Abstract
Rationale:
Spinocerebellar ataxia (SCA), a genetically inherited heterogeneous disorder, is characterized by gait ataxia, dysarthria, parkinsonism, choreic movements, dystonia, epilepsy, cognitive and psychiatric symptoms. Spinocerebellar ataxia-42 (SCA42), caused by heterozygous mutation in the calcium channel 1G (CACNA1G) gene, is a rare SCA subtype and the transmission pattern is autosomal dominant inheritance.
Patient concerns:
We presented a novel mutation (c.4721T>A; p.Met1574Lys) in 3 patients from a Chinese family using whole-exome sequencing. All patients exhibited cerebellar ataxia and the clinical manifestations were similar to those that were previously reported in the French and Japanese families. In addition, cerebral magnetic resonance imaging (MRI) showed cerebellar atrophy, and the hot cross bun sign of brainstem was found in the proband and her sister.
Diagnoses:
The clinical features and MRI findings indicated the diagnosis of SCA. Taken together, the symptoms, MRI findings, as well as whole-exome sequencing made the diagnosis of SCA42 most likely candidate.
Interventions and outcomes:
The patient was treated with cobamamide (1.5 mg once daily) for nerve nutrition and further physical therapy. At the 4-month follow-up visit, the patient's condition did not improve obviously.
Lessons:
Recently, a missense mutation in CACNA1G gene (c.5144G4A; p.Arg1715His) was identified in French and Japanese families with SCA42. However, there has been no report of SCA42 or its mutant loci in Chinese patients. Our finding showed a novel mutation in CACNA1G gene and provided important insights into the pathogenesis of SCA42.
Keywords: CACNA1G, cerebellar atrophy, hot cross bun sign, SCA42, spinocerebellar ataxia
1. Introduction
Spinocerebellar ataxia (SCA) represents a heterogeneous group of genetically inherited neurodegenerative disorders that affect the cerebellum, brainstem, and spinal cord. Its clinical manifestations are progressive cerebellar ataxia, nystagmus, dysarthria, dementia, intention tremor, abnormal muscle tone, and peripheral neuropathy.[1] Spinocerebellar ataxia-42 (SCA42), caused by the calcium channel 1G (CACNA1G) gene, is thought to be an autosomal dominant SCA subtype.[2]CACNA1G is located on chromosome 17q21 and encodes the amino acid in the voltage sensor S4 segment of the Type channel protein Cav3.1, which is highly expressed in the molecular layer of the cerebellum.[3] A heterozygous missense mutation in the CACNA1G gene (c.5144G4A; p.Arg1715His) was concurrently reported in French and Japanese families with SCA42.[4,5] To date, there has been no report of SCA42 or its mutant loci in Chinese patients. In this study, we presented a novel mutation (c.4721T>A; p.Met1574Lys) in 3 patients from a Chinese family using whole-exome sequencing.
2. Case report
A 45-year-old Han Chinese female with a white-collar job (IV-3) was referred to our hospital due to aprogressive gait abnormality over the past 2 years and dysarthria for 5 months. No apparent cause was observed at the time of symptom onset. Interestingly, it was noticed that the patient's father (III-4) and sister (IV-2) also showed symptoms of gait abnormality. There was no indication of consanguineous marriage between the patient's parents. The detailed physical examination of this patient revealed that she had dysarthria, hypomyotonia, slight hyporeflexia, and ataxia abnormality in both of her lower limbs. She was unable to walk in a straight line, but her muscle power and sensorium of the limbs were normal. Also, no deficits in superficial or deep sensation, gaze nystagmus, or Babinski and Chaddock signs were observed. Furthermore, cerebral magnetic resonance imaging (MRI) indicated cerebellar atrophy and the hot cross bun sign in the brainstem, as shown in Figure 1. The pedigree analysis of the complete family is shown in Figure 2.
Figure 1.

Magnetic resonance imaging (MRI) scans of the brain. Panels (A) and (C) show T2-weighted MRI of the patient (IV-3) brain, panel (B) shows T2-weighted MRI of the patient's sister (IV-2), while panel (D) represents the T2-weighted MRI of the patient's father (III-4).
Figure 2.

Pedigree analysis of the patient's family. Black circles (female) and squares (male) indicate family members affected with the disease, while open circles or squares indicate unaffected members. Asterisks indicate the patients used for exome sequencing. Arrows indicate the proband. MRI = magnetic resonance imaging.
The clinical presentations, examinations of relatives, and MRI findings made the diagnosis of SCA most likely candidate. Subsequently, we conducted exome sequencing analysis to confirm the diagnosis and clarify the subtypes of this disorder. The same heterozygous missense mutation (c.4721T>A; p.Met1574Lys) was observed in affected 3 family members, as shown in Figure 3. All 3 family members (III-4, IV-2, and IV-3) displayed progressive ataxia abnormality and mild-to moderate speaking difficulty. The detailed clinical manifestations were summarized in Table 1. Cerebral MRI showed cerebellar atrophy in the patient (IV-3), the patient's sister (IV-2) and their father (III-4), and the hot cross bun sign of brainstem was only observed in the patient (IV-3) and the patient's sister (IV-2).
Figure 3.

Sequencing analysis of the CACNA1G gene locus. Panel (A) shows the genetic profile of the patient (IV-3). Panel (B) represents the genetic profile of the patient's father (III-4), while panel (C) corresponds to the genetic profile of the patient's sister (IV-2). Panel (D) depicts the genetic profile of a family member not affected with the disease (IV-1).
Table 1.
Clinical characteristics of the patient and the affected family members.

Based on clinical symptoms, MRI findings, and whole-exome sequencing, the case was diagnosed with SCA42. The patient was treated with cobamamide (1.5 mg once daily) for nerve nutrition and further physical therapy. At the 4-month follow-up visit, the patient's condition did not improve obviously.
3. Discussion
SCA42, a rare autosomal dominantly inherited SCA subtype,[6] was caused by mutation in CACNA1G on chromosome 17q21, which encoded the T-type calcium channel Cav3.1. Cav3.1 is classified as a low-threshold voltage-dependent calcium channel protein and is widely expressed in the brain, especially the molecular layer of the cerebellum and inferior olive nucleus.[7] Cav3.1 plays a predominant role in the regulation of membrane potential and in the modulation of calcium signaling pathways. The mutation in the CACNA1G gene induced the amino acid change in the voltage sensor S4 segment of domain IV in Cav3.1 and thus affected the activation and inactivation of Cav3.1. Then, the mutant Cav3.1 resulted in decreased neuronal excitability in deep cerebellar nuclei neurons. Taken together, CACNA1G is regarded as a causative gene.
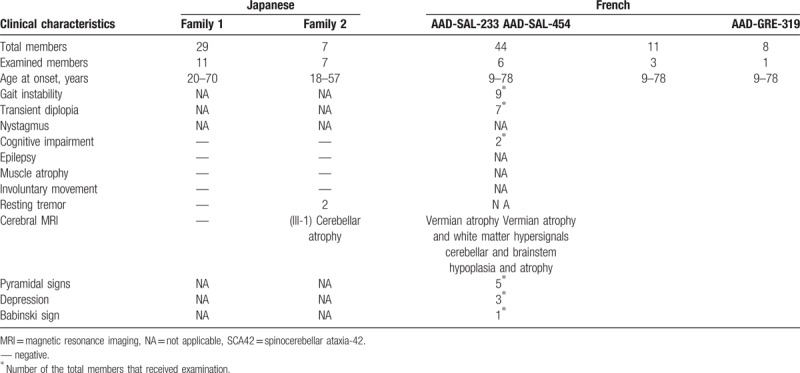
To our best knowledge, the mutation in CACNA1G gene was first identified from 3 unrelated French families in Coutelier et al's[5] study and the patients with SCA harbored the missense mutation (c.5144G>A; p.Arg1715His). Subsequently, Morino et al[4] presented the same mutation in CACNA1G gene from 2 unrelated Japanese families with slowly progressive SCA. We summarized the main characteristics of the French and Japanese families (Table 2). The age at symptom onset was highly variable and the most common manifestation was gait instability. It should be noted that the symptoms caused by the same mutation were somewhat different between the families. It may be explained by the fact that CACNA1G gene was independently identified in the different races. Further studies are warranted to clarify the clinical characteristics of the CACNA1G-dependent SCA42 in more detail.
Table 2.
Clinical characteristics of Japanese and French families with SCA42.
To date, c.5144G>A was the only reported missense mutation of CACNA1G using whole-exome sequencing. To identify other causative mutations in CACNA1G, we searched OMIM and HGMD databases, and the results indicated that SCA42 was possible to present in other mutant loci. We identified a novel mutation (c.4721T>A; p.Met1574Lys) in 3 patients with SCA from a Chinese family using whole-exome sequencing.
In the current study, the age at onset ranged from 43 to 60 years and all patients exhibited the pure form of cerebellar ataxia including gait abnormality and dysarthria. However, nystagmus was not observed. In neuroimaging, in addition to cerebellar atrophy, the hot cross bun sign of brainstem was observed in the proband and her sister, which was of particular interest to us. It was reported that the hot cross bun sign was observed in SCA1,[8] 2, 3, 7, 8,[9,10] 23,[11] and 34.[12] However, further epidemiological studies are needed to determine the prevalence of this sign in SCA42.
Currently, there are not effective therapies for SCA patients. Since the progression of the disorder is relatively slow, symptomatic treatment may contribute some to delaying further progression and improving the overall quality of life in patients. However, development of novel therapeutic approaches must be seriously considered.
In conclusion, our finding showed a novel mutation in CACNA1G gene and provided important insights into the pathogenesis of SCA42. However, further electrophysiologic studies are warranted to be conducted to support the mutant site.
Acknowledgments
The authors express their gratitude to the patient and her family.
Author contributions
Data curation: Chunkui Zhou, Lijun Zhu, Heqian Du.
Formal analysis: Li Cui.
Funding acquisition: Shaokuan Fang.
Investigation: Xinyuan Li, Chunkui Zhou, Lijun Zhu, Jing Liu.
Methodology: Xinyuan Li, Lijun Zhu, Chenglin Wang.
Project administration: Xinyuan Li, Li Cui, Heqian Du.
Resources: Xinyuan Li, Heqian Du, Jing Liu, Chenglin Wang.
Software: Xinyuan Li, Chunkui Zhou, Lijun Zhu, Heqian Du, Jing Liu.
Supervision: Li Cui, Shaokuan Fang.
Validation: Xinyuan Li, Chunkui Zhou, Li Cui, Chenglin Wang.
Visualization: Chunkui Zhou, Chenglin Wang.
Writing – original draft: Li Cui.
Writing – review & editing: Shaokuan Fang.
Footnotes
Abbreviations: CACNA1G = calcium channel 1G, MRI = magnetic resonance imaging, SCA = spinocerebellar ataxia, SCA42 = spinocerebellar ataxia-42.
XL, CZ, and LC contributed equally to this work.
Informed consent: XL and SF had received consent from the patient whose case is reported.
The authors have no conflicts of interest to disclose.
References
- [1].Durr A. Autosomal dominant cerebellar ataxias: polyglutamine expansions and beyond. Lancet Neurol 2010;9:885–94. [DOI] [PubMed] [Google Scholar]
- [2].Kimura M, Yabe I, Hama Y, et al. SCA42 mutation analysis in a case series of Japanese patients with spinocerebellar ataxia. J Hum Genet 2017;11. [DOI] [PubMed] [Google Scholar]
- [3].Calhoun JD, Hawkins NA, Zachwieja NJ, et al. Cacna1 g is a genetic modifier of epilepsy in a mouse model of Dravet syndrome. Epilepsia 2017;58:e111–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Morino H, Matsuda Y, Muguruma K, et al. A mutation in the low voltage-gated calcium channel CACNA1G alters the physiologicalproperties of the channel, causing spinocerebellar ataxia. Mol Brain 2015;8:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Coutelier M, Blesneac I, Monteil A, et al. A recurrent mutation in CACNA1G alters Cav3.1 T-Type calcium-channel conduction and causes autosomal-dominant cerebellar ataxia. Am J Hum Genet 2015;97:726–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Klockgether T. Update on degenerative ataxias. Curr Opin Neurol 2011;24:339–45. [DOI] [PubMed] [Google Scholar]
- [7].Aguado C, García-Madrona S, Gil-Minguez M, et al. Ontogenic changes and differential localization of T-type Ca(2+) channel subunits Cav3.1 and Cav3 and 2 in mouse hippocampus and cerebellum. Front Neuroanat 2016;10:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wang Y, Koh K, Takaki R, et al. Hot cross bun sign in a late-onset SCA1 patient. Neurol Sci 2016;37:1873–4. [DOI] [PubMed] [Google Scholar]
- [9].Marrannes J, Mulleners E. Hot cross bun sign in a patient with SCA-2. JBR-BTR 2009;92:263. [PubMed] [Google Scholar]
- [10].Lee YC, Liu CS, Wu HM, et al. The ’hot cross bun’ sign in the patients with spinocerebellar ataxia. Eur J Neurol 2009;16:513–6. [DOI] [PubMed] [Google Scholar]
- [11].Saigoh K, Mitsui J, Hirano M, et al. The first Japanese familial case of spinocerebellar ataxia 23 with a novel mutation in the PDYN gene. Parkinsonism Relat Disord 2015;21:332–4. [DOI] [PubMed] [Google Scholar]
- [12].Ozaki K, Doi H, Mitsui J, et al. A novel mutationin elovl4 leading to spinocerebellar ataxia (SCA) with the hot cross bun sign but lacking erythrokeratodermia: a broadened spectrum of SCA34. JAMA Neurol 2015;72:797–805. [DOI] [PubMed] [Google Scholar]


