Abstract
Background
Current guidelines on the treatment of rheumatoid arthritis (RA) recommend early therapy targeting the achievement of low disease activity (LDA) or clinical remission. Little published information is available on the success of this treatment strategy in Latin America. In a subset analysis of patients from Latin America, we compared efficacy maintenance with etanercept 50 mg once weekly (ETN50) versus placebo (PBO), on a background of methotrexate (MTX) ± other non-biologic, disease-modifying antirheumatic drugs, in patients with moderate-to-severe RA who had achieved LDA with ETN50.
Methods
In the Treat-to-Target trial, adult patients with active RA nonresponsive to MTX were treated with ETN50 for 24 weeks (Period 1). Patients achieving LDA were randomized to receive ETN50 or PBO for 28 additional weeks (Period 2). The proportion of patients maintaining LDA at week 52 and other efficacy and quality-of-life measures were assessed. Descriptive statistics are presented using last observation carried forward imputation of data.
Results
Of the 64 patients from Latin America treated in Period 1, 61 (95.3%) achieved LDA. Among patients receiving ETN50, 13/34 remained in LDA and 6/14 maintained remission at week 52 versus 6/27 and 4/10 patients receiving PBO. The median time to flare was 113 days and 33 days for the ETN50 and PBO groups, respectively. In the overall population, adverse events were reported in 37% and 43%, serious adverse events in 1% and 4%, and serious infections in 0% and 2% of patients in the ETN50 and PBO groups, respectively.
Conclusions
In patients with RA from Latin America, continuing treatment with ETN50 after achieving LDA appears to result in a higher proportion of patients maintaining LDA and remission compared with switching to PBO.
ClinicalTrials.gov Registration:
Keywords: etanercept, low disease activity, maintenance, remission, rheumatoid arthritis
1. Introduction
Rheumatoid arthritis (RA) is a chronic, progressive, systemic inflammatory disease of unknown etiology with a worldwide prevalence estimated at 0.3% to 1.2%.[1,2] It is characterized by progressive joint destruction from distal to proximal joints associated with chronic pain.[2] The limited data available indicate that the prevalence of RA is similar in Latin America to that in Western Europe or the United States of America (USA).[3,4]
Treatment with biologic agents such as tumor necrosis factor (TNF) inhibitors has significantly reduced disease and improved quality of life in patients with RA who have not responded to conventional synthetic disease-modifying antirheumatic drug (csDMARD) therapy.[5] Current guidelines, based primarily on data collected on patients from Western Europe and the USA, recommend early, intensive treatment of patients to achieve low disease activity (LDA) or clinical remission.[6] Although guidelines for diagnosis and treatment of RA are available across Latin America,[7–10] there is little published information to date on the success of these treatment strategies in this part of the world.[11]
The aim of the global Treat-to-Target trial (T2T; NCT01578850) conducted in 19 countries was to determine the efficacy of etanercept (ETN) to achieve and maintain LDA and/or remission in patients with active RA.[12] In this post-hoc subset analysis, we report our data on patients from Brazil, Colombia, and Mexico.
2. Methods
2.1. Ethics statement
This trial was approved by local ethics committees and conducted in accordance with the Declaration of Helsinki. All patients included in the study provided informed consent. The local institutional review boards and independent ethics committees that approved the T2T trial are listed in the Acknowledgments.
2.2. Study design
The details of the global T2T trial have been reported elsewhere.[12] Briefly, patients were treated with ETN 50 mg once weekly (ETN50) + methotrexate (MTX) ± other csDMARDs for 24 weeks (open-label, Period 1). Patients achieving LDA (Disease Activity Score-28 joints [DAS28]—erythrocyte sedimentation rate [ESR] <3.2) at week 24 were randomized and treated with ETN50 + MTX ± other csDMARDs or placebo (PBO) + MTX ± csDMARDs for another 28 weeks (Period 2). Patients in either treatment group who experienced a flare (defined as loss of LDA, DAS28-ESR >3.2) were switched to or continued on ETN50 (escape arm), but remained blinded to the end of the study. Data for patients from Brazil, Colombia, and Mexico were extracted from the global study and analyzed.
2.3. Patients
Adult patients with ≥1-year history of active moderate-to-severe RA disease activity (DAS28-ESR ≥3.2; ≥6 tender joint count [TJC], and ≥6 swollen joint count [SJC] or ESR ≥28 mm/h; and C-reactive protein [CRP] ≥3.5 mg/L), nonresponsive to prior MTX (≥10 mg/wk for ≥12 wks) were included in the study.
2.4. Assessments
The primary endpoint was the proportion of patients maintaining LDA without flare at week 52. Secondary endpoints included the overall proportion of patients maintaining LDA by DAS28-ESR and DAS28-CRP criteria, maintaining remission (DAS28-ESR <2.6), achieving 20% (ACR20), 50% (ACR50), 70% (ACR70), and 90% (ACR90) improvement from baseline based on American College of Rheumatology (ACR) criteria, the proportion of patients achieving normal scores on the Health Activity Questionnaire-Disability Index (HAQ-DI) at week 52, and change from baseline for other patient-reported outcome assessments.
2.5. Statistics
Descriptive statistics are presented, mean (standard deviation [SD]) for continuous data, and n (%) for binary data using last observation carried forward (LOCF). For patients who experienced a flare, the last pre-rescue observation was used for all analyses, except where otherwise indicated. No comparative statistics were performed because the subset of patients included in this analysis was small, and the statistical analysis would be underpowered and could be misinterpreted. The full study had 90% power to detect differences of 17% in the primary endpoint between the ETN50 and PBO groups with 158 subjects per group. However, with just 34 ETN50 and 27 PBO subjects in the regions considered in this paper, only large differences could be shown to be statistically significant, while smaller, yet potentially clinically meaningful differences would not reach statistical significance.
3. Results
3.1. Patients
Of the 489 patients enrolled in the global study and treated in Period 1, 64 were from Latin America with a mean (SD) disease duration of 6.4 (5.6) years and DAS28-ESR of 6.8 (0.7). Patient disposition with respect to the primary results is presented in Fig. 1. Patients randomized to ETN50 (n = 34) and PBO (n = 27) had comparable baseline demographic (Table 1) and disease (Table 2) characteristics. Although the difference was not statistically significant, more patients in the PBO group (88.9%) tested positive for anti-cyclic citrullinated protein antibodies than did patients in the ETN50 group (76.5%).
Figure 1.

Patient disposition with respect to primary endpoints. DAS28 = Disease Activity Score-28 joints, ESR = erythrocyte sedimentation rate, ETN = etanercept, LDA = low disease activity, PBO = placebo.
Table 1.
Patient characteristics at baseline (Period 1) and randomization (Period 2).

Table 2.
Patient disease characteristics at baseline (Period 1) and randomization (Period 2).

3.2. Efficacy
In Period 1, 61/64 (95.3%) achieved DAS28-ESR LDA and entered Period 2. Of these 64 patients, 24 (37.5%) achieved DAS28-ESR remission. At the end of Period 2, 13/34 (38.2%) in the ETN50 group and 6/27 (22.2%) in the PBO group remained in DAS28-ESR LDA at week 52 without experiencing a flare throughout Period 2 (Fig. 2A).
Figure 2.
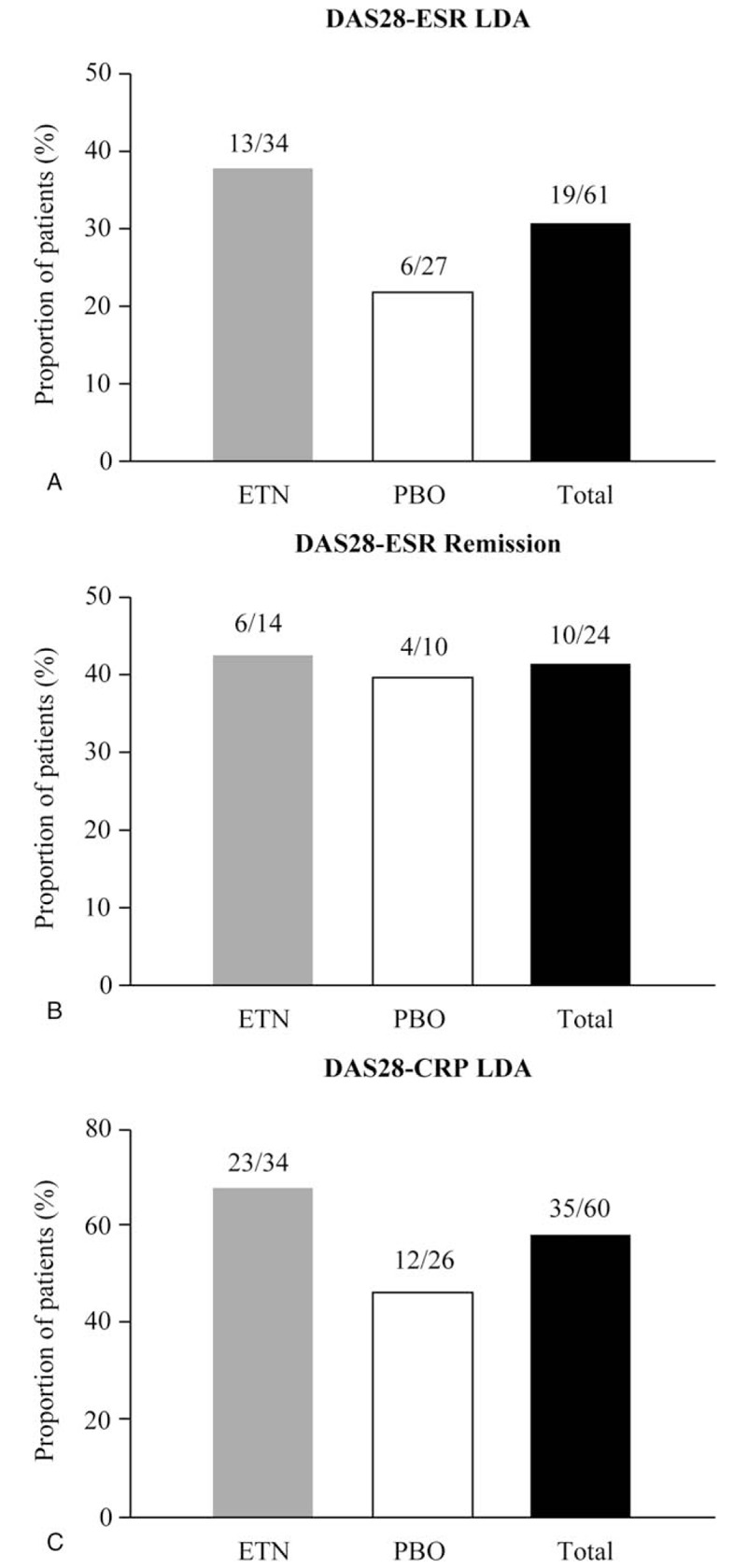
Proportion of patients at week 52 remaining in (A) DAS28-ESR LDA, (B) DAS28-ESR remission, and (C) DAS28-CRP LDA. CRP = C-reactive protein, DAS28 = Disease Activity Score-28 joints, ESR = erythrocyte sedimentation rate, ETN = etanercept, LDA = low disease activity, PBO = placebo.
At the end of Period 2, 32/61 (52.5%) patients maintained DAS28-ESR LDA using LOCF imputation of data (this includes patients who experienced a flare during Period 2, were treated with ETN50, and regained DAS28-ESR LDA by the end of Period 2); 17/34 (50.0%) and 15/27 (55.6%) were in the ETN50 and PBO groups, respectively. In addition, 6/14 (42.9%) and 4/10 (40.0%) in the ETN50 and PBO groups, respectively, maintained DAS28-ESR remission (Fig. 2B). In the ETN50 group, 23/34 (67.6%) and 12/26 (46.2%) in the PBO group were in DAS28-CRP LDA (<3.2) at the end of Period 2 (Fig. 2C).
During Period 2, 41/61 (67.2%) patients experienced a flare (Table 3) of which 21/34 (61.8%) were in the ETN50 and 20/27 (74.1%) in the PBO groups, respectively. The median (95% confidence interval [CI]) time to flare was 113 (29.0, not calculable) days in the ETN50 group and 33 (29.0, 87.0) days in the PBO group. Of patients experiencing a flare during Period 2, 4/21 (19.0%) receiving ETN50 and 9/20 (45.0%) receiving PBO were in DAS28-ESR LDA at week 52.
Table 3.
Proportion of patients in low disease activity at week 52 without post-flare censoring.

At week 52, a consistently greater proportion of patients in the ETN50 group than in the PBO group achieved predetermined efficacy endpoints (Fig. 2) which included ACR20 (85.3% vs 81.5%), ACR50 (73.5% vs 55.6%), ACR70 (52.9% vs 37.0%), ACR90 (20.6% vs 18.5%), European League Against Rheumatism (EULAR) moderate response (91.2% vs 81.5%), and EULAR good response (38.2% vs 22.2%). The proportion of patients achieving a HAQ-DI score ≤0.5 by the end of Period 2 (Fig. 3) was greater in those receiving ETN50 (21/34; 61.8%) than in those receiving PBO (10/27; 37.0%). In addition, in the ETN50 group, the proportion of patients with an improvement of >0.22 in HAQ-DI score increased slightly from 25/34 (73.5%) at week 24 to 27/34 (79.4%) at week 52 whereas this decreased in the PBO group from 26/27 (96.3%) at week 24 to 22/27 (81.5%) at week 52.
Figure 3.
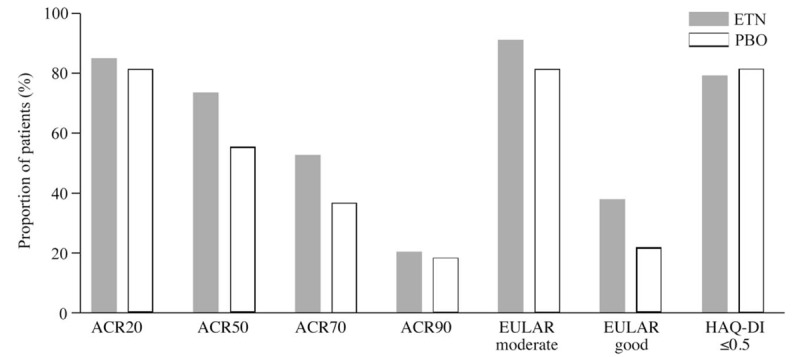
Proportion of patients achieving various endpoints at week 52. ACR = American College of Rheumatology, ETN = etanercept, EULAR = European League Against Rheumatism, HAQ-DI = Health Activity Questionnaire-Disability Index, PBO = placebo.
At week 52, the end of Period 2, there was consistently greater maintenance of improvement in the ETN50 group than in the PBO group for various predetermined efficacy and patient-reported outcome measures (Table 4) including DAS28-ESR (3.4 vs 3.9), DAS28-CRP (2.9 vs 3.2), ESR (28.7 vs 31.9), 28 TJC (3.2 vs 5.2), 28 SJC (2.4 vs 2.5), physician global assessment (1.2 vs 1.9), patient global assessment (2.2 vs 3.3), pain based on visual analog scale (21.7 vs 27.2), EuroQol-5D health state (82.7 vs 74.4), and work activity impairment (10.0% vs 12.0%).
Table 4.
Mean (SD) data for efficacy and patient-reported health-related quality-of-life scores.

3.3. Safety
Since the study was not designed for randomization based on geographic location, the number of patients in this subset analysis was too small for meaningful characterization of safety parameters. In the global study population, during Period 2, 37% of patients receiving ETN50 and 43% of patients receiving PBO reported experiencing adverse events. Serious adverse events (SAEs) were experienced by 1 (1%) and 7 (4%) patients receiving ETN50 and PBO, respectively. The patient in the ETN50 group experienced a myocardial infarction that was determined not to be treatment emergent. In the PBO group, 2 patients reported severe SAEs: 1 reported severe urinary tract infection and another reported severe injury due to a road traffic accident. Also in the PBO group, 5 patients reported moderate SAEs: 1 each of pneumonia, sinusitis, ulnar fracture, coronary artery disease, and myopathy.
4. Discussion
The global phase IV study was designed to evaluate the effectiveness of an ETN-free regimen compared with continuing ETN50 treatment on the maintenance of LDA in patients with moderate-to-severe RA who had achieved LDA on 24 weeks of ETN50 treatment.[12] The study also examined the effectiveness of an ETN-free regimen compared with continuing ETN50 treatment on the maintenance of remission in patients with moderate-to-severe RA who had achieved remission on 24 weeks of ETN50 treatment. Although information based on studies undertaken in patients from the USA and Western Europe is quite prevalent, the same cannot be said for patients from other parts of the world, including Latin America. This subset analysis was undertaken to begin to fill this gap on data among patients from Latin America and to examine whether they differed from the global study population.
At baseline, the disease characteristics of this subset were comparable to those in the global study.[12] Like those in the global study, patients in this subset had long-standing disease although slightly shorter than in the global study (6.4 vs 8.1 years) and high level of disease activity (DAS28-ESR 6.8 vs 6.4). In this subset, treatment with ETN50 was very effective, with 95.3% of patients achieving LDA in Period 1. This compared favorably with the results from the global study, where 67.7% of patients achieved LDA.[12] However, these results need to be interpreted with caution since the total number of patients is small (n = 64). At present, the underlying cause for this difference is unknown. It is possible that the difference is random, or it could be related to ethnicity. These and other possibilities would need to be investigated in larger studies focusing on this population.
The proportion of patients maintaining DAS28-ESR LDA, DAS28-CRP LDA, or DAS28-ESR remission at week 52 was higher in patients receiving ETN50 than in those receiving PBO during Period 2. A higher proportion of patients among those receiving ETN50 than among those receiving PBO achieved prespecified efficacy and quality-of-life endpoints at week 52. Patients who received ETN50 during Period 2 maintained their response on a spectrum of efficacy and quality-of-life endpoints from week 24 to week 52 better than those who received PBO. Although this post-hoc subset analysis of RA patients from Latin America was not powered for statistical analysis, treatment with ETN50 appears to result in maintenance of LDA similar to that observed for the whole study population. These data are also consistent with previous reports which demonstrated that although a lower dose of etanercept after achieving LDA or remission could maintain this status, discontinuing treatment resulted in worsening of disease symptoms.[13–17]
The median time to flare strongly favored patients receiving ETN50 (113 days) compared with those receiving PBO (33 days) in Period 2. The proportion of patients experiencing flares in both treatment groups was similar to that reported for the global study.[12] The patients enrolled in this study had a long duration of high disease activity which may have contributed to the number of patients experiencing flares.
It is unclear why some patients achieving LDA in Period 1 lost efficacy during Period 2 even though they were receiving ETN50. To be eligible for randomization and entry into Period 2, patients needed to have achieved LDA (DAS28-ESR <3.2) at week 24. It was not necessary for them to exhibit sustained efficacy. As such, it is possible that because of long disease duration the efficacy observed at that single time point was lost due to disease progression. Similarly, it is unclear why some patients receiving PBO during Period 2 continue to exhibit efficacy. This could be due to the natural course of the disease or early therapeutic intervention according to the “window of opportunity” hypothesis. Further characterizing both of these groups of patients may help elucidate prognostic factors and disease mechanisms.[18]
Although statistical analysis could not be performed between the 2 treatment groups, the higher maintenance of response by patients in the ETN50 group compared with the PBO group suggests that continuing ETN50 would be beneficial to patients. The data reported here are consistent with previous reports in patients with established RA (>12 months). It is less clear for patients with early RA (<12 months), since there is a need for further studies. It bears repeating that caution should be exercised when interpreting these results due to the small sample size of this subset. In general, data from a large clinical trial are a more reliable estimate of treatment effect than those from a subset analysis.[19] Furthermore, geographical differences among subgroups may also be a contributing factor for the results reported herein.[20] The decision on whether to continue or discontinue active therapeutic intervention should be made in the best interests of the patient and left to the treating physician and the patient. Larger studies that include predictors of sustained response with an initial anti-TNF treatment regimen and focus on the needs of the Latin American population may be needed to elucidate these issues further.
Acknowledgments
Medical writing support was provided by Mukund Nori, PhD, MBA, CMPP, of Engage Scientific Solutions and was funded by Pfizer.
The names of the Independent Ethics Committees or Institutional Review Boards that approved the T2T study are as follows: Brazil: Comitê de Ética em Pesquisa do Hospital Universitário, Comitê de Ética em Pesquisa do Hospital Heliópolis, Comitê de Ética em Pesquisa do Hospital Alberto Rassi, Comitê de Ética em Pesquisa Prof. Dr. Celso Figueirôa; China: Shanghai Changhai Hospital Ethics Committee, Ethics Committee of Guangdong General Hospital, Ethics Committee of Peking Union Medical College Hospital, Ethics Committee of Guanghua Hospital of Intergrated Traditional Chinese and Western Medicine; Colombia: Comité de Ética de la Investigación Riesgo de Fractura, Comité de Investigaciones y Etica en Investigaciones, Comité de Etica en Investigacion en el area de la salud; Czech Republic: Eticka komise Krajska nemocnice T. Bati, Eticka komise Fakultni nemocnice Olomouc a Lekarske, Etická komise pro multicentrické klinické hodnocení, Eticka komise Revmatologickeho ustavu; Egypt: Ain Shams University, Faculty of Medicine Research Ethics Committee, El Azhar University, Faculty of Medicine Ethics Committee, Ethics Committee Faculty of Medicine Alexandria University; Hungary: Egészségügyi Tudományos Tanács Klinikai Farmakológiai Etikai Bizottsága; Jordan: Jordan University of Science & Tech King Abdullah University Hospital, Lebanon: American University of Beirut Medical Center Institutional Review Board; Malaysia: Medical Research & Ethics Committee - Ministry of Health Malaysia, Medical Ethics Committee - University Malaya Medical Centre; Mexico: Comité de Ética en Investigación Av. Venustiano Grranza No. 2395 Colonia Universitaria, Comité Bioético para la investigación Clínica SC - Centro de Investigación y Atención Integral de Durango AC, Comité Bioético para la investigación Clínica SC - Centro de Alta Especialidad en Reumatología e Investigación del Potosi S.C., Comite de Etica en Investigacion del Grupo Calydes SCP - Unidad Reumatologica Las Americas S.C.P. del Centro Medico de Las Americas; Philippines: De La Salle Health Sciences Institute Independent Ethics Committee, University of the Philippines Manila Research Ethics Board, Institutional Review Board Angeles University Foundation, Institutional Review Board - Chong Hua Hospital, Institutional Ethics Review Committee St. Luke's Medical Center; Qatar: Hamad Medical Corporation Medical Research Center; Romania: Comisia Naţională de Bioetică a Medicamentului şi a Dispozitivelor Medicale; Russian Federation: Ethics Council under the Ministry of Health of the Russian Federation, Ethics Committee of GBOU VPO “Krasnoyarsk State Medical University named after professor V.F. Voyno- Yasenetskiy of Ministry of Health of Russian Federation,” Local Ethics Committee at the LLC Institute of Medical Trials, Pharma-Ethics; Taiwan: Taipei Medical University - Joint Institutional Review, The Institutional Review Board, China Medical University Hospital, Institutional Review Board of the Cathay General Hospital, Research Ethics Committee of Buddhist Tzu Chi General Hospital; Thailand: Research Ethics Committee 2, Faculty of Medicine, Chiang Mai University, Office of Human Research Ethics Committee Faculty of Medicine, Prince of Songkla University, The Khon Kaen University Ethics Committee for Human Research, Siriraj Institutional Review Board Faculty of Medicine; Ukraine: Komisiia z pytan etyky KU “Odeska oblasna klinichna likarnia,” Komisiia z pytan etyky Vinnytskoi oblasnoi klinichnoi likarni im. M.I. Pyrohova, Komisiia z pytan etyky pry Ternopilskii universytetskii likarni, Komisiia z pytan etyky pry Kyivskii oblasnii klinichnii likarni, Komisiia z pytan etyky pry Krymskii respublikanskii ustanovi “Universytetska klinika;” United Arab Emirates: Al Qassimi Clinical Research Center, Research Ethics Committee, Al Qassimi Hospital – Sharjah.
Author Contributions
All authors are responsible for the content of this manuscript. The authors together conceptualized the manuscript content, interpreted the data, reviewed multiple drafts, and approved the final version for submission.
Conceptualization: Cristiano AF Zerbini, Carlos Abud-Mendoza, Paul Mendez-Patarroyo, Mauricio De Angelo Andrade, Bonnie Vlahos, Cecilia Elena Borlenghi, Ron Pedersen.
Data curation: Ron Pedersen, Bonnie Vlahos.
Formal analysis: Ron Pedersen.
Investigation: Cristiano AF Zerbini, Carlos Abud-Mendoza, Paul Mendez-Patarroyo, Mauricio De Angelo Andrade, Bonnie Vlahos, Cecilia Elena Borlenghi.
Methodology: Cristiano AF Zerbini, Carlos Abud-Mendoza, Paul Mendez-Patarroyo, Mauricio De Angelo Andrade, Ron Pedersen, Bonnie Vlahos, Cecilia Elena Borlenghi.
Project administration: Bonnie Vlahos, Cecilia Elena Borlenghi.
Resources: Mauricio De Angelo Andrade.
Supervision: Cristiano AF Zerbini, Carlos Abud-Mendoza, Paul Mendez-Patarroyo, Ron Pedersen.
Writing – review & editing: Cristiano AF Zerbini, Carlos Abud-Mendoza, Paul Mendez-Patarroyo, Mauricio De Angelo Andrade, Ron Pedersen, Bonnie Vlahos, Cecilia Elena Borlenghi.
Footnotes
Abbreviations: ACR = American College of Rheumatology, CI = confidence interval, CRP = c-reactive protein, csDMARD = conventional synthetic disease-modifying antirheumatic drug, DAS28 = Disease Activity Score—28 joints, ESR = erythrocyte sedimentation rate, ETN = etanercept, EULAR = European League Against Rheumatism, HAQ-DI = Health Activity Questionnaire—Disability Index, LDA = low disease activity, LOCF = last observation carried forward, MTX = methotrexate, PBO = placebo, RA = rheumatoid arthritis, SAE = serious adverse events, SD = standard deviation, SJC = swollen joint count, T2T = treat-to-target, TJC = tender joint count, TNF = tumor necrosis factor, USA = United States of America.
The study was sponsored by Pfizer.
CAFZ is a consultant with Pfizer; has participated on advisory boards and speakers bureau for Pfizer; and has received research grants from Pfizer. CAM is a consultant with Bristol-Myers Squibb, and Pfizer; and has participated on speakers’ bureaus for Bristol-Myers Squibb, Merck-Serono, Pfizer, Roche, and UCB. PMP is a consultant with Pfizer. MDAA, RP, BV, and CEB are employees of Pfizer and own stock in Pfizer.
References
- [1].Halabi H, Alarfaj A, Alawneh K, et al. Challenges and opportunities in the early diagnosis and optimal management of rheumatoid arthritis in Africa and the Middle East. Int J Rheum Dis 2015;18:268–75. [DOI] [PubMed] [Google Scholar]
- [2].Kourilovitch M, Galarza-Maldonado C, Ortiz-Prado E. Diagnosis and classification of rheumatoid arthritis. J Autoimmun 2014;48–49:26–30. [DOI] [PubMed] [Google Scholar]
- [3].Mody GM, Cardiel MH. Challenges in the management of rheumatoid arthritis in developing countries. Best Pract Res Clin Rheumatol 2008;22:621–41. [DOI] [PubMed] [Google Scholar]
- [4].Ramirez LA, Rodriguez C, Cardiel MH. Burden of illness of rheumatoid arthritis in Latin America: a regional perspective. Clin Rheumatol 2015;34(suppl):S9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Conti F, Ceccarelli F, Massaro L, et al. Biological therapies in rheumatic diseases. Clin Ter 2013;164:e413–28. [DOI] [PubMed] [Google Scholar]
- [6].Singh JA, Saag KG, Bridges SL, Jr, et al. 2015 American College of Rheumatology Guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol 2016;68:1–25. [DOI] [PubMed] [Google Scholar]
- [7].Cardiel MH. Latin American Rheumatology Associations of the Pan-American League of Associations for Rheumatology (PANLAR), Grupo Latinoamericano de Estudio de Artritis Reumatoide (GLADAR). First Latin American position paper on the pharmacological treatment of rheumatoid arthritis. Rheumatology 2006;45(suppl):ii7–22. [DOI] [PubMed] [Google Scholar]
- [8].Fernandes V, de Assis TM, Queiroz CC, et al. Use of biological therapies in rheumatoid arthritis management: a comparison between the main worldwide and Brazilian recommendations. Rev Bras Reumatol 2011;51:220–30. [PubMed] [Google Scholar]
- [9].Grupo de Estudio de Artritis Reumatoidea Sociedad Argentina de Reumatologia. Actualizacion de las Guias de Practica Clinica en el Tratamiento de la Artritis Reumatoidea; 2013. Available at: http://www.reumatologia.org.ar/docs/guias_sar_2013.pdf. Accessed June 18, 2018. [Google Scholar]
- [10].Sistema general de seguridad social en salud – Colombia. Guía de Práctica Clínica, para la detección temprana, diagnóstico y tratamiento de la artritis reumatoide; 2014. Available at: http://gpc.minsalud.gov.co/gpc_sites/Repositorio/Conv_563/GPC_art_reumatoide/GPC_AR_COMPLETA.pdf. Accessed June 18, 2017. [Google Scholar]
- [11].Brenol CV, Nava JI, Soriano ER. Proper management of rheumatoid arthritis in Latin America. What the guidelines say? Clin Rheumatol 2015;34(suppl):51–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Pavelka K, Akkoc N, Al-Maini M, et al. Maintenance of remission with combination etanercept-DMARD therapy versus DMARDs alone in active rheumatoid arthritis: results of an international treat-to-target study conducted in regions with limited biologic access. Rheumatol Int 2017;37:1469–79. [DOI] [PubMed] [Google Scholar]
- [13].Emery P, Hammoudeh M, FitzGerald O, et al. Sustained remission with etanercept tapering in early rheumatoid arthritis. New Engl J Med 2014;371:1781–92. [DOI] [PubMed] [Google Scholar]
- [14].Smolen JS, Nash P, Durez P, et al. Maintenance, reduction, or withdrawal of etanercept after treatment with etanercept and methotrexate in patients with moderate rheumatoid arthritis (PRESERVE): a randomised controlled trial. Lancet 2013;381:918–29. [DOI] [PubMed] [Google Scholar]
- [15].van Herwaarden N, den Broeder AA, Jacobs W, et al. Down-titration and discontinuation strategies of tumor necrosis factor–blocking agents for rheumatoid arthritis in patients with low disease activity. Cochrane Database Syst Rev 2014;CD010455. [DOI] [PubMed] [Google Scholar]
- [16].van Vollenhoven RF, Ostergaard M, Leirisalo-Repo M, et al. Full dose, reduced dose or discontinuation of etanercept in rheumatoid arthritis. Ann Rheum Dis 2016;75:52–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Wiland P, Dudler J, Veale D, et al. The effect of reduced or withdrawn etanercept-methotrexate therapy on patient-reported outcomes in patients with early rheumatoid arthritis. J Rheumatol 2016;43:1268–77. [DOI] [PubMed] [Google Scholar]
- [18].Nagy G, van Vollenhoven RF. Sustained biologic-free and drug-free remission in rheumatoid arthritis, where are we now? Arthritis Res Ther 2015;17:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Yusuf S, Wittes J, Probstfield J, et al. Analysis and interpretation of treatment effects in subgroups of patients in randomized clinical trials. JAMA 1991;266:93–8. [PubMed] [Google Scholar]
- [20].Yusuf S, Wittes J. Interpreting geographic variations in results of randomized, controlled trials. N Engl J Med 2016;375:2263–71. [DOI] [PubMed] [Google Scholar]


