Abstract
The aim of this study was to examine the expression of serological markers in patients with inflammatory bowel disease in China, and determine the diagnostic utility of serological markers, individually and in combination, for the diagnosis and differential diagnosis of Crohn's disease (CD).
Serum samples were obtained from 160 participants in Eastern China. Among the participants, 98 were diagnosed with CD, 33 had ulcerative colitis (UC), and 29 were healthy controls (HC). The serum samples were tested for the presence of antibodies against outer membrane porin C (anti-OmpC), Pseudomonas fluorescens bacterial sequence I2 (anti-I2), anti-laminarin (anti-L), anti-chitin (anti-C), anti-chitobioside carbohydrate antibody (ACCA), anti-laminaribioside carbohydrate antibody (ALCA), anti-mannobioside carbohydrate antibody (AMCA), and anti-Saccharomyces cerevisiae antibody (ASCA) by indirect enzyme-linked immunosorbent assay (ELISA).
Individually, anti-C, anti-L, ASCA-IgG, and ALCA lacked diagnostic value in the differentiation of CD. ASCA-IgA remained the most accurate marker for the diagnosis of CD, with an area under the curve (AUC) of 0.77; however, its sensitivity and specificity were both lower than 75%. Among the combinations of the 5 markers with significant diagnosing ability for CD, combinations with any 2 of the 3 markers, ASCA IgA, AMCA, and ACCA positive, provided the best accuracy in the diagnosis and differential diagnosis of CD (sensitivity and specificity both above 75%) and had the highest Youden index.
Serological antibodies, when considered in combination, have remarkable value in the diagnosis and differential diagnosis of CD. Especially, the combination of any 2 of the 3 markers, ASCA-IgA, AMCA, ACCA positive, appears to be optimal.
Keywords: Crohn's disease, diagnosis, differential diagnosis, serological antibodies
1. Introduction
Although the mechanisms underlying the development of Chrohn's disease (CD) are incompletely understood, an increasing amount of data has demonstrated that the etiology of CD stems from an inappropriate response of the mucosal immune system to the gut microbiota in genetically susceptible individuals.[1,2] Anti-Saccharomyces cerevisiae antibody (ASCA) is the most well-known serologic marker in commercial use, with a sensitivity of approximately 60%.[3] However, values as low as 39% and 44% have also been reported.[4–6] Therefore, the identification of additional seromarkers to improve the diagnosis and differentiation of CD would be highly beneficial.
Currently, a range of auto-antibodies, such as anti-mannobioside carbohydrate antibody (AMCA), anti-chitobioside carbohydrate antibody (ACCA), anti-laminaribioside carbohydrate antibody (ALCA), anti-laminarin (anti-L), anti-chitin (anti-C), and antibodies to microbiota-derived antigens, have been pinpointed as potentially beneficial in the diagnosis of CD. The scientific literature and physicians’ experiences all suggest that serological panels examining multiple antibodies are useful in the differential diagnosis of CD versus ulcerative colitis (UC) and other intestinal diseases with which CD is often confused.[7–9] However, a conclusive plan of action cannot be yet devised from the current findings, which suffer from small sample sizes, particularly in the studies that have been conducted in China. Furthermore, previous studies have examined only a limited range of serological markers, which demonstrate a lower positive response rate in clinical practice (e.g., ASCA) as opposed to that in trials. In light of these prevalent issues, the multicenter study presented herein examined the utility of several blood-based markers in the proper diagnosis of CD.
2. Patients and methods
2.1. Case and control identification
This study was approved by the Ethics Committee of The First Affiliated Hospital of Zhejiang Chinese Medical University, and informed consent was obtained from all participants. The participants were recruited between 2012 and 2015 from 5 centers in Eastern China, The First Affiliated Hospital of Zhejiang University of Traditional Chinese Medicine, Sir Run Run Shaw Hospital, The First Hospital of Zhejiang Province, The Second Affiliated Hospital of Zhejiang University School of Medicine, and Suzhou Municipal Hospital. The final cohort comprised 160 individuals. At recruitment, cases were diagnosed as CD or UC based on findings from endoscopy, histopathology, surgery, and/or radiologic reports by local physicians. In addition, healthy individuals were randomly selected from the same 5 centers in approximately the same timeframe. The healthy individuals and the patients with UC comprised the control population. Clinical data of the participants are shown in Table 1.
Table 1.
Clinical data of subjects.
2.2. Antibody test
Serum samples, obtained from consecutive participants, were stored in liquid nitrogen containers and shipped on dry ice to Herui Pharmaceuticals (Suzhou, Jiangsu, China), where they were analyzed for ASCA-IgA, ASCA-IgG, AMCA, ALCA, ACCA, anti-L, anti-C, anti-OmpC, and anti-I2 by indirect enzyme-linked immunosorbent assay (ELISA). Specifically, the antigen was diluted in a binding solution, with a final concentration ranging from 1 to 100 μg/mL, and 100 μL of the diluted antigen was placed into individual wells. The plate was sealed and incubated for 2 hours at room temperature. The antigen was then aspirated off and washed 4 times with 200 μL of washing solution. Subsequently, 200 μL of blocking solution was added into each well and the samples were incubated at room temperature for 30 minutes to overnight. After aspirating off the blocking solution, the primary antibody was diluted in a dilution buffer, with a final concentration in accordance with the manufacturer's instructions, and 100 μL of the diluted horseradish peroxidase (HRP)-conjugated antibody was added into each well. The plate was then sealed and incubated at room temperature for 1 hour. The antibody was then aspirated off and washed 4 times with 200 μL of washing solution. HRP-conjugated secondary antibody was diluted in a dilution buffer, with a final concentration in accordance with the manufacturer's instructions and 100 μL of the diluted HRP-conjugated antibody was added into each well. The plate was sealed and incubated at room temperature for 1 hour. The antibody was again aspirated off and washed 4 times with 200 μL of washing solution. An electrochemiluminescence (ECL) substrate was prepared in accordance with the manufacturer's instructions and 50–100 μL of the substrate was added into each well. Finally, the ELISA plate was placed on a titer plate shaker and incubated for 2 minutes at room temperature. The light signal (relative light units) was read using a PerkinElmer VICTOR X3 Multilabel Plate Reader, Perkin Elmer Inc, Waltham, MA.
The laboratory technicians were not informed of the case/control status of the samples.
2.3. Statistical analysis
The independent sample t-test was used to evaluate differences between the CD and control groups in the serum levels of each marker. To evaluate the accuracy of each serological marker, receiver operating characteristic (ROC) curves were constructed by plotting the sensitivity versus the 1-specificity according to the titers of the individual markers. Furthermore, to determine whether a combination of markers had improved predictive accuracy, new ROC curves were constructed for the combined markers. All statistical analyses were performed using SPSS 17.0 (SPSS Inc., Chicago). A P-value < .05 was considered statistically significant.
3. Results
3.1. Presence of antibodies
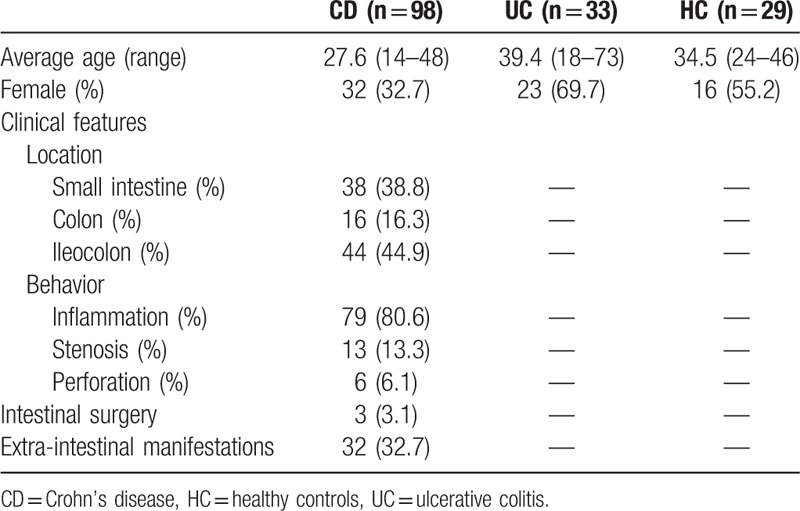
The serum levels of all tested antibodies were significantly higher in the CD group compared to those in the control group (comprised of patients with UC and healthy individuals) (P < .001), with the exception of anti-C and anti-L (P = .95, .32). Data are shown in Table 2 and Figure 1.
Table 2.
The differences of the individual marker between the 2 groups.
Figure 1.
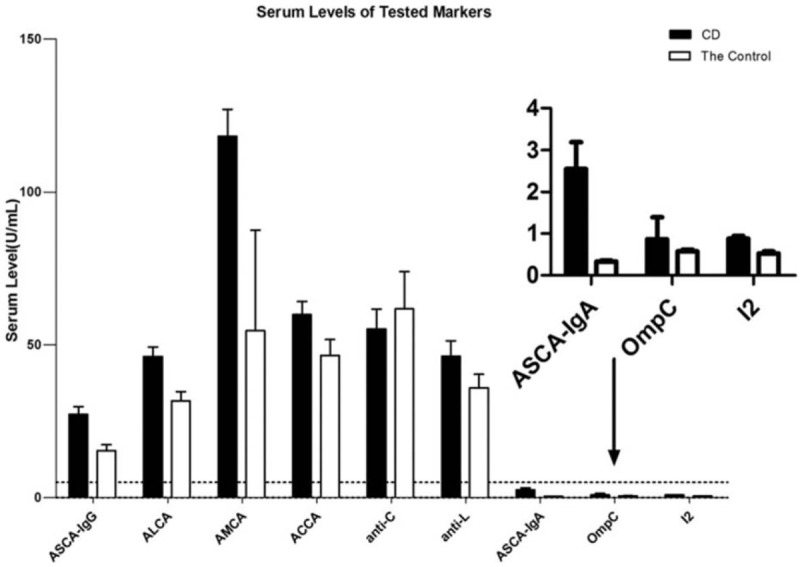
Serum levels of anti-Saccharomyces cerevisiae antibody (ASCA) IgA and IgG, anti-laminaribioside carbohydrate antibody (ALCA), anti-mannobioside carbohydrate antibody (AMCA), anti-chitobioside carbohydrate antibody (ACCA), anti-chitin (anti-C), anti-laminarin (anti-L), antibody against outer membrane porin C (anti-OmpC), antibody against the Pseudomonas fluorescens bacterial sequence I2 (anti-I2) in patients with Crohn's disease and the control. The box plots indicated the average of the serum levels and the whiskers above the box indicated the variance. ACCA = anti-chitobioside carbohydrate antibody, anti-C = anti-chitin, anti-L = anti-laminarin, ALCA = anti-laminaribioside carbohydrate antibody, AMCA = anti-mannobioside carbohydrate antibody, ASCA = anti-Saccharomyces cerevisiae antibody.
3.2. Diagnosis and differentiation abilities of individual markers
According to the ROC curves (Fig. 2), all of the serum markers reached statistical significance in the screening and diagnosis of CD (P < .05). However, ASCA-IgG and ALCA were not accurate predictors of CD due to their low areas under the curve (AUCs; <0.70). Furthermore, for all individual markers, the cutoff values were lower than the standard values offered by pure reagents, with the exception of anti-I2 (Table 3).
Figure 2.
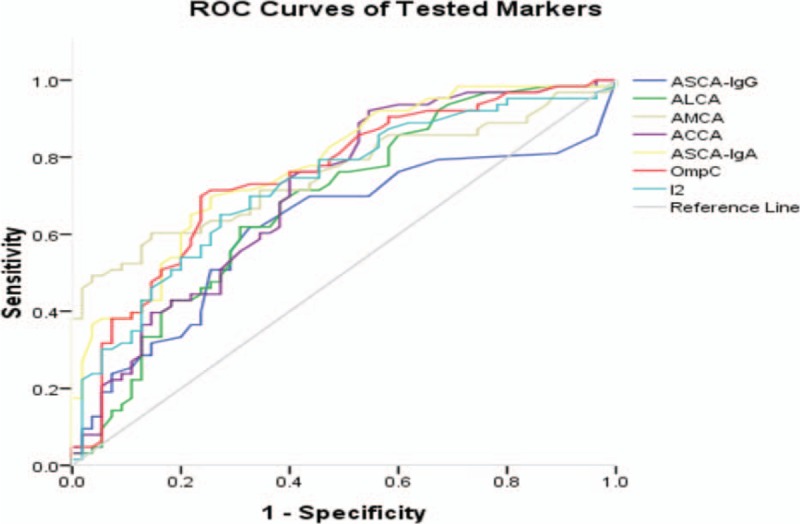
Receiver operating characteristic (ROC) curves of anti-Saccharomyces cerevisiae antibody (ASCA) IgA and IgG, anti-laminaribioside^^ carbohydrate antibody (ALCA), anti-mannobioside carbohydrate antibody (AMCA), anti-chitobioside carbohydrate antibody (ACCA), antibody against outer membrane porin C (anti-OmpC), antibody against the Pseudomonas fluorescens bacterial sequence I2 (anti-I2) for discriminating Crohn's disease and the control. Under the reference line, the area under the curve (AUC) =0.5. AUC < .6, low diagnostic accuracy. ACCA = anti-chitobioside carbohydrate antibody, anti-C = anti-chitin, ALCA = anti-laminaribioside carbohydrate antibody, AMCA = anti-mannobioside carbohydrate antibody, ASCA = anti-Saccharomyces cerevisiae antibody, ROC = receiver operating characteristic.
Table 3.
Validity of each marker for diagnosis of Crohn's disease.
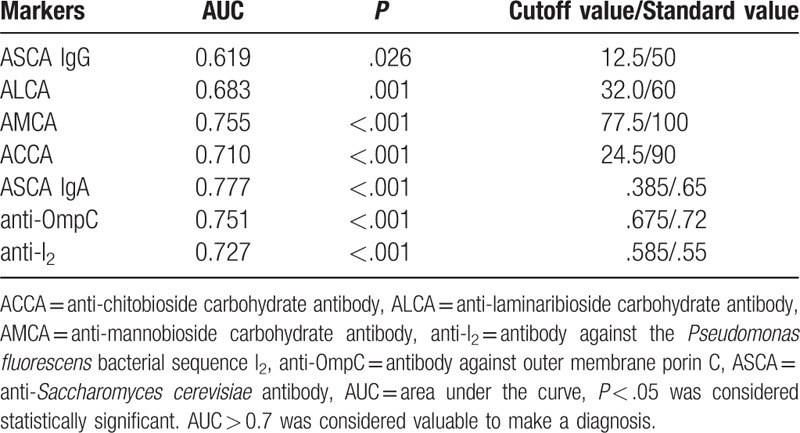
The higher the specificity and positive predictive value (PPV), the lower the sensitivity and negative predictive value (NPV). Individually, ASCA-IgG, ASCA-IgA, ALCA, AMCA, ACCA, anti-OmpC, and anti-I2 lacked sufficient sensitivity for the screening of CD when based on the standard value. Nevertheless, the use of the cutoff values according to ROC curves led to moderate improvements in sensitivity while maintaining a relatively high specificity (Table 4).
Table 4.
Sensitivity, specificity, PPV, and NPV of each individual marker.
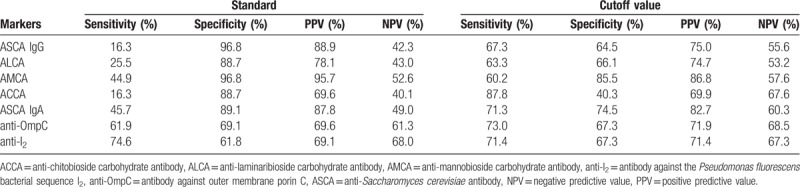
Using the regression coefficient of each individual marker to determine its relative contribution, the ASCA-IgA level was determined to have a higher predictive accuracy (AUC, 0.78) than those of the other individual markers (AUCs ranging from 0.62 to 0.76). ASCA-IgA had both the lowest rate of false negatives and a PPV above 70%, rendering it the most reliable individual marker for the diagnosis and differentiation of CD.
3.3. Diagnosis and differentiation abilities of different combinations of ASCA IgA, AMCA, ACCA, anti-OmpC, and anti-I2
Based on the above results, the diagnostic value of different combinations of ASCA IgA (as it was determined to be the more reliable individual marker) with 4 other markers that also showed diagnostic value (ASCA IgA, AMCA, ACCA, anti-OmpC, and anti-I2,) was evaluated (Tables 5–8, Figs. 3–6). Combinations with any 2 of the 3 markers, ASCA IgA, AMCA, and ACCA positive, were observed to provide the best accuracy, with sensitivity and specificity both above 75%, and had the highest Youden index (sensitivity + specificity-1). The remaining other combinations had either low sensitivity or lack of specificity, or both.
Table 5.
Validity of 2 markers combination for diagnosis of CD.
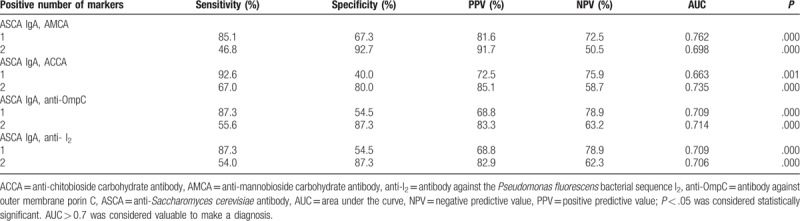
Table 8.
Validity of the 5 markers combination for diagnosis of CD.
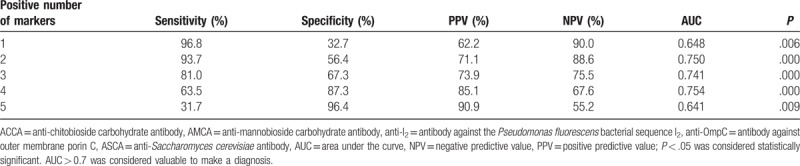
Figure 3.
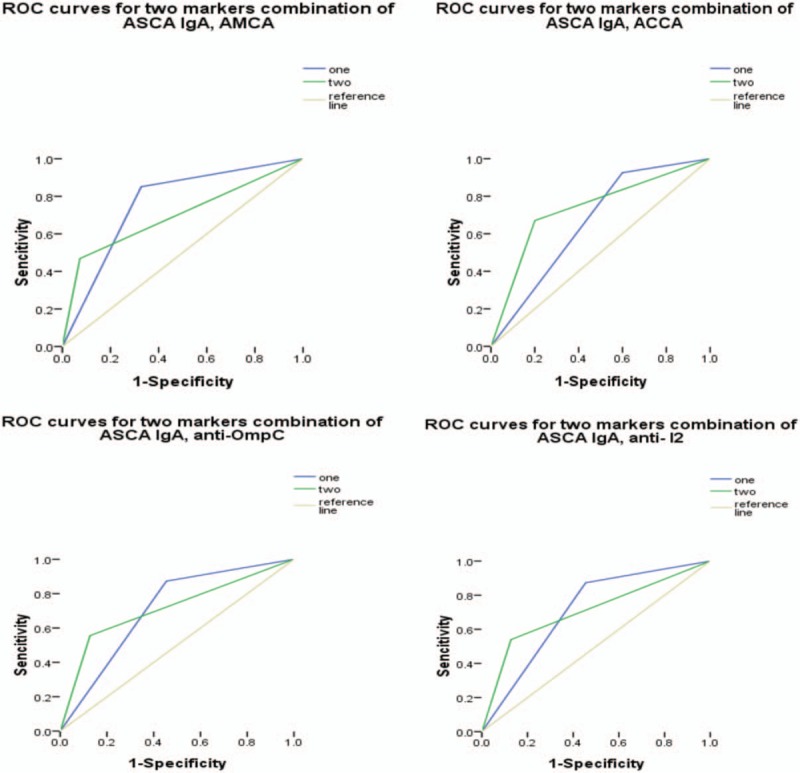
Receiver operating characteristic curves for the different combinations of anti-Saccharomyces cerevisiae antibody (ASCA) IgA with any one of the remaining 4 markers. Under the reference line, the area under the curve (AUC) =.5. AUC < .6, low diagnostic accuracy. ASCA = anti-Saccharomyces cerevisiae antibody, AUC = area under the curve.
Figure 6.
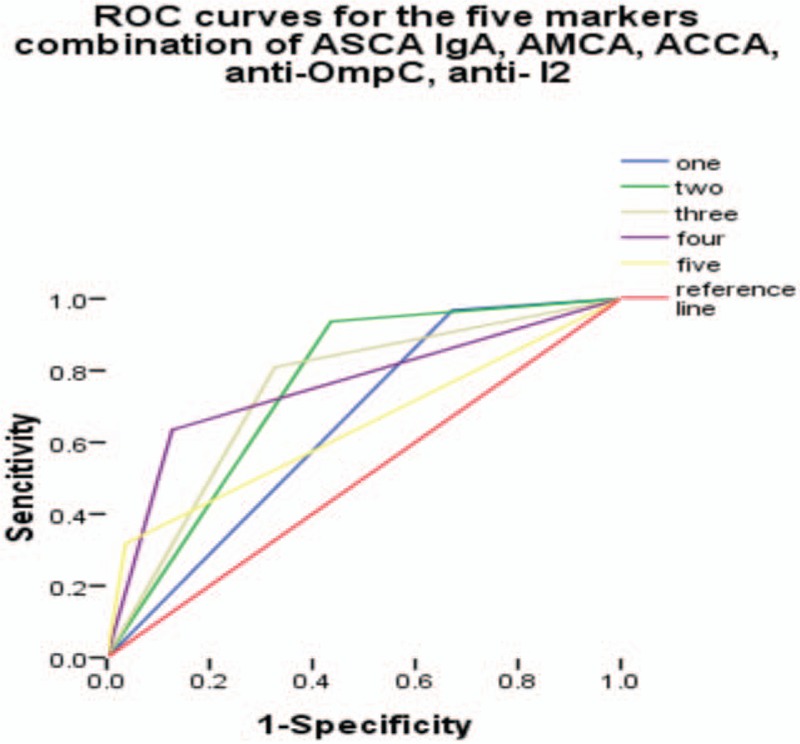
Receiver operating characteristic curves for the different combinations of anti-Saccharomyces cerevisiae antibody (ASCA) IgA with the remaining 4 markers. Under the reference line, the area under the curve (AUC) =0.5. AUC < 0.6, low diagnostic accuracy. ASCA = anti-Saccharomyces cerevisiae antibody, AUC = area under the curve.
Table 6.
Validity of 3 markers combination for diagnosis of CD.
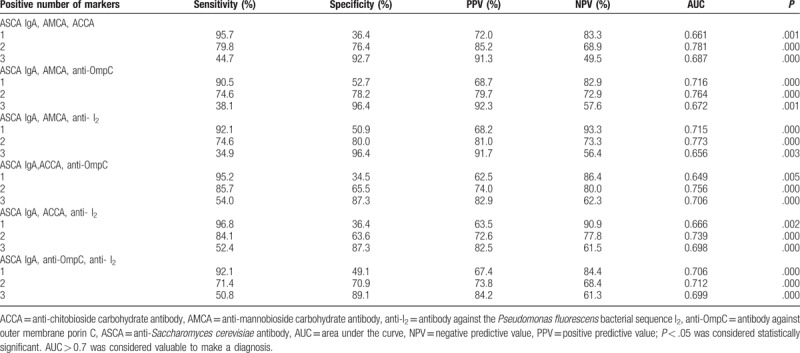
Table 7.
Validity of 4 markers combination for diagnosis of CD.
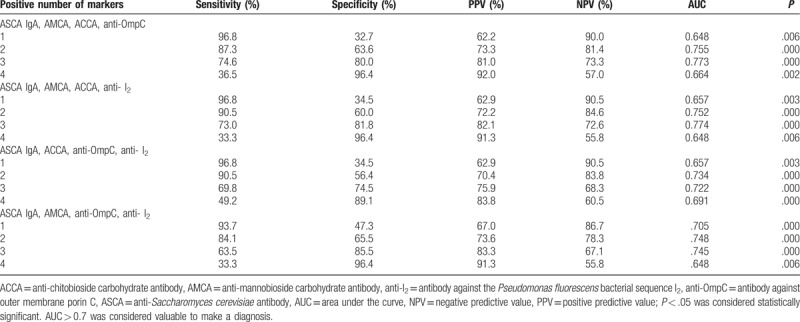
Figure 4.
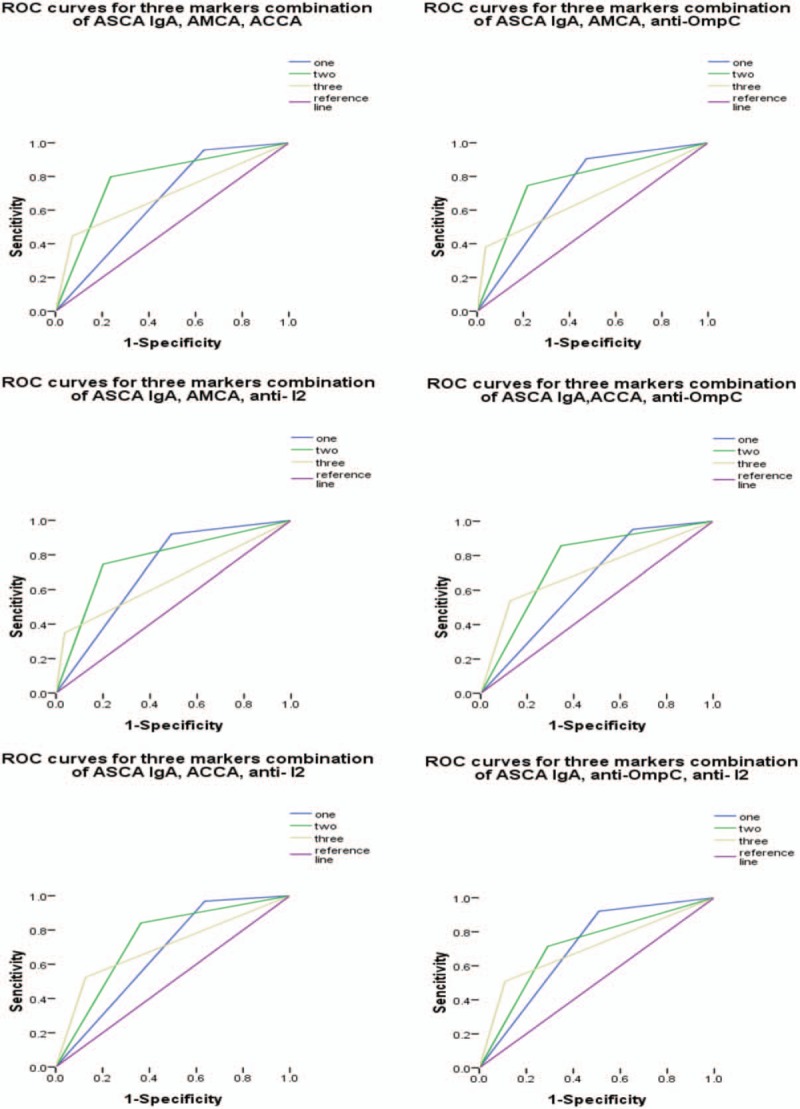
Receiver operating characteristic curves for the different combinations of anti-Saccharomyces cerevisiae antibody (ASCA) IgA with any 2 of the remaining 4 markers. Under the reference line, the area under the curve (AUC) =0.5. AUC < 0.6, low diagnostic accuracy. ASCA = anti-Saccharomyces cerevisiae antibody, AUC = area under the curve.
Figure 5.
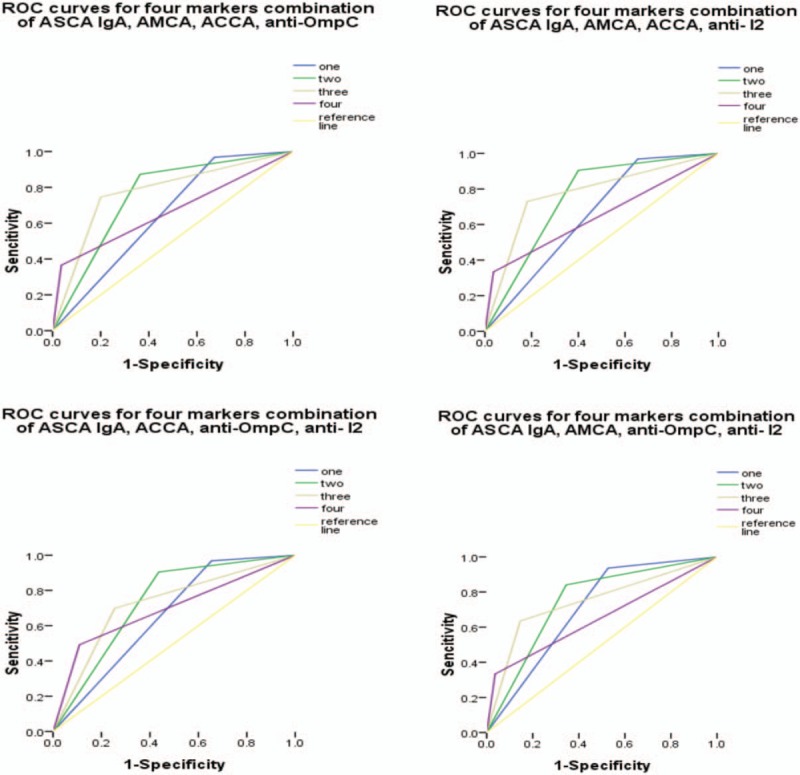
Receiver operating characteristic curves for the different combinations of anti-Saccharomyces cerevisiae antibody (ASCA) IgA with any 3 of the remaining 4 markers. Under the reference line, the area under the curve (AUC) =0.5. AUC < 0.6, low diagnostic accuracy. ASCA = anti-Saccharomyces cerevisiae antibody, AUC = area under the curve.
4. Discussion
Patients suffering from CD often cycle between relapse and remission periods, which takes both a physical and psychological toll on the patients’ quality of life. Therefore, CD has long been regarded as a prominent public health issue in the Western world. Recently however, a rising incidence of CD in developing countries has rendered CD a worldwide issue. In China, the prevalence of CD between the years of 2003 and 2007 was 8.5 times higher than that between 1989 and 1993.[10]
The expanding global prevalence of CD necessitates more robust and accurate tools for diagnosing patients with the disease. However, a definite diagnosis is difficult to establish using current techniques. A diagnosis of indeterminate colitis (IC) is applied to 10% to 17% of patients with colitis, as rigorous diagnostic methods are lacking.[11] Currently, the diagnosis of CD relies heavily on disease history reviews, clinical histories, comprehensive physical examinations, laboratory tests, imaging, endoscopy, and pathological examinations.[12–14] Endoscopy, along with the histopathology of biopsies, remains the gold standard for CD diagnosis. However, these procedures are invasive and rely heavily on the operators’ experience for an accurate diagnosis.
Thus, the search for noninvasive markers to minimize the use of endoscopic and radiologic examinations has become a focus area of CD research. CD is often characterized by the production of several serological antibodies with distinct antigenic specificities, including microbial antibodies and autoantibodies. Overviews of the current clinical data regarding the use of antibody markers in the diagnosis of CD reveal ASCA as the most reliable and specific marker. Already in commercial use, ASCA boasts high sensitivity ranges, from 50% to 80%, with a specificity of over 90%.[15,16] With regards to other markers, the diagnostic specificities of AMCA, ALCA, and ACCA for CD are all greater than 80%.[7] The overexpressions of anti-OmpC, anti-I2, anti-C, and anti-L have also been identified as key markers.[11,17–20] However, various international studies have revealed inconsistent findings, and only a limited number of studies have examined the response of antibodies to microbial antigens and autoantibodies in patients residing in non-Western countries. Limited studies have shown that serological markers increase significantly in Japanese patients with CD; however, the titers and positive rates are lower than those in Western patients with CD. The same has also been observed in China, indicating that the expression of biomarkers is globally heterogeneous and may be affected by ethnicity, dietary habits, or environmental conditions.[21] The present study also confirmed the discrepancies stated above, as for all markers, the cutoff values of were lower than those observed in other countries. This implies that we need to develop new reference values of the serum antibodies.
Furthermore, the evaluation of any one of the antibodies alone was insufficient in the diagnosis and differential diagnosis of CD, due to a lack of adequate sensitivity and specificity (lower than 75%). Thus, we examined the diagnostic value of different combinations of the antibodies. We found that combinations with any 2 of the 3 markers, ASCA IgA, AMCA, and ACCA positive, provided the most accuracy, with an AUC of 0.78 and a sensitivity and specificity of more than 75%. In contrast, the other tested combinations had either low fidelity (AUC < 0.70) or lacked adequate sensitivity and specificity (< 75%), or both.
5. Conclusion
The increasing prevalence of CD in the global community highlights the need for proper diagnostic methods. Serological antibodies, when considered individually, lack rigorous predictive accuracy and demonstrate low specificity and sensitivity; nevertheless, when considered in combination, significant increases in the ability to diagnosis and differential diagnosis CD were observed. Especially, the combination of any 2 of the 3 markers, ASCA IgA, AMCA, and ACCA, appears to be optimal, providing a new avenue for the diagnosis of CD in clinical practice.
Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing.
Author contributions
Formal analysis: Conghua Ji.
Project administration: Yihong Fan.
Supervision: Bin Lv.
Writing – original draft: Fang Yao.
Footnotes
Abbreviations: ACCA = anti-chitobioside carbohydrate antibody, ALCA = anti-laminaribioside carbohydrate antibody, AMCA = anti-mannobioside carbohydrate antibody, anti-C = antichitin, anti-I2 = antibody against the Pseudomonas fluorescens associated sequence I2, anti-L = anti-laminarin, anti-OmpC = antibody against outer membrane porin C of Escherichia coli, ASCA = anti-Saccharomyces cerevisiae antibody, AUC = area under the curve, CD = Crohn's disease, ECL = electrochemiluminescence, ELISA = enzyme-linked immunosorbent assay, HC = healthy controls, HRP = horseradish peroxidase, IC = indeterminate colitis, NPV = negative predictive value, PPV = positive predictive value, ROC = receiver operating characteristic, UC = ulcerative colitis.
Funding
1. Natural Science Foundation of China (81473506)
2. Funding project of Zhejiang Co-construction Foundation (WKJ-ZJ-1531)
3. Natural Science Foundation of Zhejiang Province (LY17H290009)
4. Funding Project of Medical science and Technology Planning Foundation of Zhejiang Province (2015RCA021)
5. Chinese Traditional Medicine Science Planning Programs of Zhejiang Province (2016ZB04, 2017ZA056)
Conflict-of-interest statement: We declare that we have no financial or personal relationships with other individuals or organizations that would inappropriately influence our work. There is no professional or other personal interest of any nature in any product or service.
References
- [1].De Souza HSP. Etiopathogenesis of inflammatory bowel diseases: today and tomorrow. Curr Opin Gastroenterol 2017;33:222–9. PMID: 28402995. [DOI] [PubMed] [Google Scholar]
- [2].Podolsky DK. Inflammatory bowel disease. N Engl J Med 2002;347:417–29. PMID: 12167685. [DOI] [PubMed] [Google Scholar]
- [3].Dotan I, Fishman S, Dgani Y, et al. Antibodies against laminaribioside and chitobioside are novel serologic markers in Crohn's disease. Gastroenterology 2006;131:366–78. PMID: 16890590. [DOI] [PubMed] [Google Scholar]
- [4].Reumaux D, Sendid B, Poulain D, et al. Serological markers in inflammatory bowel diseases. Best Pract Res Clin Gastroenterol 2003;17:19–35. PMID: 12617880. [DOI] [PubMed] [Google Scholar]
- [5].Rutgeerts P, Vermeire S. Clinical value of the detection of antibodies in the serum for diagnosis and treatment of inflammatory bowel disease. Gastroenterology 1998;115:1006–9. PMID: 9786723. [DOI] [PubMed] [Google Scholar]
- [6].Sandborn WJ, Loftus EV, Jr, Colombel JF, et al. Evaluation of serologic disease markers in a population-based cohort of patients with ulcerative colitis and Crohn's disease. Inflamm Bowel Dis 2001;7:192–201. PMID: 11515844. [DOI] [PubMed] [Google Scholar]
- [7].Kuna AT. Serological markers of inflammatory bowel disease. Biochem Med (Zagreb) 2013;23:28–42. PMID: 23457764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kaul A, Hutfless S, Liu L, et al. Serum anti-glycan antibody biomarkers for inflammatory bowel disease diagnosis and progression: a systematic review and meta-analysis. Inflamm Bowel Dis 2012;18:1872–84. PMID: 22294465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ferrante M, Henckaerts L, Joossens M, et al. New serological markers in inflammatory bowel disease are associated with complicated disease behavior. Gut 2007;56:1394–403. PMID: 17456509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wang YF, Ouyang Q, Hu RW. Progression of inflammatory bowel disease in China. J Dig Dis 2010;11:76–82. PMID: 20402832. [DOI] [PubMed] [Google Scholar]
- [11].Castillo S, Ramaiah B, Blum S, et al. The value of serologic markers in indeterminate colitis: a prospective follow-up study. Gastroenterology 2003;125:999PMID: 12974272. [DOI] [PubMed] [Google Scholar]
- [12].Bernstein CN, Fried M, Krabshuis JH, et al. World Gastroenterology Organization Practice Guidelines for the diagnosis and management of IBD in 2010. Inflamm Bowel Dis 2010;16:112–24. PMID: 19653289. [DOI] [PubMed] [Google Scholar]
- [13].Bernstein CN. Treatment of IBD: where we are and where we going. Am J Gastroenterol 2015;110:114–26. PMID: 25488896. [DOI] [PubMed] [Google Scholar]
- [14].Nielsen OH, Coskun M, Steenholdt C, et al. The role and advances of immunomodulator therapy for inflammatory bowel disease. Expert Rev Gastroenterol Hepatol 2015;9:177–89. PMID: 25101818. [DOI] [PubMed] [Google Scholar]
- [15].Cruyssen BV, Peeters H, Hoffman IE, et al. CARD15 polymorphisms are associated with anti-Saccharomyces cerevisiae antibodies in caucasian Crohn's disease patients. Clin Exp Immunol 2005;140:354–9. PMID: 15807862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kaila B, Orr K, Bernstein CN. The anti-Saccharomyces cerevisiae antibody assay in a province-wide practice: accurate in identifying cases of Crohn's disease and predicting inflammatory disease. Can J Gastroenterol 2005;19:717–21. PMID: 16341311. [DOI] [PubMed] [Google Scholar]
- [17].Landers CJ, Cohavy O, Misra R, et al. Selected loss of tolerance evidenced by Crohn's disease-associated immune response to auto-and microbial antigens. Gastroenterology 2002;123:689–99. PMID: 12198693. [DOI] [PubMed] [Google Scholar]
- [18].Sutton CL, Yang H, Li Z, et al. Familial expression of anti-Saccharomyces cerevisiae mannan antibodies in affected and unaffected relatives of patients with Crohn's disease. Gut 2000;46:58–63. PMID: 10601056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Zholudev A, Zurakowski D, Young W, et al. Serologic testing with ANCA, ASCA, and anti-OmpC in children and young adults with Crohn's disease and ulcerative colitis: diagnostic value and correlation with disease phenotype. Am J Grastroenterol 2004;99:2235–41. PMID: 15555007. [DOI] [PubMed] [Google Scholar]
- [20].Oshitani N, Hato F, Kitagawa S, et al. Distinct elevation of levels of anti-Cacnorhabditis elegans antibody in sera of patients with inflammatory bowel disease. Clin Diagn Lab Immnol 2003;10:856–61. PMID: 12965916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hisabe T, Matsui T, Sakurai T, et al. Anti-Saccharomyces cerevisiae antibodies in Japanese patients with inflammatory bowel disease: diagnostic accuracy and clinical value. J Gastroenterol 2003;38:121–6. PMID: 12640524. [DOI] [PubMed] [Google Scholar]


