Abstract
Background:
The aim of this study was to evaluate the correlation between endothelial nitric oxide synthase (eNOS) polymorphism (−786T>C) and migraine susceptibility in a meta-analysis.
Methods:
A literature search was performed for case–control studies from inception to July 30, 2018 focusing on eNOS polymorphism (−786T>C) and risk of migraine. From 454 full-text articles, 6 were included in this study. Heterogeneity was assessed with the I2 index and quality assessment was performed using the Newcastle–Ottawa scale.
Results:
CC genotype was not related to higher susceptibility of migraine compared with TT+ TC genotypes with significant difference (fixed effects model; OR = 1.27; 95% CI = 0.90–1.80; P = .17; I2 = 18%). However, subgroup analysis showed CC variant increase the risk for migraine compared with TT+ TC genotypes in Caucasian populations (fixed effects model; OR = 1.62; 95% CI = 1.03–2.56; P = .04; I2 = 18%), which could not be observed in non-Caucasian populations (fixed effects model; OR = 0.88; 95% CI = 0.51–1.53; P = .66; I2 = 0%). There was no significant difference for other genotypes and alleles between patients with migraine and healthy controls (all P > .05).
Conclusion:
This meta-analysis indicated that CC variant increases the risk for migraine compared with TT + TC genotypes in Caucasian populations.
Keywords: endothelial nitric oxide synthase, migraine, polymorphism, susceptibility
1. Introduction
Migraine is a chronic paroxysmal neurological disorder characterized by attacks of moderate or severe headache and reversible neurological and systemic symptoms that affects approximately 12% to 16% of the population.[1,2] Migraine often cause significant disability and impaired quality of life, adversely affecting the activities of daily living and work-related productivity in many patients.[3] Migraine is clinically diagnosed according to the criteria set out by the International Headache Society (IHS).[4] Migraine often begins with premonitory symptoms hours or days before the onset of pain. The most common premonitory symptoms include fatigue, impaired concentration, and neck stiffness. Furthermore, other psychological (anxiety, depression, irritability), arousal (drowsiness), neurological (photophobia), and cranial parasympathetic symptoms (lacrimation), and general symptoms (e.g., yawning, increased urination, nausea, diarrhoea) can occur before the onset of pain.[2] The IHS classifies migraine as migraine with aura (MA), representing about one-third of all migraine, or migraine without aura (MO) that accounts for approximately two-thirds of all migraine in the population.[2,4,5] Although researches have been performed for many years, the complex pathogenetic mechanisms of migraine still remain unclear. Migraine is considered to be as a result of combination of environmental and genetic aspects.
Nitric oxide (NO) is a signaling molecule with a short half-life, which is synthesized from L-arginine by 3 isozymes of nitric oxide synthase (NOS), including neuronal NOS (nNOS), endothelial NOS (eNOS), and inducible NOS (iNOS).[6] It exerts a variety of physiological effects such as regulating blood pressure via smooth muscle relaxation, and functioning as a neurotransmitter.[7] NO in the migraine pathogenesis has been reported in several studies.[8,9] Polymorphism of NOS could affect production of NO, which may be connected with migraine. The associations between a single nucleotide polymorphism (SNP) of NOS and migraine were widely studied.[10–20] Although nNOS is predominately expressed in neurons of the central and peripheral nervous systems, studies evaluating the association between nNOS polymorphism and migraine were limited. There was no marked association between nNOS polymorphisms (276C>T, rs2682826; −421+145599C>T, rs693534; 853–2109C>A, rs7977109) and migraine susceptibility.[10,11] Conversely, a series of studies reporting the relationships between SNPs of iNOS, eNOS genes, and risk of migraine.[12–20] There was no association between bi-allelic tetranucleotide polymorphism of iNOS and migraine.[12] The 2087G>A (rs2297518) and the −1026C>A (rs2779249) polymorphisms in the iNOS gene affect the susceptibility to MA when their effects are combined within haplotypes, whereas the 2087G>A affects the susceptibility to aura in migraine patients.[13] A significant interaction between iNOS 2087G>A and eNOS 2512+242G>A/C (rs743506) polymorphisms in migraine patients compared to control group, suggesting that this combination affect migraine susceptibility.[14] Three clinically relevant polymorphisms of eNOS have been studied: SNP in the promoter region (−786T>C, rs2070744), an SNP in exon 7 (+894G>T, rs1799983), and the variable number of tandem repeats (VNTR) in intron 4.[21,22] Borroni et al[15] reported that eNOS (+894G>T) polymorphism was an independent risk for MA. However, a later study could not verify the conclusion.[16] A recent meta-analysis indicated that “T” allele of the eNOS +894G > T variant increased the risk of migraine among non-Caucasians.[17] The substitution of thymine by cytosine in the 5′ promoter region at nucleotide—786 (−786T>C) results in a significant reduction in eNOS gene promoter activity and in decreased basal NO production.[16] Similarly, there are some controversies for the connection between eNOS polymorphism (−786T>C) and migraine risk.[14,18–20] This meta-analysis was carried out to evaluate the relationship between eNOS (−786T>C) polymorphism and migraine susceptibility.
2. Patients and methods
2.1. Literature search
Two authors independently performed literature search for published studies from inception to July 30, 2018 using PubMed, Cochrane Database, EMBASE, Web of science, Google scholar, and China National Knowledge Infrastructure (CNKI). The keywords used were “nitric oxide synthase,” or “NOS,” or “endothelial nitric oxide synthase,” or “NOS3,” or “eNOS” and “migraine,” or “migraine disorder.” The titles and abstracts of the resulting articles were examined and unrelated articles were excluded. If an article was selected for inclusion, the references were reviewed for additional studies. This study was approved by the ethics committee of the First Hospital of Jilin University.
2.2. Inclusion and exclusion criteria
An article was relevant if it reported original data from case–control study, regardless of language, investigating the correlations between eNOS polymorphism (−786T>C) and migraine susceptibility. Controls were healthy individuals without migraine randomly selected from general populations after excluding those with underlying diseases (e.g., cardiovascular, renal, hepatic, gastrointestinal, pulmonary, endocrine, auto-immune, psychiatric diseases),[1,5,14,19] or whose health was established by medical diagnosis.[18,20] Studies were excluded if one of the following existed: studies with insufficient genotyping data of patients or control group; case reports; review articles; experiment researches; case-only studies. Disagreements between the 2 reviewers regarding inclusion of a study were resolved through consensus.
2.3. Data extraction and quality assessment
Two independent reviewers extracted the data and assessed the quality of each study using the Newcastle–Ottawa scale (NOS). In this scale 9 points represent the highest quality. In case of disagreements, the original article was evaluated by a third reviewer.
2.4. Data analysis
RevMan 5.2 (Cochrane database) was used to analyze the data. The results were reported as odds ratios (OR) with 95% confidence intervals (CI). Heterogeneity between studies was assessed by using the I2 statistic: values of 25%, 50%, and 75% represent mild, moderate, and severe heterogeneity, respectively. Based on results of the heterogeneity test, a fixed effect model was used if P > .10, while a random effects model was performed if P ≤ 0.10. Begg's funnel test and Egger's test were applied to evaluate publication bias across studies with Stata (version 12.0). A P < .05 was considered statistically significant.
3. Results
3.1. Search results
We followed the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement for reporting our results. Six studies of full-text articles were selected for inclusion in this meta-analysis. Our selection process for studies included in the analysis was shown in Figure 1.
Figure 1.
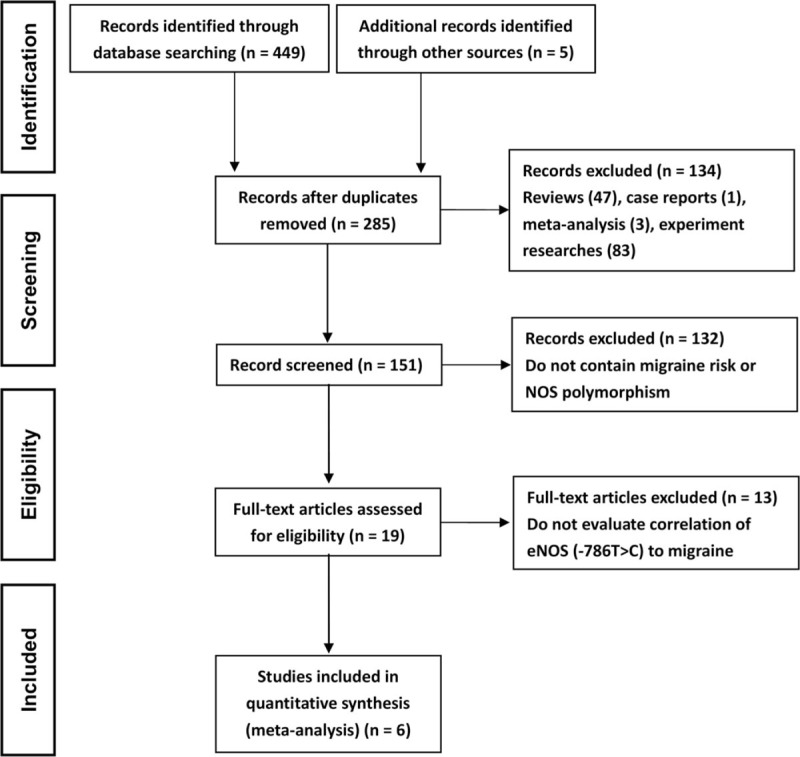
Flow chart of literature selection in this study.
3.2. Characteristics of the included studies
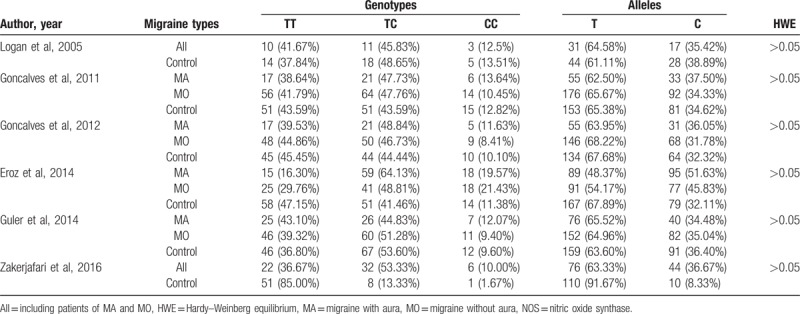
The characteristics of the included studies, which were published between 2005 and 2017, were presented in Table 1. Two studies were from Brazil,[14,19] 2 from Turkey,[1,5] 1 from the United Kingdom,[18] and the last one was from Iran.[20] A total of 763 patients with migraine and 560 healthy controls were included in this meta-analysis. The frequencies of genotypes and alleles were summarized in Table 2. All studies had a Newcastle–Ottawa scale score ≥7, with an average of 7.5 (Table 1).
Table 1.
Characteristics of the included studies.
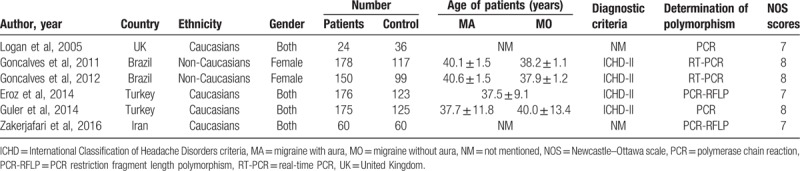
Table 2.
The detailed frequencies of genotypes and alleles of NOS.
3.3. Correlation of eNOS polymorphism −786T>C and migraine susceptibility
Compared with patients with TT genotype, those with CC + TC genotypes did not have higher risk for incidence of migraine with statistical significance (random effects model; OR = 1.66; 95% CI = 0.88–3.13; P = .12; I2 = 86%). CC genotype was not associated with higher susceptibility of migraine compared with TT+ TC genotypes with significant difference (fixed effects model; OR = 1.27; 95% CI = 0.90–1.80; P = .17; I2 = 18%) (Fig. 2A). Similar result was observed when analyzed patients with C allele and those with T allele (random effects model; OR = 1.30; 95% CI = 0.91–1.85; P = .15; I2 = 76%). However, subgroup analysis showed CC variant increase the risk for migraine compared with TT+ TC genotypes in Caucasian populations (fixed effects model; OR = 1.62; 95% CI = 1.03–2.56; P = .04; I2 = 18%), which could not be observed in non-Caucasian patients (fixed effects model; OR = 0.88; 95% CI = 0.51–1.53; P = .66; I2 = 0%) (Fig. 2B). We also analyzed the relationship of eNOS polymorphism and migraine subtypes, MA and MO, no significant difference was observed (Fig. 3: TT vs TC + CC; Fig. 4: CC vs TC + TT).
Figure 2.
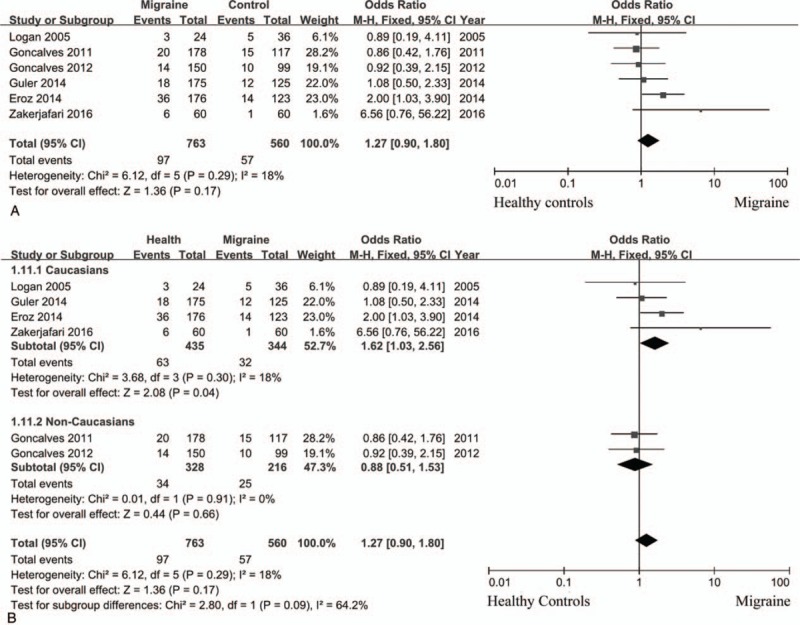
eNOS (−786T>C) polymorphism and the risk of migraine (CC vs TT + TC). (A) the overall results; (B) different populations.
Figure 3.
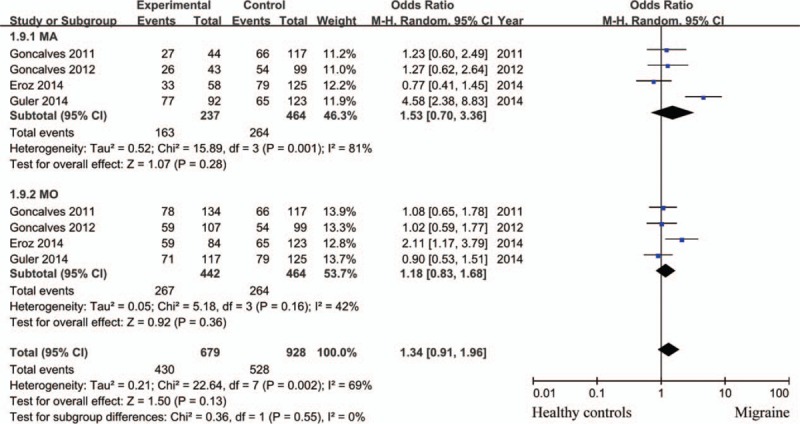
eNOS (−786T>C) polymorphism and the risk of migraine subtypes (TT vs TC + CC).
Figure 4.
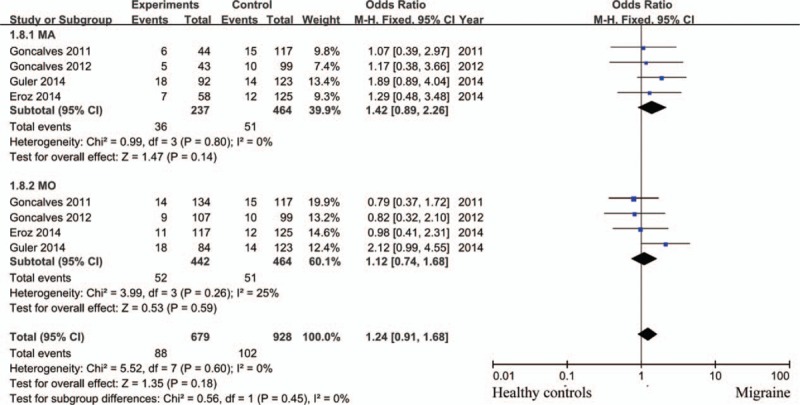
eNOS (−786T>C) polymorphism and the risk of migraine subtypes (CC vs TC + TT).
3.4. Heterogeneity
Considering heterogeneity existed in this study, we used Galbraith plots to explore the heterogeneity sources (Fig. 5). Low Newcastle–Ottawa scale score[20] and different genetic backgrounds[5] were the main causes for heterogeneity. After removal of these 2 studies, as concluded above, both CC + CT genotypes (fixed effects model; OR = 0.99; 95% CI = 0.76–1.30; P = .95; I2 = 0%) and C allele (fixed effects model; OR = 0.98; 95% CI = 0.81–1.20; P = .86; I2 = 0%) did not increase the risk of migraine compared with TT genotype and T allele, respectively.
Figure 5.
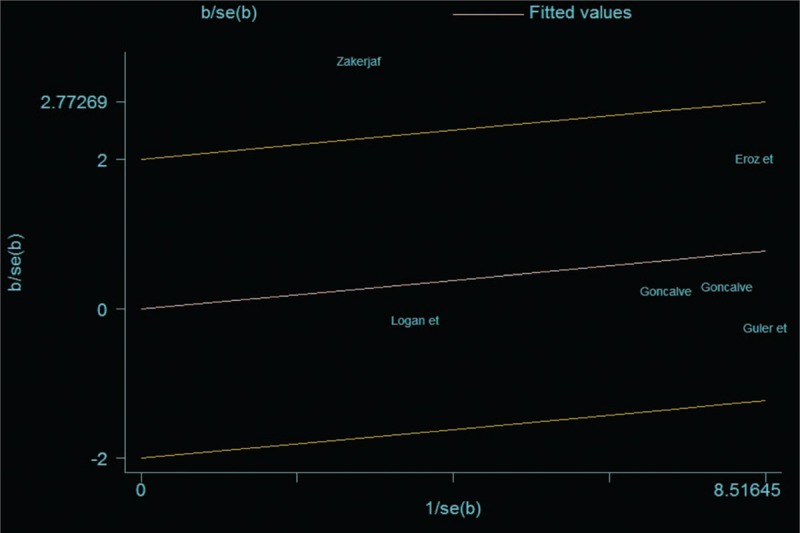
Galbraith plots for studies included in this meta-analysis.
3.5. Publication bias
Publication bias was determined using Begg's funnel plots and Egger's test. Neither Egger's test nor Begg's funnel plots indicated any significant publication bias (P = .452 and 0.317, respectively) (Fig. 6).
Figure 6.
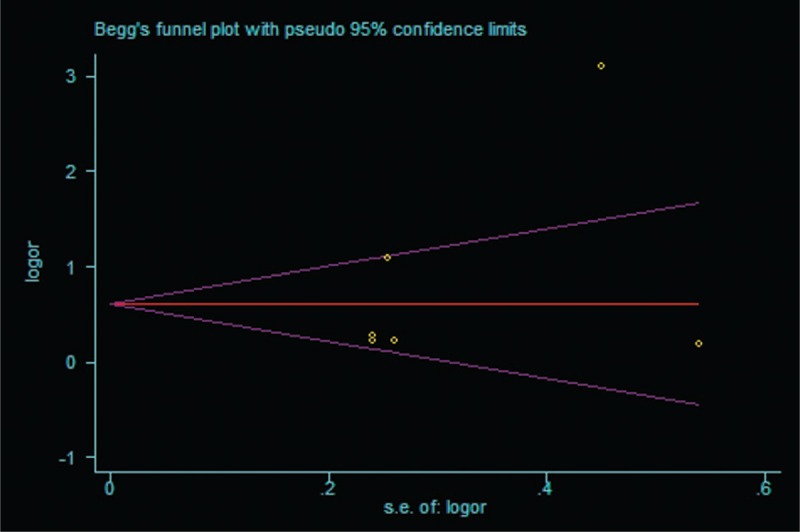
Begg's funnel plot for publication bias.
4. Discussion
NO regulates a host of physiological functions, including vascular tone, pain sensation, neurotransmission, and as an immune defence mechanism.[23] NO activates soluble guanylate cyclase and subsequently increases cGMP. cGMP can induce gene expression via the induction of transcription factors such as mitogen activated protein (MAP) kinases or NF-κB.[24] Although the aetiology and pathogenesis of migraine remain unclear, NO is believed to play a key role in migraine pathogenesis. Migraine was initiated by NO donors, glyceryl trinitrate (GTN), or isosorbide dinitrate, which indicated that NO may participate in the pathological process of migraine.[24,25] GTN did initiate migraine headaches but not the aura in migraineurs with aura in most studies. This indicated that NO is involved in the pain mechanism in both types of migraines but not in the initiation of the aura.[24] Further study demonstrated that not NO itself but the elevation of cGMP by NO or other mediators is necessary to induce migraine.[26] Inhibition of NOS in spontaneous migraine attacks leads to the attenuation of symptoms in two-thirds of the patients.[27] Thus, NO seems not only be involved in the initiation of migraine attacks but also in the maintenance of these attacks. Calcitonin gene related peptide (CGRP) is a key player in migraine pathophysiology.[28] CGRP levels were elevated following GTN-induced migraine.[29] Pretreatment of trigeminal ganglia cultures with NO donors was also found to increase CGRP release in response to both depolarization and inflammatory mediators, which may have occurred due to NO donors increasing CGRP promoter activity.[30,31] While NO donors have some functions independent of CGRP, their function in migraine may strongly relate to promoting CGRP production and release, adding to the CGRP-influenced pathways in migraine.[31]
NO was synthesized by NOS from L-arginine. The activity of NOS has an important impact on the production of NO, and polymorphism of NOS gene leads to variant activity of NOS. In this study, we carried out a meta-analysis to evaluate the correlation of eNOS polymorphism (−786T>C) and migraine risk. The overall results did not indicate associations between eNOS polymorphism and migraine. However, subgroup analysis showed CC variant increase the risk for migraine compared with TT + TC genotypes in Caucasian populations.
When compared between CC + TC genotypes and TT genotype, between T allele and C allele, significant heterogeneity existed. Random effects model indicated that there was no significant difference between patients and healthy controls. First, we carried out subgroup analysis by populations. Lack of association was observed between eNOS polymorphism and migraine susceptibility in non-Caucasians (fixed effects model; OR = 1.10; 95% CI = 0.78–1.56; P = .58; I2 = 0%). Second, using Galbraith plots, source of heterogeneity was identified. After removal of studies with significant heterogeneity, fixed model showed that both CC + CT genotypes and C allele did not increase migraine susceptibility compared with TT genotype and T allele, respectively.
For migraine subtypes, 4 studies were included.[1,5,14,19] We tried to contact the authors of the remaining 2 studies for details. However, no reply was received. Strategies mentioned above were used to cope with study of high heterogeneity. Again no relationship was observed between eNOS gene polymorphism and the risk of migraine subtypes regardless of subgroup analysis, fixed or random effects model usage.
Our study has several strengths. First, this is the largest study to date evaluating the correlation between eNOS −786T>C polymorphism and risk of migraine. Second, patients and controls of different genetic backgrounds made it possible to analyze the associations in different populations. However, our study also has several limitations. First, due to apparent heterogeneity across studies in some cases, the findings from our study should be dealt with some caution. Second, we did not include academic dissertations and conference papers, so there may have been bias in provision of data. Third, there are other factors that affect the incidence of migraine, for example, gender,[32] medication overuse,[33] and so on. However, we could not evaluate the influence of these factors on the association between eNOS polymorphism and migraine susceptibility with studies included in this meta-analysis. Furthermore, a tendency of higher risk for migraine susceptibility could be observed in this meta-analysis (e.g., CC genotype vs TC + TT genotypes, C allele vs T allele). Although statistical significance could not be acquired with current studies, a larger sample may reach a positive result. Hence, further study is still needed.
Although the association of eNOS −786T>C polymorphism and migraine susceptibility were reported in previous studies. This meta-analysis indicated that CC variant increase the risk for migraine compared with TT+ TC genotypes in Caucasian populations. More studies are still needed to verify such conclusion.
Acknowledgments
We thank the Department of Geriatric Medicine, the First Hospital of Jilin University, for their assistance in this work. This work was supported by the Program from Health commission of Jilin Province (2014Q030).
Author contributions
DH, WZH, DB, HYN, and ZHY conceived the study. DH, WZH, DB, HYN, and ZHY designed the study and analyzed the data. DH, DB, and ZHY wrote this manuscript. All authors discussed and revised the manuscript before submission.
Conceptualization: Han Dong.
Data curation: Han Dong, Bin Dong, Ya Nan Hu, Hui Ying Zhao.
Formal analysis: Han Dong, Zhi Hao Wang, Bin Dong.
Funding acquisition: Han Dong.
Investigation: Han Dong, Bin Dong, Ya Nan Hu, Hui Ying Zhao.
Methodology: Han Dong, Zhi Hao Wang, Bin Dong, Ya Nan Hu, Hui Ying Zhao.
Resources: Zhi Hao Wang.
Software: Han Dong, Zhi Hao Wang, Bin Dong.
Supervision: Hui Ying Zhao.
Validation: Ya Nan Hu.
Writing – original draft: Han Dong.
Writing – review & editing: Han Dong.
Footnotes
Abbreviations: CI = confidence intervals, CNKI = China National Knowledge Infrastructure, eNOS = endothelial NOS, IHS = International Headache Society, iNOS = inducible NOS, MA = migraine with aura, MO = migraine without aura, nNOS = neuronal NOS, NO = nitric oxide, NOS = nitric oxide synthase, OR = odds ratios, PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses, SNP = single nucleotide polymorphism.
This work was supported by the Program from Health commission of Jilin Province (2014Q030).
The authors have no conflicts of interest to disclose.
References
- [1].Güler S, Gürkan H, Tozkir H, et al. An investigation of the relationship between the eNOS gene polymorphism and diagnosed migraine. Balkan J Med Genet 2015;17:49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Dodick DW. Migraine. Lancet 2018;391:1315–30. [DOI] [PubMed] [Google Scholar]
- [3].Ichikawa M, Katoh H, Kurihara T, et al. Clinical response to valproate in patients with migraine. J Clin Neurol 2016;12:468–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders. Cephalalgia 2018;38:1–211. [DOI] [PubMed] [Google Scholar]
- [5].Eröz R, Bahadir A, Dikici S, et al. Association of endothelial nitric oxide synthase gene polymorphisms (894G/T, −786T/C, G10T) and clinical findings in patients with migraine. Neuromol Med 2014;16:587–93. [DOI] [PubMed] [Google Scholar]
- [6].Bandara N, Gurusinghe S, Lim SY, et al. Molecular control of nitric oxide synthesis through eNOS and caveolin-1 interaction regulates osteogenic differentiation of adipose-derived stem cells by modulation of Wnt/β-catenin signaling. Stem Cell Res Ther 2016;7:182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Thomas DD. Breathing new life into nitric oxide signaling: a brief overview of the interplay between oxygen and nitric oxide. Redox Biol 2015;5:225–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Olesen J, Iversen HK, Thomsen LL. Nitric oxide supersensitivity: a possible molecular mechanism of migraine pain. Neuroreport 1993;4:1027–30. [DOI] [PubMed] [Google Scholar]
- [9].Zhao YL, Wang YL, Ying G. Effect of nitric oxide on migraine attack. Chin J Clin Rehab 2006;10:138–9. [Google Scholar]
- [10].Alaşehirli B, Akçalı A, Demiryürek AT, et al. Lack of association between the C276T polymorphism of the neuronal nitric oxide synthase gene and migraine. Int J Neurosci 2013;123:50–4. [DOI] [PubMed] [Google Scholar]
- [11].García-Martín E, Martínez C, Serrador M, et al. Neuronal nitric oxide synthase (nNOS, NOS1) rs693534 and rs7977109 variants and risk for migraine. Headache 2015;55:1209–17. [DOI] [PubMed] [Google Scholar]
- [12].Lea RA, Curtain RP, Shepherd AG, et al. No evidence for involvement of the human inducible nitric oxide synthase (iNOS) gene in susceptibility to typical migraine. Am J Med Genet 2001;105:110–3. [PubMed] [Google Scholar]
- [13].de OS, Mansur T, Gonçalves FM, et al. Inducible nitric oxide synthase haplotype associated with migraine and aura. Mol Cell Biochem 2012;364:303–8. [DOI] [PubMed] [Google Scholar]
- [14].Gonçalves FM, Luizon MR, Speciali JG, et al. Interaction among nitric oxide (NO)-related genes in migraine susceptibility. Mol Cell Biochem 2012;370:183–9. [DOI] [PubMed] [Google Scholar]
- [15].Borroni B, Rao R, Liberini P, et al. Endothelial nitric oxide synthase (Glu298Asp) polymorphism is an independent risk factor for migraine with aura. Headache 2006;46:1575–9. [DOI] [PubMed] [Google Scholar]
- [16].Toriello M, Oterino A, Pascual J, et al. Lack of association of endothelial nitric oxide synthase polymorphisms and migraine. Headache 2008;48:1115–9. [DOI] [PubMed] [Google Scholar]
- [17].Chen M, Tang W, Hou L, et al. Tumor necrosis factor (TNF) -308G>A, nitric oxide synthase 3 (NOS3) +894G>T polymorphisms and migraine risk: a meta-analysis. PLoS One 2015;10:e0129372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Logan JF, Chakravarthy U, Hughes AE, et al. Evidence for association of endothelial nitric oxide synthase gene in subjects with glaucoma and a history of migraine. Invest Ophthalmol Vis Sci 2005;46:3221–6. [DOI] [PubMed] [Google Scholar]
- [19].Gonçalves FM, Martins-Oliveira A, Speciali JG, et al. Endothelial nitric oxide synthase haplotypes associated with aura in patients with migraine. DNA Cell Biol 2011;30:363–9. [DOI] [PubMed] [Google Scholar]
- [20].Zakerjafari M, Dehkordi FF, Yaghoubi H. Evaluation of NOS3 T-C 786 gene polymorphism in Iranian patients affected by migraine and normal individuals. Adv Biores 2016;7:16–9. [Google Scholar]
- [21].Casas JP, Cavalleri GL, Bautista LE, et al. Endothelial nitric oxide synthase gene polymorphisms and cardiovascular disease: a HuGE review. Am J Epidemiol 2006;164:921–35. [DOI] [PubMed] [Google Scholar]
- [22].Luizon MR, Izidoro-Toledo TC, Simoes AL, et al. Endothelial nitric oxide synthase polymorphisms and haplotypes in Amerindians. DNA Cell Biol 2009;28:329–34. [DOI] [PubMed] [Google Scholar]
- [23].Pradhan AA, Bertels Z, Akerman S. Targeted nitric oxide synthase inhibitors for migraine. Neurotherapeutics 2018;15:391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Neeb L, Reuter U. Nitric oxide in migraine. CNS Neurol Disord Drug Targets 2007;6:258–64. [DOI] [PubMed] [Google Scholar]
- [25].Bednarczyk EM, Wack DS, Kassab MY, et al. Brain blood flow in the nitroglycerin (GTN) model of migraine: measurement using positron emission tomography and transcranial Doppler. Cephalalgia 2002;22:749–57. [DOI] [PubMed] [Google Scholar]
- [26].Kruuse C, Thomsen LL, Jacobsen TB, et al. The phosphodiesterase 5 inhibitor sildenafil has no effect on cerebral blood flow or blood velocity, but nevertheless induces headache in healthy subjects. J Cereb Blood Flow Metab 2002;22:1124–31. [DOI] [PubMed] [Google Scholar]
- [27].Lassen LH, Ashina M, Christiansen I, et al. Nitric oxide synthase inhibition in migraine. Lancet 1997;349:401–2. [DOI] [PubMed] [Google Scholar]
- [28].Lassen LH, Haderslev PA, Jacobsen VB, et al. CGRP may play a causative role in migraine. Cephalalgia 2002;22:54–61. [DOI] [PubMed] [Google Scholar]
- [29].Juhasz G, Zsombok T, Modos EA, et al. NO-induced migraine attack: strong increase in plasma calcitonin gene-related peptide (CGRP) concentration and negative correlation with platelet serotonin release. Pain 2003;106:461–70. [DOI] [PubMed] [Google Scholar]
- [30].Eberhardt M, Hoffmann T, Sauer SK, et al. Calcitonin gene-related peptide release from intact isolated dorsal root and trigeminal ganglia. Neuropeptides 2008;42:311–7. [DOI] [PubMed] [Google Scholar]
- [31].Kaiser EA, Russo AF. CGRP and migraine: could PACAP play a role too? Neuropeptides 2013;47:451–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Stovner LJ, Andree C. Prevalence of headache in Europe: a review for the Eurolight project. J Headache Pain 2010;11:289–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Antonaci F, Ghiotto N, Wu S, et al. Recent advances in migraine therapy. Springerplus 2016;5:637. [DOI] [PMC free article] [PubMed] [Google Scholar]


