3.4 MHz was used to complete all examinations in 30 minutes. Ultrasound technicians who were unaware of the experiments executed all the checkups. Left ventricular end-diastolic diameter resulting from the left ventricular long axis was measured by heart ultrasonography. The improved Simpson method was used to measure left ventricular end-diastolic volume index (LVEDVD) and left ventricular end systolic volume index (LVESVD). Subsequently, the left ventricular ejection fraction (LVEF) was calculated as follows:
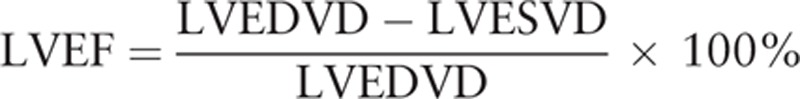 |
2.6. Statistical analysis
SPSS16.0 statistical analysis package (SPSS, IL) was used for data processing and statistical analysis. The serum NT-proBNP levels were expressed as median and interquartile range (IQR). The IQR was the interval from P2.5 to P97.5 (2.5th to 97.5th percentiles) according to different age groups. The Pearson correlation analysis was used to investigate the correlation between serum NT-proBNP levels and age of 1031 healthy children (0–18 years), and the Spearman correlation analysis was used to investigate the correlation between serum cardiac function indexes and echocardiographic indexes (LVEFs) of 392 children with HFMD. The receiver operating characteristics (ROC) curve was used to analyze the diagnostic level of NT-proBNP in the severe group (third and fourth stages) and the deceased group with HFMD. P values <.05 were considered to be statistically significant.
3. Results
3.1. Relationship between NT-proBNP levels and age in healthy children
The Pearson correlation analysis demonstrated that the serum NT-proBNP levels were negatively correlated with the age of 1031 healthy children. The levels decreased with age in healthy children (r = –0.230, P < .05) (Fig. 1).
Figure 1.
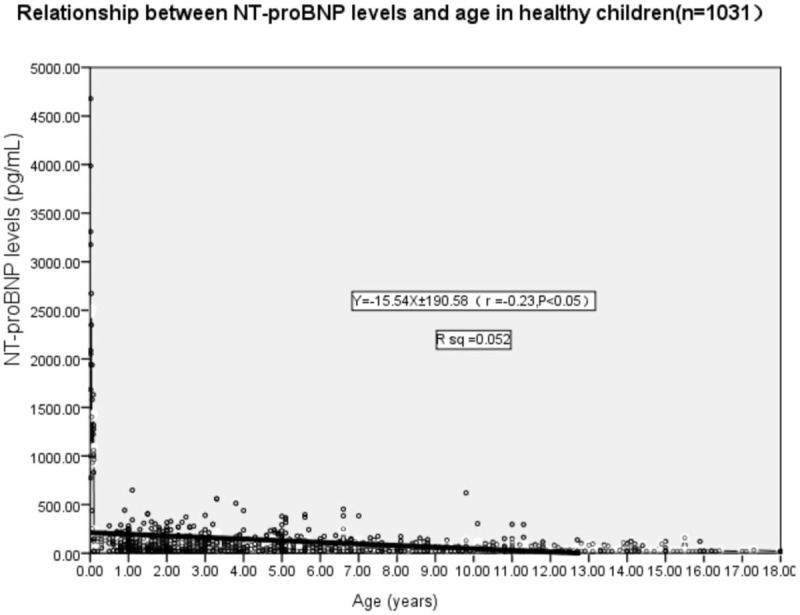
Pearson correlation analysis displayed a negative correlation between serum NT-proBNP level and age in 1031 healthy children (0–18 years). The serum NT-proBNP level decreased with age in healthy children (r = −0.230, P < .05). NT-proBNP = N-terminal probrain natriuretic peptide.
3.2. Median (2.5th–97.5th percentiles) NT-proBNP levels in healthy children aged 0 to 18 years
The serum NT-proBNP levels in the newborn group aged 0 to <1 month (n = 80) was 250 to 3987 pg/mL. The median was 1360 pg/mL, which was significantly higher than that in other age groups (P < .01) (Fig. 2).
Figure 2.
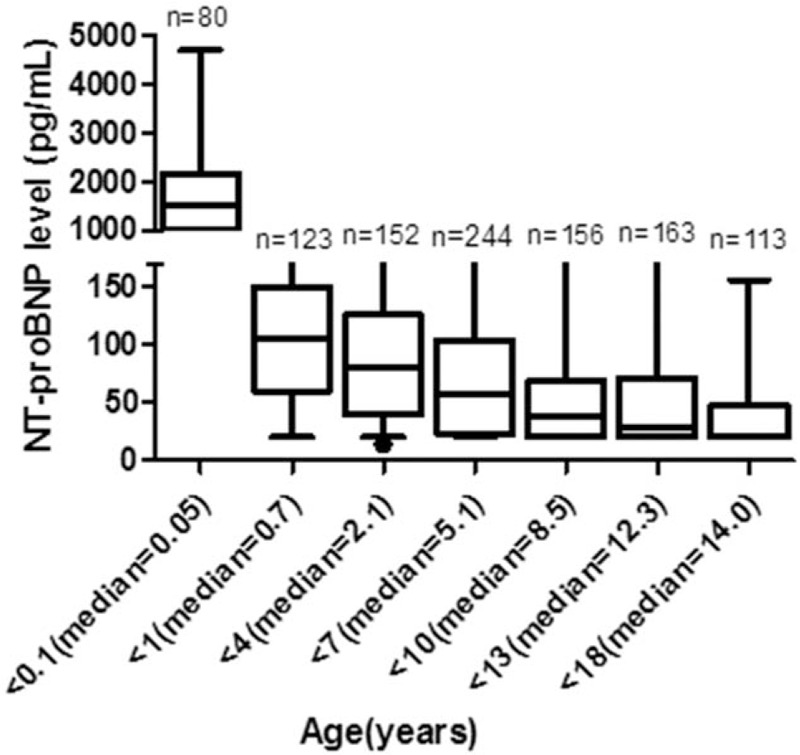
Median (2.5th–97.5th percentiles) NT-proBNP levels of healthy children aged 0 to 18 years. NT-proBNP = N-terminal probrain natriuretic peptide.
3.3. Median (2.5th–97.5th percentiles) NT-proBNP levels of different sex in healthy children aged 0 to 18 years
The results of t test showed no significant difference in the serum NT-proBNP level of different sexes, although the median NT-proBNP level of baby boys (1669.5 pg/mL) seemed to be higher than that of baby girls (1270.5 pg/mL) in the newborn group (P = .422) (Fig. 3).
Figure 3.
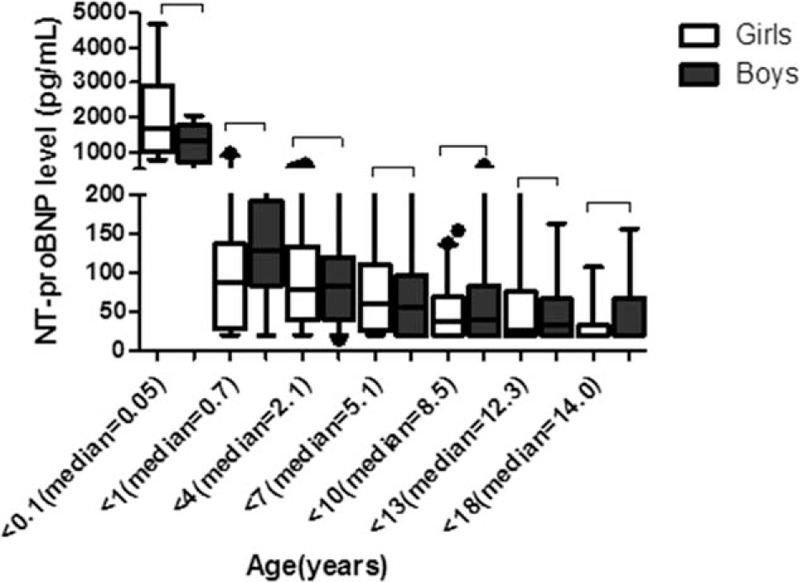
Median (2.5th–97.5th percentiles) NT-proBNP levels of different sexes in healthy children aged 0 to 18 years. NT-proBNP = N-terminal probrain natriuretic peptide.
3.4. Median (25th–75th percentiles) NT-proBNP levels and LVEF of patients with different HFMD stages
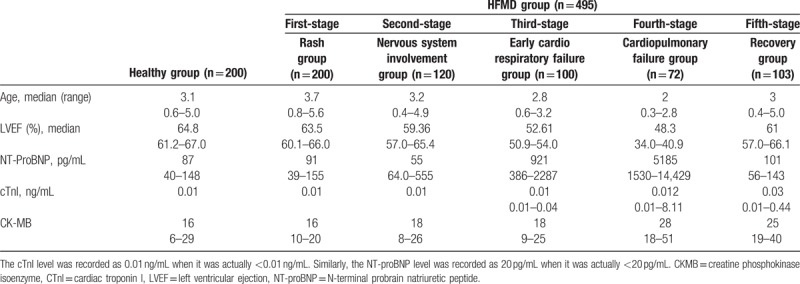
A total of 392 children with HFMD were enrolled in the study. Detections were conducted 495 times, which included 100 times for the first stage, 120 times for the second stage, 100 times for the third stage, 72 times for the fourth stage, and 103 times for the fifth stage. Each detection included the levels of serum NT-proBNP, cTnI, and CK-MB and the echocardiographic LVEF value. The control group comprised 200 healthy children (aged 6 months to 5 years); the age matched the age of children with HFMD (Table 1).
Table 1.
Median (25th–75th percentiles) NT-proBNP levels and LVEF of patients with different HFMD stages.
3.5. Spearman correlation analysis of serum cardiac function indicator and LVEF
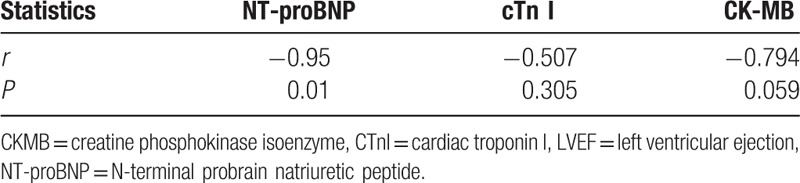
The results of Spearman statistical analysis showed that NT-proBNP levels were negatively correlated with LVEF (r = –0.95, P < .05). The increase in serum NT-proBNP level was associated with the worsening of HFMD, but the levels of cTnI and CK-MB showed no correlation with LVEF. The results are shown in Table 2.
Table 2.
Spearman correlation analysis of serum cardiac function indicator and LVEF.
3.6. ROC curves of the NT-proBNP levels
An ROC analysis was conducted to examine the diagnostic utility of NT-proBNP levels for distinguishing between mild HFMD (first and second stages), severe HFMD, and deceased groups.
Differences were considered to be significant for the third stage of HFMD (early cardiorespiratory) and above (Fig. 4A), fourth stage of HFMD (cardiorespiratory failure) and above (Fig. 4B), and deceased group with severe HFMD (Fig. 4C) (P < .05). The cutoff value was 400, 1500, and 5000 pg/mL, respectively. The area under the ROC curve, sensitivity, and specificity were 0.92, 92.00%, and 80.49%, respectively, for the third stage (Fig. 4A); and 0.93, 93.18%, and 78.26%, respectively, for the fourth stage (Fig. 4B).
Figure 4.
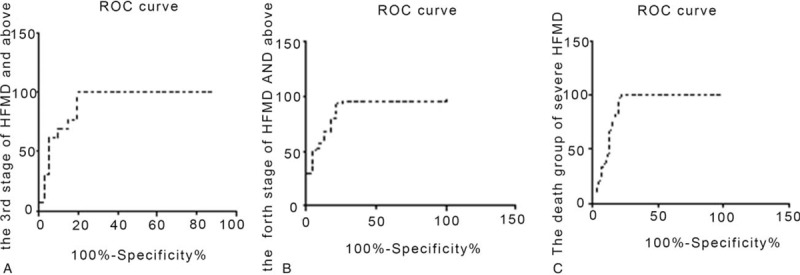
Receiver operating characteristic curves of the NT-proBNP levels. (A) The third stage (early cardiorespiratory) of HFMD and above; (B) the fourth stage (cardiorespiratory failure) of HFMD and above; (C) the deceased group of severe HFMD. HFMD = Hand, foot, and mouth disease, NT-proBNP = N-terminal proBNP.
Further, 12 of 72 children with severe HFMD complicated with HF (the fourth stage) died. The median (P25, P75) serum NT-proBNP level of the deceased group was 5850 pg/mL (5030, 14,660). The ROC curve showed that the serum NT-proBNP level was 5000 pg/mL in the deceased group with severe HFMD. The area under the ROC curve, sensitivity, and specificity were 0.89, 91.67%, and 80.00%, respectively, for the deceased group (Fig. 4C).
4. Discussion
Several studies explored the normal reference values of serum NT-proBNP, but satisfactory results were not achieved. The NT-proBNP reference values in children are quite different from those in adults. Galasko et al[10] performed a study on 2285 subjects and showed that the NT-proBNP reference values were 42.5 to 106.4 pg/mL in men and 111.0 to 215.9 pg/mL in women (median age 38 years [26–53], 56% women, [P2.5, P97.5]). Sugimoto et al[11] reported the normal reference values of serum NT-proBNP in 232 healthy children aged 4 months to 14 years. Schwachtgen et al[12] reported that the median NT-proBNP reference value in children aged 0 to 10 years, 10 to 13 years, and 13 to 18 years was 173.6, 118.5, and 61.1 pg/mL, respectively. Nir et al[13] reported that the neonatal serum NT-proBNP level was the highest at birth, but decreased a week or a few weeks later. The premature serum level of NT-proBNP was 530 to 3220 pg/mL, 490 to 2740 pg/mL, and 373 to 1863 pg/mL 1 day, 3 days, and 7 days after birth, respectively. The findings of all these studies provided evidence for the application of NT-proBNP in children, but they still had shortcomings, such as small sample size and imperfect age classification system. Most importantly, the study subjects were not Chinese children. The present study could effectively address the aforementioned limitations. The median (reference range) NT-proBNP value of different age groups, including neonatal period (0 to <1 month), infant period (1 month to <1 year), toddler period (1 year to <4 years), preschool age (4 years to <7 years), early school-age (7 years to <10 years), middle school-age (10 years to <13 years), and adolescence (13 years to <18 years) was 1360.0 (250.0–3987.0) pg/mL, 106.0 (20.0–532.0) pg/mL, 80.0 (20.0–324.0) pg/mL, 58.0 (20.0–374.0) pg/mL, 38.0 (20.0–163.0) pg/mL, 29.0 (20.0–296.0) pg/mL, and 20.0 (20.0–145.0) pg/mL, respectively. The NT-proBNP levels slightly decreased with age from infancy to adolescence in healthy children. The results of this study showed that the serum NT-proBNP levels were negatively correlated with the age of children (0–18 years) (r = –0.230, P < .05). Different age groups require different NT-proBNP reference values.
The serum NT-proBNP levels in the healthy neonatal group were higher than those in other age groups. The levels decreased gradually with the increase in days of birth. The reasons for this physiologic fluctuation in the levels are still unclear. It is hypothesized that the removal of placenta, and thereby significant redistribution of blood volume to the heart, causes a volume overload and an increase in the afterload at the same time. A rapid increase in pulmonary blood flow with lung expansion further adds to the stimulus. Finally, renal immaturity may contribute to the decreased clearance of NT-proBNP during the first week of life. As a result, the NT-proBNP levels are significantly elevated in newborns and drop rapidly over the first 2 weeks of life. The NT-proBNP levels of boys and girls were not significantly different (P > .05). Moreover, they were not the same as those of adults. Some studies reported that NT-proBNP levels were influenced by sex.[14–19] Because androgens suppress BNP secretion, the NT-proBNP reference values are lower in men than in women. However, the effects of androgens are not significant in children.[20] No sex-related differences were found in the present study.
A total of 392 children with HFMD and 1031 healthy children were enrolled in this study. In the present study, cTnI and CK-MB were not apparently elevated in the first stage (HFMD rash group), second stage (nervous system involvement group), and third stage (cardiorespiratory failure group). However, the NT-proBNP levels were significantly elevated in the third stage (the phase of early HF in HFMD with a median of 921 pg/mL) and in the fourth stage (the phase of HF in HFMD with a median of 5185 pg/mL). They did not significantly increase with a median of 55 pg/mL in the second stage—the phase of nervous system involvement. The results showed that HF, not myocarditis, occurred in the early stage of HFMD in patients. cTnI is found in cardiomyocytes because it is an acknowledged marker for myocardial injury. If a myocardial injury occurs, cTnI is released steadily and slowly from the structural protein of myocardial cells. However, cTnI isn’t elevated in second stage and the third stage. The elevated NT-proBNP level is a biomarker for predicting HF in severe HFMD. Evidence also showed that serum NT-proBNP was an effective biomarker to discriminate the second stage from the third stage of HFMD (P > .05). Timely and accurate confirmation of the stage of HFMD in patients is the critical step in reducing HFMD mortality.
Long et al[21] also reported that the levels of myocardial enzymes did not change significantly in patients with severe HEV71 infection, but the patients had a complicated cardiovascular failure. ECG detection was used to screen HF in the early stage of severe HFMD. ECG should be performed beside the bed. Although it is the gold standard for the clinical evaluation of HF, ECG is a complicated procedure. In contrast, the procedure of serological biomarker testing has several advantages: it is fast, simple, precise, and can be performed with large samples. The correlation analysis in this study showed that only the NT-proBNP level was negatively correlated with LVEF (r = –0.95, P < .05). The NT-proBNP level is the critical marker to reflect the left ventricular systolic function and assess the HF levels. However, the levels of cTnI and CK-MB had no correlation with LVEF (P > .05). Therefore, the NT-proBNP level is a good biomarker to diagnose HF in HFMD.
The NT-proBNP level is also a biomarker to diagnose acute HF in HFMD. Ogawa et al[22] reported that the levels were elevated only 1 hour after HF. Troponin in peripheral blood generally increases in 5 to 8 hours after injury, reaches the peak in 12 to 24 hours, and maintains a high level for 7 to 10 days. The NT-proBNP levels elevated earlier than the levels of common biomarkers of myocardial injury. Therefore, the NT-proBNP level elevated in the peripheral blood, but the levels of cTnI and CK-MB did not elevate in the early stage of severe HFMD.
NT-proBNP levels could predict the severity of HFMD. The results of the ROC curve analysis of the third stage (the phase of early HF of HFMD) and the fourth stage (the phase of HF of HFMD) showed that children with HFMD had high risks of being complicated with HF or had already been complicated with HF when the serum NT-proBNP levels were greater than 400 pg/mL or 1500 pg/mL, respectively. When the NT-proBNP level of children with HFMD was >5000 pg/mL, it indicated that the children had the risk of death. Some of the children died on the first day or the next day when they were admitted to the hospital. The survey showed that the median (P25 and P75) of serum NT-proBNP level of 12 death cases was 5850 pg/mL (5030 and 14,660). However, the levels of a few children with severe HFMD were even elevated to >10,000 pg/mL but reverted to normal after the successful therapy. In addition, patients with severe HFMD show characteristics such as elevated body temperature, quickened pulse, reduced LVEF, and loaded ventricular volume or pressure. Therefore, cardiorespiratory failure is a major reason for death in children with severe HFMD. Deng et al[23] and Qiu et al[24] also reported similar results in children.
The pathogenic mechanism of HF caused by HEV71 infection is unclear. Because HEV71 infection involves brainstem and autonomic nerve dysfunction, leading to a “sympathetic storm,” excessive secretion of catecholamines (the hormones epinephrine and norepinephrine) significantly increases catecholamine concentration in the blood.[25–27] This may lead to increased blood flow to the heart and ventricular preload, resulting in increased NT-proBNP secretion and release. NT-proBNP promotes vasodilatation and natriuresis and inhibits ventricular remodeling. Finally, the activation of inflammatory pathways, particularly the release of cytokines, contributes to ventricular remodeling.[28] Moreover, neurogenic HF, pulmonary edema, and pneumorrhagia occur.
Different treatment methods apply to different stages of HFMD in children. Antiviral treatment and strategies for cardiopulmonary function involvement are critical in therapy. Ribavirin is the priority antiviral drug for children with mild HFMD. However, the treatment of severe HFMD in children is the key. Liquid management and control of intracranial pressure via dehydration treatment should be adopted for children with severe neurologic involvement performance, and mannitol is used to control startle and jitter. For the third phase of children with HFMD, intravenous immunoglobulin is used to neutralize the virus and inhibit nonspecific inflammatory response. High-dose immunoglobulin (1 g/[kg·d]) can rapidly alleviate symptoms, shorten the course of the disease, prevent illness development, and reduce the incidence of severe complications. Endotracheal intubation and ventilator-assisted ventilation are considered for children with breathing rhythm changes in HFMD. Milrinone is the priority antiviral drug for treating heart failure. Sodium nitroprusside supplementation can be more effective in reducing the heart rate and blood pressure levels and increasing ejection fraction and left cardiac output. For critical children with HF, the blood purification technology is proposed to reduce inflammatory substances in the blood and the ECMO technique is used to improve ventilation if mechanical ventilation, vasoactive agents, and fluid therapy are all ineffective.
The clinical factors that influenced NT-proBNP levels were age, sex, renal function, obesity, and the assay methods used.[14–19] In particular, obesity was known to elevate the NT-proBNP levels. The weight of children was ignored in the present study. Therefore, obesity should be stratified more carefully using body mass index. The second limitation of this study was that it comprised only 12 patients with HFMD who died. The third limitation of this study was the lack of international standard for a symptom-based HFMD grading system in children.
5. Conclusions
The detection of serum NT-proBNP levels is simple and rapid. The present study showed that NT-proBNP levels could reflect the severity of HFMD and discriminate the second stage from the third stage of HFMD effectively. Particularly, NT-proBNP is a useful biomarker to predict the early stage of severe HFMD in children with HF. It is also useful in risk stratification for children with HFMD. Different ages fit with different normal reference values of NT-proBNP in children. Detecting serum NT-proBNP levels in children with HFMD might contribute to early prevention and treatment of severe HFMD and reduce the fatality rate.
Acknowledgments
The authors thank Yimin Zhu, Xuejun Ma, Xinping Zhang, Zeshu Nin, Hao Bo for their assistance related to the study. They thank the language editing services “MedSci Publishing Group Language Editing” for checking the spelling and grammar in the manuscript.
Author contributions
Sai Li performed the studies, and participated in designing and drafting the manuscript. Suwu Yi and Zhou Zhou helped in data collection and statistical analysis. Zhenghui Xiao and Junming Luo participated in clinical consultation. Boli Nie and Bin Hu conduct the experiment. Shiping Wang and Liya Mo reviewed the manuscript. All authors read and approved the final manuscript.
Formal analysis: Sai Li.
Funding acquisition: Sai Li.
Investigation: Sai Li, Zhenghui Xiao, Liping Li, Bin Hu, Zhou Zhou, Suwu Yi, Junming Luo, Ling Xie, Boli Nie, Shiping Wang, Liya Mo.
Methodology: Sai Li, Zhenghui Xiao, Liping Li, Bin Hu, Zhou Zhou, Suwu Yi, Junming Luo, Ling Xie, Boli Nie, Shiping Wang, Liya Mo.
Resources: Liya Mo.
Validation: Sai Li.
Writing – original draft: Sai Li, Zhenghui Xiao, Liping Li, Liya Mo.
Writing – review & editing: Sai Li, Zhenghui Xiao, Liping Li, Bin Hu, Zhou Zhou, Suwu Yi, Junming Luo, Ling Xie, Boli Nie, Shiping Wang, Liya Mo.
Footnotes
Abbreviations: CKMB = creatine phosphokinase isoenzyme, CTnI = cardiac troponin I, HEV71 = human enterovirus 71, HF = heart failure, HFMD = hand, foot, and mouth disease, LVEF = left ventricular ejection, NT-proBNP = N-terminal probrain natriuretic peptide.
This work was supported by the National Natural Science Foundation of China [Grant No. 81672051] and the Science Foundation of Hunan Health and Family [Grant number B2013-107].
All authors declare that they have no conflicts of interest.
References
- [1].Kok CC. Therapeutic and prevention strategies against human enterovirus 71 infection. World J Virol 2015;4:78–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Zeng M, El Khatib NF, Tu S, et al. Seroepidemiology of Enterovirus 71 infection prior to the 2011 season in children in Shanghai. J Clin Virol 2012;53:285–9. [DOI] [PubMed] [Google Scholar]
- [3].National Bureau of Statistics of the People's Republic of China. Statistical Yearbook of China; 2012 (China Statistics Press, 2013). [Google Scholar]
- [4].Escobar C, Santiago-Ruiz JL, Manzano L. Diagnosis of heart failure in elderly patients: a clinical challenge. Eur J Heart Fail 2011;13:1152author reply 1152-1153. [DOI] [PubMed] [Google Scholar]
- [5].Hummel A, Empe K, Dorr M, et al. De novo acute heart failure and acutely decompensated chronic heart failure. Dtsch Arztebl Int 2015;112:298–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ahmad T, O’Connor CM. Therapeutic implications of biomarkers in chronic heart failure. Clin Pharmacol Ther 2013;94:468–79. [DOI] [PubMed] [Google Scholar]
- [7].Levin ER, Gardner DG, Samson WK. Natriuretic peptides. N Engl J Med 1998;339:321–8. [DOI] [PubMed] [Google Scholar]
- [8].Pemberton CJ, Johnson ML, Yandle TG, et al. Deconvolution analysis of cardiac natriuretic peptides during acute volume overload. Hypertension 2000;36:355–9. [DOI] [PubMed] [Google Scholar]
- [9].Tang WH, Francis GS, Morrow DA, et al. National Academy of Clinical Biochemistry Laboratory Medicine Practice Guidelines: clinical utilization of cardiac biomarker testing in heart failure. Clin Biochem 2008;41:210–21. [DOI] [PubMed] [Google Scholar]
- [10].Galasko GI, Lahiri A, Barnes SC, et al. What is the normal range for N-terminal pro-brain natriuretic peptide? How well does this normal range screen for cardiovascular disease? Eur Heart J 2005;26:2269–76. [DOI] [PubMed] [Google Scholar]
- [11].Sugimoto M, Manabe H, Nakau K, et al. The role of N-terminal pro-B-type natriuretic peptide in the diagnosis of congestive heart failure in children. Correlation with the heart failure score and comparison with B-type natriuretic peptide. Circ J 2010;74:998–1005. [DOI] [PubMed] [Google Scholar]
- [12].Schwachtgen L, Herrmann M, Georg T, et al. Reference values of NT-proBNP serum concentrations in the umbilical cord blood and in healthy neonates and children. Z Kardiol 2005;94:399–404. [DOI] [PubMed] [Google Scholar]
- [13].Nir A, Lindinger A, Rauh M, et al. NT-pro-B-type natriuretic peptide in infants and children: reference values based on combined data from four studies. Pediatr Cardiol 2009;30:3–8. [DOI] [PubMed] [Google Scholar]
- [14].Costello-Boerrigter LC, Boerrigter G, Redfield MM, et al. Amino-terminal pro-B-type natriuretic peptide and B-type natriuretic peptide in the general community: determinants and detection of left ventricular dysfunction. J Am Coll Cardiol 2006;47:345–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wang TJ, Larson MG, Levy D, et al. Impact of age and sex on plasma natriuretic peptide levels in healthy adults. Am J Cardiol 2002;90:254–8. [DOI] [PubMed] [Google Scholar]
- [16].Kawai K, Hata K, Tanaka K, et al. Attenuation of biologic compensatory action of cardiac natriuretic peptide system with aging. Am J Cardiol 2004;93:719–23. [DOI] [PubMed] [Google Scholar]
- [17].Daniels LB, Maisel AS. Natriuretic peptides. J Am Coll Cardiol 2007;50:2357–68. [DOI] [PubMed] [Google Scholar]
- [18].Hammerer-Lercher A, Ludwig W, Falkensammer G, et al. Natriuretic peptides as markers of mild forms of left ventricular dysfunction: effects of assays on diagnostic performance of markers. Clin Chem 2004;50:1174–83. [DOI] [PubMed] [Google Scholar]
- [19].Fradley MG, Larson MG, Cheng S, et al. Reference limits for N-terminal-pro-B-type natriuretic peptide in healthy individuals (from the Framingham Heart Study). Am J Cardiol 2011;108:1341–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Nir A, Bar-Oz B, Perles Z, et al. N-terminal pro-B-type natriuretic peptide: reference plasma levels from birth to adolescence. Elevated levels at birth and in infants and children with heart diseases. Acta Paediatr 2004;93:603–7. [DOI] [PubMed] [Google Scholar]
- [21].Long L, Xu L, Xiao Z, et al. Neurological complications and risk factors of cardiopulmonary failure of EV-A71-related hand, foot and mouth disease. Sci Rep 2016;6:23444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ogawa A, Seino Y, Yamashita T, et al. Difference in elevation of N-terminal pro-BNP and conventional cardiac markers between patients with ST elevation vs non-ST elevation acute coronary syndrome. Circ J 2006;70:1372–8. [DOI] [PubMed] [Google Scholar]
- [23].Deng HL, Zhang YF, Li YP, et al. N-terminal pro-brain natriuretic peptide levels associated with severe hand, foot and mouth disease. BMC Infect Dis 2016;16:585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Qiu J, Lu X, Liu P, et al. N-terminal pro-B-type natriuretic peptide for the prognostic prediction of severe enterovirus 71-associated hand, foot, and mouth disease. Int J Infect Dis 2017;54:64–71. [DOI] [PubMed] [Google Scholar]
- [25].Maron MB, Holcomb PH, Dawson CA, et al. Edema development and recovery in neurogenic pulmonary edema. J Appl Physiol (1985) 1994;77:1155–63. [DOI] [PubMed] [Google Scholar]
- [26].Fu YC, Chi CS, Lin NN, et al. Comparison of heart failure in children with enterovirus 71 rhombencephalitis and cats with norepinephrine cardiotoxicity. Pediatr Cardiol 2006;27:577–84. [DOI] [PubMed] [Google Scholar]
- [27].Lu WH, Hsieh KS, Lu PJ, et al. Hexamethonium reverses the lethal cardiopulmonary damages in a rat model of brainstem lesions mimicking fatal enterovirus 71 encephalitis. Crit Care Med 2013;41:1276–85. [DOI] [PubMed] [Google Scholar]
- [28].Knudson JD, Cabrera AG. The pathophysiology of heart failure in children: the Basics. Curr Cardiol Rev 2016;12:99–103. [DOI] [PMC free article] [PubMed] [Google Scholar]


