Abstract
Women with Asherman syndrome (AS) have damaged endometrium and reduced blood flow to the uterus and placenta which may lead to low birth weight and several obstetric complications.
The objective is to determine the association between low birth weight and obstetrical complications in women with AS compared to women with normal intrauterine cavity.
A retrospective case-control study was conducted in Women's Specialized Hospital, King Fahad Medical City, from December 2008 to December 2015. Pregnant women with AS undergoing hysteroscopic adhesiolysis who presented to our clinic were matched for age, parity, body mass index, methods of conception, and gestational age to pregnant women without AS based on a 1:3 ratio. The main outcome measure included birth weight and obstetrical complications.
The study included 56 women with 14 cases and 42 controls. Pregnant women with AS had significantly lower birth weight (2.23 ± 0.28 kg) compared with pregnant women without AS (3.13 ± 0.383 kg) (P < .001 odds ratio [OR] 0.029, 95% confidence interval [CI] 0.006–0.148, P = .001). Complications of delivery including retained placenta, placenta previa, and fetal death were significantly higher in patients with AS compared with controls 28.6% 7.1%, and 7.1% compared to 4.8%, 0%, and 0%, respectively. This was statistically significant (P < .001).
Pregnant women with AS delivered low birth weight newborns and had more obstetrical complications as compared with pregnant women with normal cavity.
Keywords: Asherman syndrome, low birth weight, obstetrical complications
1. Introduction
Asherman syndrome (AS) is defined by the presence of intrauterine permanent adhesions, obliterating the uterine cavity partially or completely. It usually occurs after trauma to the basalis layer of the endometrium after endometrial curettage.[1] It was first described by Heinrich Fritsch in 1894 after that Joseph G. Asherman who described the pathology and symptoms of this syndrome.[2]
Patients with AS may present with amenorrhea with or without severe dysmenorrhea, oligomenorrhea, infertility, or recurrent miscarriages.[3] The syndrome occurs most frequently after repeated curettage for incomplete abortion (50%), postpartum hemorrhage (24%), and elective abortion (17.5%). Others less common etiologic factors include myomectomy, hysterotomy, diagnostic curettage, cesarean section, and tuberculosis.[3–5] Direct visualization of the uterus via hysteroscopy is the most reliable method for diagnosis. Other methods are sonohysterography (SHG), hysterosalpingogram (HSG), and magnetic resonance imaging (MRI).[6–8] Hysteroscopic adhesiolysis is the treatment of choice for the management of intrauterine adhesions.[9]
Several obstetric complications have been reported in women with corrected AS like miscarriages,[10–13] abnormal placentation,[14–19] cervical incompetency,[15,20] intrauterine growth retardation (IUGR),[21] premature birth,[22–24] uterine rupture,[25–27] and preeclampsia may also be another complication after AS.
This retrospective study has been designed to determine the associations between low birth weight in women with AS post hysteroscopic adhesiolysis and women who had normal intrauterine cavity post hysteroscopy.
2. Materials and methods
An analytical retrospective case-control study was carried out, and it had included 56 pregnant women who attended Women's Specialized Hospital, King Fahad Medical City, in Riyadh, Saudi Arabia, between 2008 and December 2015. Fourteen women who had undergone hysteroscopic adhesiolysis for intrauterine adhesions and eventually became pregnant were considered cases. Three control subjects were matched to each study patient; a ratio of cases: controls of 1:3 (n = 42).
The birth weight of the infants of 42 pregnant women was examined and compared to pregnant women who had previous hysteroscopic adhesiolysis. Each pregnant case was matched to age, parity, body mass index (BMI), previous curettage, postpartum hemorrhage, abortion, myomectomy, cesarean section and endometritis, method of conception either spontaneous or assisted reproductive techniques (ovulation induction, intrauterine insemination (IUI) or in vitro fertilization (IVF) treatment), gestational age (either preterm, less than 37 + 0 weeks, or term delivery), the mode of delivery either cesarean sections or vaginal delivery. Other main outcome measures including birth weight and obstetrical complications (retained placenta, placenta previa, fetal death, and postpartum hemorrhage) were analyzed.
Hysteroscopic classification of AS (mild if filmy adhesion occupying less than one-quarter of uterine cavity and ostial areas and upper fundus minimally involved or clear, moderate if one-fourth to three-fourth of cavity involved and ostial areas and upper fundus partially involved and no agglutination of uterine walls or severe if more than three-fourth of cavity involved and occlusion of both ostial area and upper fundus and agglutination of uterine walls) were recorded.[9]
We excluded women who had high blood pressure, heart diseases, infections such as rubella, cytomegalovirus, toxoplasmosis, syphilis, kidney disease, lung disease, sickle cell anemia, smoking, drinking alcohol, or abusing drugs. In addition, we excluded fetuses with chromosomal defects, previous history intrauterine growth retardation and multiple gestations (twins, triplets, or more) in both groups.
All categorical variables age group, Marsh classification, curettage, postpartum hemorrhage (PPH), and previous Cesarean sections were presented as numbers and percentages. Continuous variables height, weight, BMI, and weight of baby were expressed as mean ± S.D. Chi-square /Fisher exact test was applied according to whether the cell expected frequency is smaller than 5 and determine the significant relationship between Asherman syndrome and study variables. Independent sample t test was used to see the mean significant difference among AS and birth weight and other study parameters. Binary logistic regression was applied to determine the significant predictors/risk factors which were associated with AS. P value of less than .05 was considered statistically significant. All data was entered and analyzed through statistical package SPSS version 22. Institutional review board approval was granted for the study (15–440).
3. Results
The study included 56 women (14 cases of AS and 42 control subjects). Their age ranged between 21 and 39 years with a mean of 30.5 years and (±SD) of (±5 years). Their body mass index ranged between 22.86 and 35.30 kg/m2 with a mean of 29.77 kg/m2 and (±SD) of (±3.17 kg/m2). Their parity ranged between 0 and 5 with a median of 1. The weight of their babies ranged between 1.6 and 3.8 kg with a mean of 2.91 kg and (±SD) of (±0.53 kg).
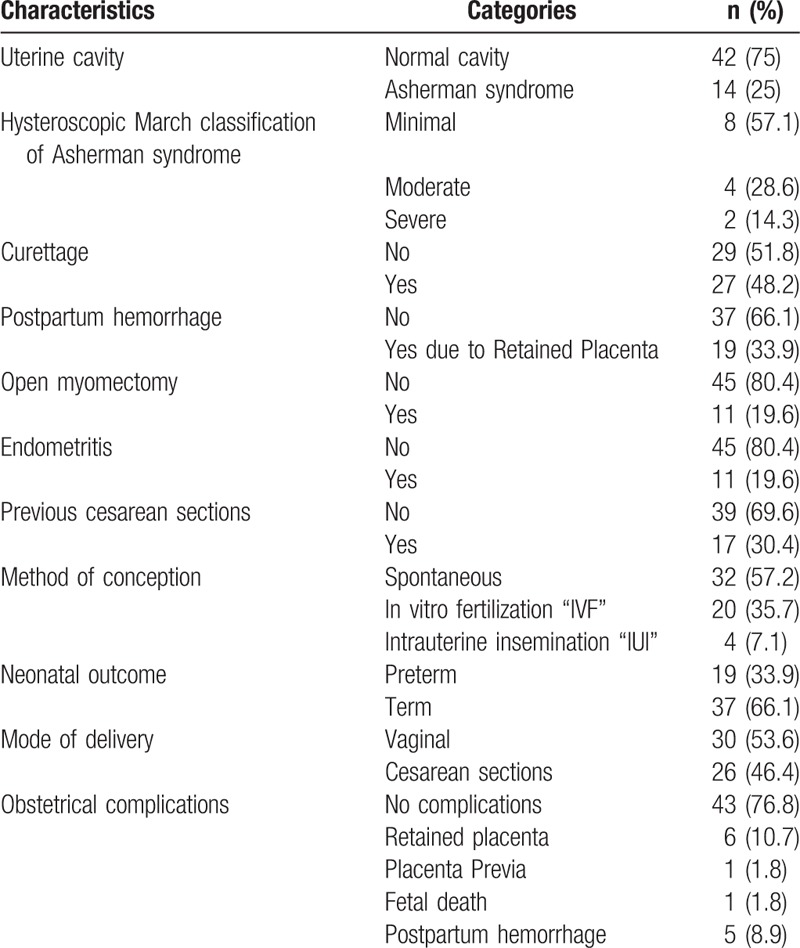
Table 1 shows that according to March classification, cases of AS were classified as minimal (57.1%), moderate (28.6%), and severe (14.3%). Curettage and postpartum hemorrhage due to retained placenta were reported among 27 (48.2%) and 19 (33.9%) of the participants, respectively. Open myomectomy and endometritis were observed separately among 11 (19.6%) of the participants. Previous cesarean section was reported among 17 (30.4%) of the participants. Regarding the methods of conception it was spontaneous among 32 (57.2%) of the participants whereas IVF and IUI were reported among 20 (35.7%) and 4 (7.1%) of the participants, respectively. Nineteen (33.9%) of the participants had preterm delivery. Twenty-six (46.4%) of the participants delivered through cesarean section. Obstetrical complications were encountered among 13 (23.2%) of the participant, mainly retained placenta among 6 (10.7%) of the participant.
Table 1.
Clinical characteristics of patients (n = 56).
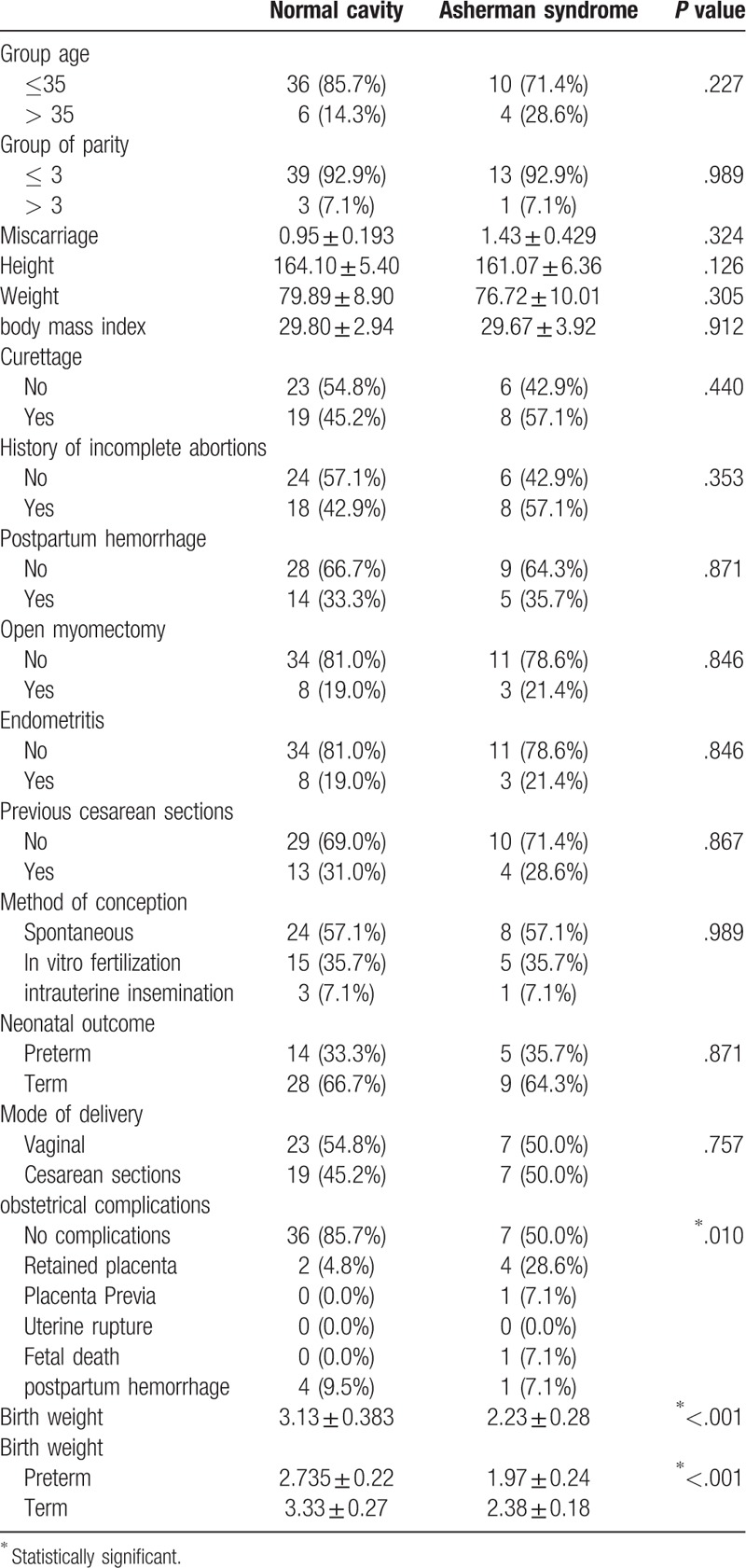
From Table 2, it is evident that among studied factors that could be associated with AS, only obstetrical complications and birth weight of the baby were significantly associated with AS. Complications were reported among 50% of women with AS compared with 15% of those without AS, P = .010. Patients with AS have a mean ± SD low birth weight of 2.23 ± 0.280 kg of their babies compared with 3.13 ± 0.383 kg for babies of women who did not have AS. The difference was statistically significant, P < .001. Similarly, birth weight was significantly lower among babies of women with AS either delivered preterm (2.735 ± 0.22 vs 1.97 ± 0.24 kg) or term (3.33 ± 0.27 vs 2.38 ± 0.18 kg), P < .001.
Table 2.
Association between demographic characteristics and Asherman syndrome.
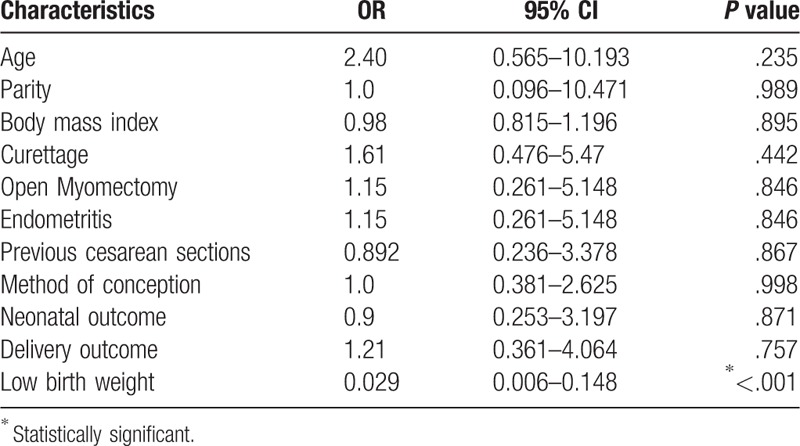
Logistic regression analysis revealed that the only significant predictor for AS was the birth weight as an increase in the birth weight by 1 g was associated with a decrease in the likelihood of AS by 2.9% (adjusted OR = 0.029; 95% CI: 0.006–0.148), P < .001. All other studied variables were not significantly associated with AS (Table 3).
Table 3.
Independent risk factors associated with Asherman syndrome.
4. Discussion
Online search yielded very few studies analyzing the health of infants born to women with a history of AS or even the impact of the syndrome on pregnancies and these data were mainly from case reports. Our study aims to determine the associations between low birth weight in women with Asherman syndrome posthysteroscopic adhesiolysis in comparison with women who had normal intrauterine cavity posthysteroscopy.
Among women with a past history of AS, the rate of preterm delivery ranged between 17.9% and 50%.[14,24,28] In accordance with that, the present study reported a rate of 35.7%.
In the present study, the spontaneous conception rate with AS was 57.1% (8 out of 14). It has been reported in an earlier study that the rate was 45.5% (133 out of 292).[29]
In pregnant women with Asherman syndrome, the defective placentation may lead to IUGR.[23,30] Women with a previous history of AS have damaged endometrium, and therefore a reduced blood flow to the uterus and placenta. This will eventually lead to poor placental perfusion.[23] The current study revealed a significant association between AS and low birth weight. A previous retrospective case-control study found no difference in pregnancy outcome aside from low birth weight in pregnancies with and without intrauterine adhesions.[31]
The defective uterine endometrium and the obliterated uterine cavity may also predispose women to ectopic tubal and cervical pregnancies.[32] In the current study, the reported complications were retained placenta, placenta previa, fetal death, and postpartum hemorrhage. Retained placenta and placenta previa were also reported by Feng et al[33] in their study as outcomes of intrauterine adhesions.
Women who have had previous intrauterine surgeries or procedures particularly surgical curettage are at higher risk for AS.[29] This was also found in our present study where the rate of curettage was higher, although not significant as a result of the relatively small sample size, among cases with AS compared with their matched control subjects (57.1% vs 45.2%).
There are 3 stages of AS, Stage I (mild), Stage II (moderate), and Stage III (severe), which indicates the higher the extent of endometrial cavity involvement, the higher the stage.[9] According to March classification, cases of AS in the present study were classified as minimal (57.1%), moderate (28.6%), and severe (14.3%). Outcome of AS depends mainly on the degree of Intra-uterine adhesions. Klatsky et al[34] reported that if intrauterine adhesions are only mild and filmy they can stretch with uterine growth during pregnancy. They also documented that the prediction of the outcome of AS is challenging, and thus close monitoring of the pregnancy by an obstetrician is essential in order to screen for potential complications in patients with previous history of AS.
Everett[35] reported that, in the general population, in 550 women who conceived, 67 pregnancies (12%) ended in miscarriage. The spontaneous miscarriage rate after treatment of intrauterine adhesions was around 20%. It is unclear whether this represents an increase in the risk of early miscarriage after treatment of AS. In the present study, we matched controls for the miscarriage to explore the influence of infant birth weight regardless of the miscarriage.
Among the limitations of this study is the fact that the study sample was relatively small as it was limited to Women's Specialized Hospital, which could affect the generalizability of results. We relied on records in the current study out of which some were incomplete particularly regarding babies’ information (The Neonatal Intensive Care Unit admission, early neonatal death, developmental assessment) and umbilical artery Doppler during pregnancy. Despite these limitations, we found a relationship between AS and low birth weight as well as the presence of obstetrical complications, and thus close monitoring of the pregnancy is essential in order to screen for potential complications in patients of AS. In the light of the study's results, we recommended conducting a study with adequate sample size to increase the study's power and thus more generalizable results.
5. Conclusion
Pregnant women with AS delivered low birth weight newborns and had more obstetrical complications as compared with pregnant women with normal cavity.
Author contributions
Conceptualization: Dania Al-Jaroudi.
Data curation: Saeed Baradwan, Afnan Baradwan.
Formal analysis: Saeed Baradwan, Afnan Baradwan, Mohammed Bashir.
Investigation: Saeed Baradwan, Mohammed Bashir.
Methodology: Saeed Baradwan, Afnan Baradwan, Mohammed Bashir, Dania Al-Jaroudi.
Project administration: Saeed Baradwan.
Resources: Saeed Baradwan.
Software: Saeed Baradwan, Mohammed Bashir, Dania Al-Jaroudi.
Supervision: Saeed Baradwan, Mohammed Bashir, Dania Al-Jaroudi.
Validation: Saeed Baradwan, Dania Al-Jaroudi.
Visualization: Saeed Baradwan, Dania Al-Jaroudi.
Writing – original draft: Saeed Baradwan, Afnan Baradwan, Dania Al-Jaroudi.
Writing – review & editing: Saeed Baradwan, Dania Al-Jaroudi.
Footnotes
Abbreviations: AS = Asherman syndrome, BMI = body mass index, HSG = hysterosalpingogram, IUGR = intrauterine growth retardation, IUI = intrauterine insemination, IVF = in vitro fertilization, MRI = magnetic resonance imaging, PPH = postpartum hemorrhage, SHG = sonohysterography.
The authors have no conflicts of interest to disclose.
References
- [1].March CM. Intrauterine adhesions. Obstet Gynecol Clin North Am 1995;22:491–505. [PubMed] [Google Scholar]
- [2].Asherman JG. Traumatic intra-uterine adhesions. J Obstet Gynaecol Br Emp, 1950;57:892–6. [DOI] [PubMed] [Google Scholar]
- [3].Schenker JG. Etiology of and therapeutic approach to synechia uteri. Eur J Obstet Gynecol Reprod Biol 1996;65:109–13. [DOI] [PubMed] [Google Scholar]
- [4].Valle RF, Sciarra JJ. Intrauterine adhesions: hysteroscopic diagnosis, classification, treatment, and reproductive outcome, Am. J Obstet Gynecol 1996;158:1459–70. [DOI] [PubMed] [Google Scholar]
- [5].Orhue AA, Aziken ME, Igbefoh JO. A comparison of two adjunctive treatments for intrauterine adhesions following lysis. Int J Gynaecol Obstet 2003;82:49–56. [DOI] [PubMed] [Google Scholar]
- [6].Soares SR, Barbosa dos Reis MM, Camargos AF. Diagnostic accuracy of sonohysterography, transvaginalsonography, and hysterosalpingography in patients with uterine cavity diseases. Fertil Steril 2000;73:406–11. [DOI] [PubMed] [Google Scholar]
- [7].Schlaff WD, Hurst BS. Preoperative sonographic measurement of endometrial pattern predicts outcome of surgical repair in patients with severe Asherman's syndrome. Fertil Steril 1995;63:410–3. [DOI] [PubMed] [Google Scholar]
- [8].Confino E, Friberg J, Giglia RV, et al. Sonographic imaging of intrauterine adhesions. Obstet Gynecol 1985;66:596–8. [PubMed] [Google Scholar]
- [9].March C, Israel R. Intrauterine adhesions secondary to elective abortion. Hysteroscopic diagnosis and management. Obstet Gynecol 1976;48:422–9. [PubMed] [Google Scholar]
- [10].Tam WH, Lau WC, Cheung LP, et al. Intrauterine adhesions after conservative and surgical management of spontaneous abortion. J Am Assoc Gynecol Laparosc 2002;9:182–5. [DOI] [PubMed] [Google Scholar]
- [11].Friedler S, Margalioth EJ, Kafka I, et al. Incidence of postabortion intra-uterine adhesions evaluated by hysteroscopy: a prospective study. Hum Reprod 1993;8:442–4. [DOI] [PubMed] [Google Scholar]
- [12].Liu X, Fan G, Jin Z, et al. Lower uterine segment pregnancy with placenta increta complicating first trimester induced abortion: diagnosis and conservative management. Chin Med J 2003;116:695–8. [PubMed] [Google Scholar]
- [13].Ecker JL, Sorem KA, Soodak L, et al. Placenta increta complicating a first-trimester abortion. A case report. J Reprod Med 1992;37:893–5. [PubMed] [Google Scholar]
- [14].Zikopoulos KA, Kolibianakis EM, Platteau P, et al. Live delivery rates in subfertile women with Asherman's syndrome after hysteroscopic adhesiolysis using the resectoscope or the Versapoint system. Reprod Biomed Online 2004;8:720–5. [DOI] [PubMed] [Google Scholar]
- [15].Fernandez H, Al-Najjar F, Chauveaud-Lambling A, et al. Fertility after treatment of Asherman's syndrome stage 3 and 4. J Minim Invasive Gynecol 2006;13:398–402. [DOI] [PubMed] [Google Scholar]
- [16].Al-Serehi A, Mhoyan A, Brown M, et al. Placenta accreta: an association with fibroids and Asherman syndrome. J Ultrasound Med 2008;27:1623–8. [DOI] [PubMed] [Google Scholar]
- [17].Feng ZC, Huang YL, Sun JF, et al. Diagnostic and therapeutic hysteroscopy for traumatic intrauterine adhesion. Clinical analysis of 70 patients. Chin Med J (Engl) 1989;102:553–8. [PubMed] [Google Scholar]
- [18].Kayem G, Davy C, Goffinet F, et al. Conservative versus extirpative management in cases of placenta accrete. Obstet Gynecol 2004;104:531–6. [DOI] [PubMed] [Google Scholar]
- [19].Goldenberg M, Schiff E, Achiron R, et al. Managing residual trophoblastic tissue; hysteroscopy for directing curettage. J Reprod Med 1997;42:26–8. [PubMed] [Google Scholar]
- [20].Capella-Allouc S, Morsad F, Rongières-Bertrand C, et al. Hysteroscopic treatment of severe Asherman's syndrome and subsequent fertility. Hum Reprod 1999;14:1230–3. [DOI] [PubMed] [Google Scholar]
- [21].Fonseca EB, Celik E, Parra M, et al. Fetal Medicine Foundation Second Trimester Screening Group. Progesterone and the risk of preterm birth among women with a short cervix. N Engl J Med 2007;375:462–9. [DOI] [PubMed] [Google Scholar]
- [22].Yasmin H, Adeghe JH. Severe early-onset intrauterine growth restriction (IUGR) in a woman with Asherman's syndrome. J Obstet Gynaecol 2004;24:312–4. [DOI] [PubMed] [Google Scholar]
- [23].Martin JA, Hamilton BE, Sutton PD, et al. Births: Final Data for 2006. National Vital Statistics Reports 2008;7:57. [PubMed] [Google Scholar]
- [24].Yu D. Factors affecting reproductive outcome of hysteroscopic adhesiolysis for Asherman's syndrome. Fertil Steril 2007;89:715–22. [DOI] [PubMed] [Google Scholar]
- [25].Meis PJ, Klebanoff M, Thom E, et al. Prevention of recurrent preterm delivery by 17 Alpha-HydroxyprogesteroneCaproate. N Engl J Med 2003;348:2379–85. [DOI] [PubMed] [Google Scholar]
- [26].Deaton JL, Maier D, Andreoli J., Jr Spontaneous uterine rupture during pregnancy after treatment of Asherman's syndrome. Am J Obstet Gynecol 1989;160(5, part 1):1053–4. [DOI] [PubMed] [Google Scholar]
- [27].Shiau CS, Hsieh CC, Chiang CH, et al. Intrapartum spontaneous uterine rupture following uncomplicated resectoscopic treatment of Asherman's syndrome. Med J 2005;28:123–7. [PubMed] [Google Scholar]
- [28].McComb P, Wagner B. Simplified therapy for Asherman's syndrome. Fertil Steril 1997;68:1047–50. [DOI] [PubMed] [Google Scholar]
- [29].Schenker JG, Margalioth EJ. Intrauterine adhesions: an updated appraisal. Fertil Steril 1982;37:593–610. [DOI] [PubMed] [Google Scholar]
- [30].Hare AA, Olah KS. Pregnancy following endometrial ablation: a review article. J Obstet Gynaecol 2005;25:108–14. [DOI] [PubMed] [Google Scholar]
- [31].Ball RH, Buchmeier SE, Longnecker M. Clinical significance of sonographically detected uterine synechiae in pregnant patients. J Ultrasound Med 1997;16:465–9. [DOI] [PubMed] [Google Scholar]
- [32].Klyszejko C, Bogucki J, Klyszejko D, et al. Cervical pregnancy in Asherman's syndrome [article in Polish]. Ginekol Pol 1987;58:46–8. [PubMed] [Google Scholar]
- [33].Feng ZC, Yang B, Shao J, et al. Diagnostic and therapeutic hysteroscopy for traumatic intrauterine adhesions after induced abortions: clinical analysis of 365 cases. Gynaecol Endosc 1999;8:95–8. [Google Scholar]
- [34].Klatsky PC, Tran ND, Strachowski L. A pregnancy complicated by endometrial scarring. Fertil Steril 2009;91:2707–8. [DOI] [PubMed] [Google Scholar]
- [35].Everett C. Incidence and outcome of bleeding before the 20th week of pregnancy: prospective study from general practice. BMJ 1997;315:32–4. [DOI] [PMC free article] [PubMed] [Google Scholar]


