Abstract
Rationale:
We present the first case of enterovirus (EV) D68, lineage B3 infection, associated with acute flaccid myelitis (AFM) in Taiwan. AFM caused by EV D68 is relatively rare. This report highlights the importance of clinical recognition of the disease and discusses treatments that can benefit such patients.
Patient concerns:
A 5-year-old boy experienced sudden onset of acute flaccid paralysis (AFP) involving left arm after fever and respiratory symptoms for 3 days.
Diagnoses:
Magnetic resonance imaging (MRI) of the spinal cord revealed signal changes over segments C1 to T5 on a T2-weighted image (T2WI), compatible with the diagnosis of AFM. The EV D68 strain, cultured from the throat of the patient was identified.
Interventions:
We administered intravenous immunoglobulin (IVIG, 1g/kg, twice), pulse steroid therapy (methylprednisolone, 30 mg/kg, twice) and oral prednisolone (1mg/kg/day). Rehabilitation was also arranged.
Outcomes:
The patient still had mild muscle atrophy over left arm after following-up for 1 year.
Lessons:
Early diagnosis and prompt management are essential for managing this kind of patient. IVIG, pulse therapy, and oral prednisolone may play crucial roles in controlling its clinical course.
Keywords: acute flaccid myelitis, acute flaccid paralysis, enterovirus D68, Taiwan
1. Introduction
Enterovirus (EV) D68 was first isolated in 1962 in Berkeley, California, the United States.[1] Four children had severe respiratory tract infection and pneumonia associated with EV D68. In 2014, there was an outbreak of EV D68 infection occurred in the United States.[2] Another outbreak of EV D68, B3 lineage, occurred in Sweden in 2016.[3] Most of them suffered from respiratory tract symptoms, whereas some patients had severe respiratory infection that required endotracheal intubation and intensive unit care. Among them, a few polio-like acute flaccid myelitis (AFM) cases presenting as acute flaccid paralysis (AFP) have also been reported in the literature.[1–3] Mortality due to AFM caused by EV D68 is very low[3]; however, the prognosis was guarded. Many patients continued to experience sequelae of flaccid paralysis, or even bowel or bladder dysfunction.
In Taiwan, the Centers of Disease Control had 32 specimens that tested positive for EV D68 during the period 2014 to 2015. All patients had respiratory symptoms without paralysis. The patient group consisted of 19 male and 13 female patients. The age ranged from 0 to 49 years. More than half (59.4%) patients were aged younger than 5 years. Herein, we present the first case of AFM caused by EV D68 infection in Taiwan in 2016.
2. Case report
A 5-year-old boy, who was previously healthy, experienced fever (up to 38.8°C) on August 13, 2016. He also experienced headache, dizziness, vomiting, productive cough, nasal obstruction, and rhinorrhea. Azithromycin was prescribed at another hospital. On August 15, 2016, left arm weakness was noted in the patient. Transient right lower leg pain was noted on the next day. On August 17, 2016, he experienced right leg weakness (grade 4). Urination and defecation were normal. Therefore, he was admitted.
After admission, a physical examination revealed that the muscle power of the left arm was grade 1 and that of the left forearm was grade 3. The muscle power of the right arm and left leg was normal. The muscle power of the right thigh was grade 4. The left scapula showed a winging sign. The deep tendon reflexes (DTRs) of the left biceps, brachioradialis, and triceps were 1+. The left deltoid DTR was 2+. The cremasteric reflex and anal reflex were normal. Sensory dermatomes were also normal. He had neck pain with nuchal rigidity. Brudzinski sign and Kernig sign were positive. He had no ankle clonus or spasticity. The Babinski sign was negative. Other physical examinations indicated lymphadenopathy over the neck. There was no infected throat, vesicles, or ulcers in the oral cavity. The breathing sound was slightly coarse. No rales, rhonchi, or wheezing was heard. The abdomen was soft, tympanic, and without tenderness. The abdominal bowel sound indicated hypoactivity.
Blood laboratory data revealed a normal leukocyte count and normal C-reactive protein level. A chest x-ray showed increased infiltration of both lungs. A neck x-ray showed no bone or mass lesion. Cerebral spinal fluid (CSF) data showed pleocytosis (270/μL) with a predominance of polymorphonuclear (PMN) leukocytes (PMNL: 69%; lymphocytes: 31%). The CSF glucose level was normal (48 mg/dL), and the CSF protein level was slightly elevated (83 mg/dL). The observed opening pressure was 20 cm H2O. The serum IgG level was 2300 mg/dL, and the CSF IgG level was 33.9 mg/dL. The IgG index was 0.682, which was within normal range. We also obtained virus cultures from the blood, CSF, throat, and stools of the patient. Vancomycin and cefotaxime were added to the treatment regimen. A brain and neck computed tomography (CT) on August 17, 2016 revealed no mass lesions. The patient complained of neck and back pain on the next day. Brudzinski sign and Kernig sign remained positive. Increased intracranial pressure was suspected and glycerin was administered. The pain was relieved gradually after glycerin infusion. Brudzinski sign and Kernig sign were negative after treatment. T2-weighted magnetic resonance imaging (MRI) of the brain and neck on August 19, 2016 revealed a swelling change over C1–T5 of the spinal cord (Figs. 1 and 2). The patient was diagnosed with meningomyelitis. Moreover, the muscle power of the left leg decreased. Intravenous immunoglobulin (IVIG) 1 g/kg was started on the same day. We also changed vancomycin to ciprofloxacin due to suspected mycoplasmal infection.
Figure 1.
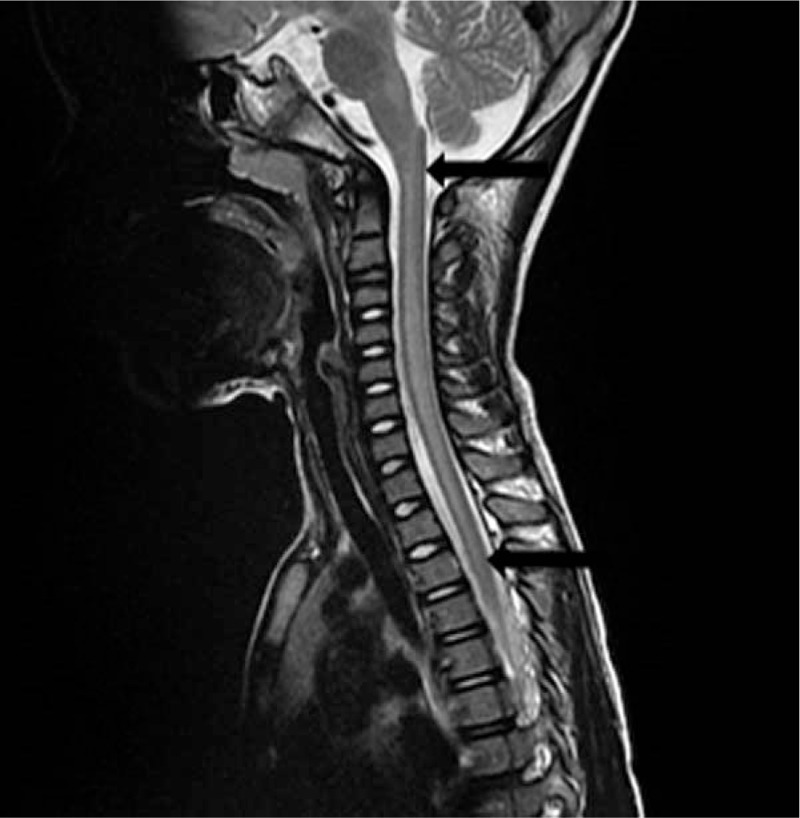
Sagittal T2WI MRI of the spinal cord showing enhanced signal over C1–T5 (arrow). MRI = magnetic resonance imaging, T2WI = T2-weighted image.
Figure 2.
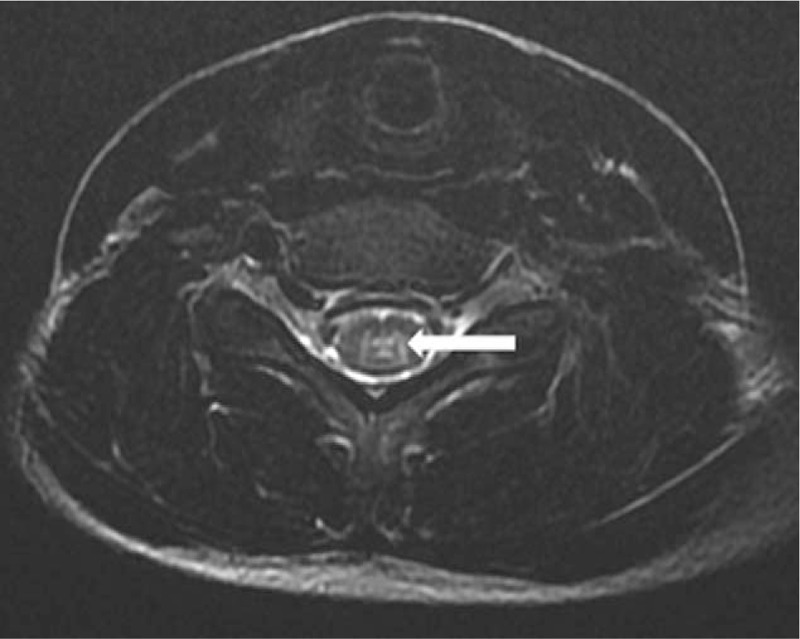
Transverse T2WI MRI of the sixth segment of the cervical spinal cord showing the involvement of gray matter (arrow). MRI = magnetic resonance imaging, T2WI = T2-weighted image.
After IVIG 1 g/kg administration, the muscle power of his hand improved to normal. Pulse steroid therapy was delivered from August 20, 2016, for 3 days. Rehabilitation was started then. The muscle power of the distal upper extremity improved to grade 4. Then, IVIG 1 g/kg was administered again on August 23, 2016. Bacterial cultures from the blood, CSF, and throat of the patient showed no growth.
No fever was noted after admission. The muscle power improved after medical treatment and rehabilitation. On discharge (August 25, 2016), the muscle power levels of the left hand, left forearm, and left arm were grades 5, 4, and 2, respectively. The winging of the left scapula improved. The DTR over the left upper extremity decreased slightly. The muscle power in both lower extremities was grade 5. The patient could ascend and descend stairs unassisted with alternate feet. Finally, the Centers for Disease Control in Taiwan reported that the throat viral culture was identified as EV D68 (B3 lineage). After discharge, the patient again experienced myalgia in both legs and weakness of left leg 2 days later. Subsequently, pulse steroid therapy was used for 3 days. Then, oral prednisolone (1 mg/kg/d) was prescribed and tapered weekly for approximately 4 weeks. The muscle power of the left proximal upper extremity improved to grade 4. He had sequel of mild left arm weakness (grade 4+) after following up for 1 year (Fig. 3).
Figure 3.
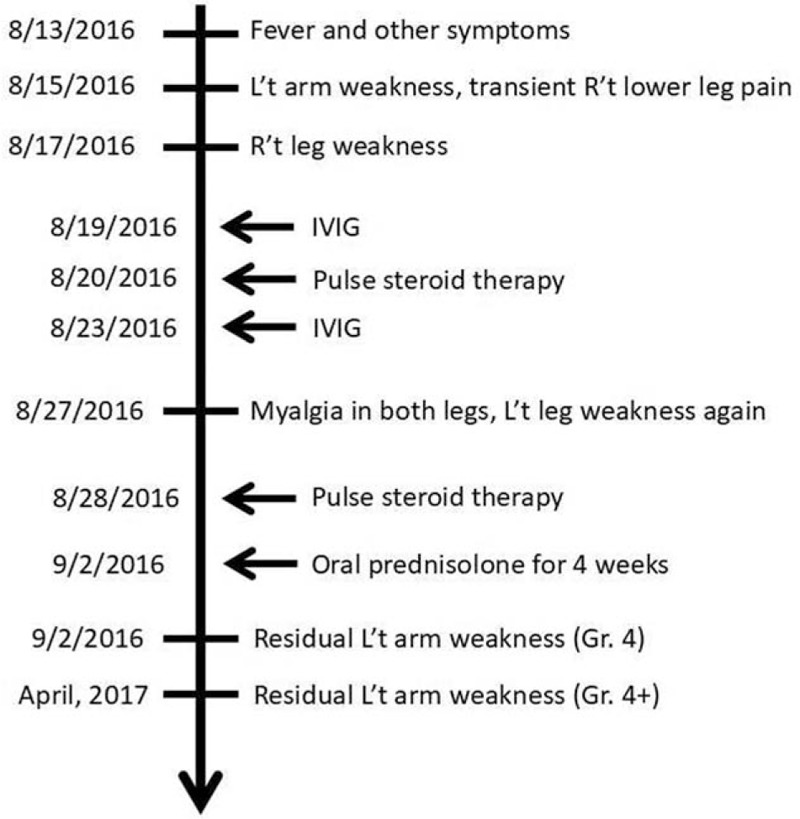
Timeline of clinical course of the case.
This study was approved by ethic committee of Cathay General Hospital and with the informed consent from the parent for publication.
3. Discussion
Enteroviruses are small single-stranded RNA viruses belonging to the Picornaviridae family. EV D68 is a unique genotype that differs slightly from most EVs because it shares several physiochemical properties with human rhinoviruses.[1] EV D68 differs from other EVs in its temperature sensitivity. It grows in cell cultures at 33°C instead of 37°C. Therefore, it has mainly been isolated from respiratory samples and has very rarely been reported in stools.
EV D68 infection in children causes a wide range of respiratory disorders such as pharyngitis, bronchitis, severe pneumonia, or even respiratory failure.[1,4–7] Healthy adults usually experience a milder range of respiratory symptoms. Cough and fever are common symptoms of EV D68 infection. The risk factors for severe respiratory disease, both in children and adults, are an immunocompromised state, chronic obstructive pulmonary disease, and asthma.[1,4,5,7] The neurological features of EV D68 infection, including AFP due to AFM, present in both children and adults. Our patient had nuchal rigidity, indicating the presence of meningitis. The swelling change and signal attenuation of spinal cord MRI showed the existence of myelitis.
No specific treatment is available for AFM associated with EV D68 infection. Most patients have been treated with IVIG 2 g/kg administered once, pulse steroid therapy, or a combination of both.[3,8] Esposito et al[9] reported a case of a healthy 4-year-old boy from Italy who experienced fever and respiratory prodromes before the onset of AFM. In that case, AFM involved the dorsal portion of the medulla oblongata, pontine tegmentum, and cervical dorsal spinal cord. The nasopharyngeal aspirate and bronchoalveolar lavage obtained from the patient tested positive for EV-D68 B3 lineage. Plasmapheresis was performed and IVIG (1 g/kg/d) was administered during the first 3 days starting on the same day that weakness was reported. Intravenous steroid therapy (30 mg/kg) was discontinued after 5 days. Furthermore, oral prednisolone (2 mg/kg/d) was administered for 4 weeks, and then tapered over an additional period of 2 weeks. After 4 weeks of treatment, the signs and symptoms of AFM were significantly reduced, although some weakness and tingling remained in the patient's limbs. In our case, we used IVIG 1 g/kg/dose twice and pulse steroid therapy (30 mg/kg) between the 2 IVIG administrations from the fifth day after reporting weakness. As the weakness progressed, another dose of intravenous pulse steroid (30 mg/kg) was administered. Plasmapheresis was not attempted in our case, in contrast to the case of Esposito et al. Both IVIG and pulse steroid therapy were used in both cases.[9] In our case, the patient exhibited progression of weakness despite a high level of IgG. Therefore, a second pulse steroid dose was prescribed during the second admission. From both cases, IVIG and pulse steroid therapy seem to be effective when used during the early stages of AFM. In the interim consideration report of CDC[10] in 2014, there are no indication for use of corticosteroid, no clear indication for efficacy of IVIG, and the use of plasmapheresis is discouraged, in the treatment of AFM. These suggestions were based on the initial experience from the clinical events in 2014. They might be modified if more patients and data were collected. Further studies on evidence-based or big data collections are mandatory before a consensus is accomplished. The outcomes of Colorado children with AFM followed up for 1 year showed persisted weakness in 6 of 8 patients.[11]
AFM still had long-term functional effects.
4. Conclusions
The occurrence of AFP due to EV D68 infection, particularly in summer or autumn, warrants more attention than it currently receives. Early general clinical management of children with AFM should be the basic standards of care for children with severe neurological disease, and physical and occupational therapy should be attempted as soon as the child is stable in physical condition to optimize functional outcomes. Treatment with IVIG and/or pulse steroid therapy may be considered earlier to improve the corresponding prognosis.
Acknowledgments
We thank for the Centers for Disease Control in Taiwan for virus isolation and identification.
Author contributions
Methodology: Chiao-Wei Lo.
Resources: Su-Ching Hu.
Supervision: Kun-Long Hung.
Writing – original draft: I-Ju Chen.
Footnotes
Abbreviations: AFM = acute flaccid myelitis, AFP = acute flaccid paralysis, CSF = cerebral spinal fluid, DTRs = deep tendon reflexes, EV = enterovirus, IgG = immunoglobulin G, IVIG = intravenous immunoglobulin, MRI = magnetic resonance imaging, PMNL = polymorphonuclear leukocytes, T2WI = T2-weighted image.
The authors report no conflicts of interest.
References
- [1].Holm-Hansen CC, Midgley SE, Fischer TK, et al. Global emergence of enterovirus D68: a systematic review. Lancet Infect Dis 2016;16:e64–75. [DOI] [PubMed] [Google Scholar]
- [2].Orvedahl A, Padhye A, Barton K, et al. Clinical characterization of children presenting to the hospital with enterovirus D68 infection during the 2014 outbreak in St. Louis. Pediatr Infect Dis J 2016;35:481–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Dyrdak R, Grabbe M, Hammas B, et al. Outbreak of enterovirus D68 of the new B3 lineage in Stockholm, Sweden, August to September 2016. Euro Surveill 2016;21: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Greninger AL, Naccache SN, Messacar K, et al. A novel outbreak enterovirus D68 strain associated with acute flaccid myelitis cases in the USA (2012-14): a retrospective cohort study. Lancet Infect Dis 2015;15:671–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Midgley CM, Watson JT, Nix WA, et al. Severe respiratory illness associated with a nationwide outbreak of enterovirus D68 in the USA (2014): a descriptive epidemiological investigation. Lancet Respir Med 2015;3:879–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lam HY, Wong AT, Tsao YC, et al. Prevalence and phylogenetic characterization of human enterovirus D68 among children with respiratory infection in Hong Kong. Diagn Microbiol Infect Dis 2016;85:174–6. [DOI] [PubMed] [Google Scholar]
- [7].Schuster JE, Miller JO, Selvarangan R, et al. Severe enterovirus D68 respiratory illness in children requiring intensive care management. J Clin Virol 2015;70:77–82. [DOI] [PubMed] [Google Scholar]
- [8].Nelson GR, Bonkowsky JL, Doll E, et al. Recognition and management of acute flaccid myelitis in children. Pediatr Neurol 2016;55:17–21. [DOI] [PubMed] [Google Scholar]
- [9].Esposito S, Chidini G, Cinnante C, et al. Acute flaccid myelitis associated with enterovirus-D68 infection in an otherwise healthy child. Virol J 2017;14:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Centers for Disease Control and Prevention (CDC). Acute flaccid myelitis: interim considerations for clinical management; 2014. Available at: https://www.cdc.gov/acute-flaccid-myelitis/downloads/interim-considerations-afm.pdf. Accessed September 2014. [Google Scholar]
- [11].Martin JA, Messacar K, Yang ML, et al. Outcomes of Colorado children with acute flaccid myelitis at 1 year. Neurology 2017;89:129–37. [DOI] [PMC free article] [PubMed] [Google Scholar]


