Supplemental Digital Content is available in the text
Keywords: biopsy, cancer, complications, prostate, robotic, surgery
Abstract
The aim of our study was to investigate the effects of prostate biopsy on perioperative outcomes of robotic-assisted laparoscopic prostatectomy (RALP).
A total of 181 patients who underwent the RALP in our institution have been retrospectively reviewed, patients were divided into different groups according to the interval of biopsy to RALP and core numbers of biopsy. Perioperative outcomes including estimated blood loss (EBL), operative time (OT), surgical margin status, postoperative drainage, hospital stay, and perioperative complications were served as endpoints.
Interval of biopsy to RALP was not significantly correlated with any perioperative outcomes, while the biopsy core numbers had significant correlation with the EBL. In logistic regression analysis, the biopsy core numbers were associated with higher risk of positive surgical margins. Body mass index (BMI) was also a significant factor related to OT.
Delay of the RALP after biopsy was not applicable in the era of RALP and surgeons could be more freely in selecting the time of RALP. Besides, further studies should focus on how to improve the diagnostic efficiency of prostate cancer without increasing the incidence of surgical complications.
1. Introduction
Worldwide, prostate cancer is one of the most prevalent malignant tumors in men. It was reported that 60.3 thousand new cases would be diagnosed in China in 2015.[1] In United States, it was estimated that a total of 161,360 new cases would be diagnosed in 2017.[2] According to the recent guidelines, radical prostatectomy (RP) is the standard treatment for localized prostate cancer.[3] Robotic-assisted laparoscopic prostatectomy (RALP) was first introduced in 2000 by Binder and Kramer.[4] RALP provides a magnified surgical field, 3-dimensional vision, and precise control which help surgeons to dissect the surgical planes more precisely and reduce the damage to the surrounding tissues.[5,6] Now it has been a favorable option for RP since its oncological and functional outcomes are at least as much as that in open or laparoscopic techniques and even has an advantage in reducing the perioperative morbidity and the risk of positive surgical margins.[7]
Currently, most cases of prostate cancer were diagnosed by the prostate biopsy. Prostate biopsy was traditionally thought to be inducing periprostatic inflammation and hematoma, which was supported by the magnetic resonance imaging finds.[8] Thus, surgeons typically recommended that retropubic RP should be performed at least 4 to 6 weeks after biopsy to allow prostate recovery from the inflammation and hematoma induced by biopsy. This concept, however, may not suitable for cases of RALP as RALP has several advantages over the open technique. And some studies have also shown that early RALP did not affect the perioperative outcomes of surgery and might even be beneficial in reducing the operative time (OT).[7,9,10] Meanwhile, urologist also considered that both the core numbers and the approaches of biopsy may affect the outcomes of RP.[11,12] It was widely agreed that sextant biopsy is inadequate and 10 to 14 cores were suggested for improving the detection of prostate cancer[13] which resulted in an increasing of biopsy core numbers in the past decades. Although the short term complications from biopsy were similar, it was still unclear that whether an extend biopsy core number would result in worse surgical outcomes of RALP when compared to conventional sextant biopsies. Transperineal biopsy (TP) and transrectal biopsy (TR) are the 2 primary approaches of prostate biopsy. A recent meta-analysis showed that both cancer detection rate and relevant complications were similar between TP and TR.[14]
In our study, we retrospectively reviewed a series of patients who underwent RALP in our institution to determine whether the interval between the prostate biopsy and RALP (IBBP), and the biopsy core numbers were associated with the perioperative outcomes of RALP. A prior sample size and post hoc power analysis were performed by G∗POWER.[15] Odds ratio (OR) was assumed as 0.98, results were listed in Supplement Table 1.
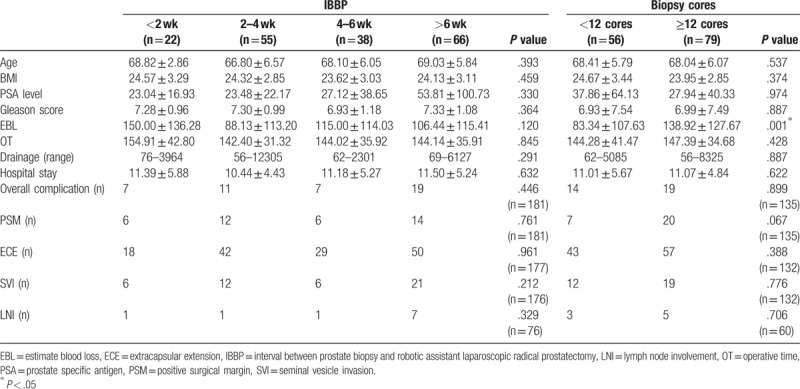
There were not significant differences of perioperative outcomes between different groups (all P > .05, Table 1), which indicates that a short interval of biopsy to RALP did not render any adverse effects on the perioperative outcomes. However, we still found that higher biopsy core numbers may result in higher estimated blood loss (EBL, 83.34 vs 138.92 mL, P = .001).
Table 1.
Comparison of perioperative outcomes in different groups.
2. Materials and methods
2.1. Patients, outcomes, and techniques
After approved by our Institutional Review Board, 398 patients between December 2014 and March 2017 were identified from medical record system in our institute by the key words: “prostate cancer,” “da van ci,” and “robotic.” These patients were further screened under inclusion and exclusion criteria. Inclusion criteria were underwent prostate biopsy before surgery; underwent RALP in our institute; and prostate cancer proven by pathological diagnosis. Exclusion criteria were incomplete data about demographics and perioperative information; with a history of incontinence, radiotherapy, chemotherapy, hormone therapy, or other pelvic surgery before RALP. Finally, a total of 181 patients were enrolled into this study.
Demographic, operative, and clinical data including patient age, body mass index (BMI), prostate volume (calculated by multiply the long, width, and height), IBBP, and core numbers of prostate biopsy, OT (including the preparation of the surgery system), EBL (reported by the anesthetist), transfusion rate, postoperative drainage, hospital stay, pre- and postoperative prostate special antigen (PSA), perioperative complications, and pathologic data were recorded. Biochemical recurrence was defined as 2 consecutive PSA values of >0.2 ng/mL after RALP. Complications were classified according to the Clavien–Dindo system.[16] All surgeries were performed by 2 experienced surgeons who have overcame the learning curve (GJM and SLA) in our institute with a standard transperitoneal approach and interfacial technique, as reported by Patel et al.[17] Hemostatic agents were routinely and postoperatively used in patients without contraindications. According to the 2017 EAU Guidelines, 10- to 12-core biopsy is recommended in diagnosis of prostate cancer.[3] A TP with at least 12 cores was performed by one experienced surgeon (ZYJ) with the modified procedures for all patients in our institute.[4] However, there were still some patients did not accept the biopsy with at least 12 cores, since these biopsies were performed in other institutes. So, we separated the biopsy cores into 2 groups (<12 and ≥12 cores). According to the previous studies, patients were divided into 4 groups based on IBBP: less than 2 weeks, 2 to 4 weeks, 4 to 6 weeks, and higher than 6 weeks.[7]
2.2. Statistical analysis
Variables were recorded as X ± SD or range for each characteristic. Perioperative variables were analyzed using the t test, Kruskal–Wallis test, Mann–Whitney U test, Wilcoxon Rank Sum test, Pearson chi-square, or Fisher exact tests. Spearman correlation was used for nonparametric data. Logistic regression was applied for analyzing the association between clinical variables and selected perioperative outcomes, results were summarized with ORs and 95% confidence intervals (95% CIs). Factors with P < .1 in univariate regression analysis were involved in multivariate analysis. All testes were 2 sides, P value < .05 was considered to indicate statistical significance. Bonferroni adjusted pairwise comparisons were also conducted using Mann–Whitney U tests. All analyses were performed by SPSS software version 23.0 (IBM, New York) and R-3.3.2.[18]
3. Results
3.1. Patient demographics
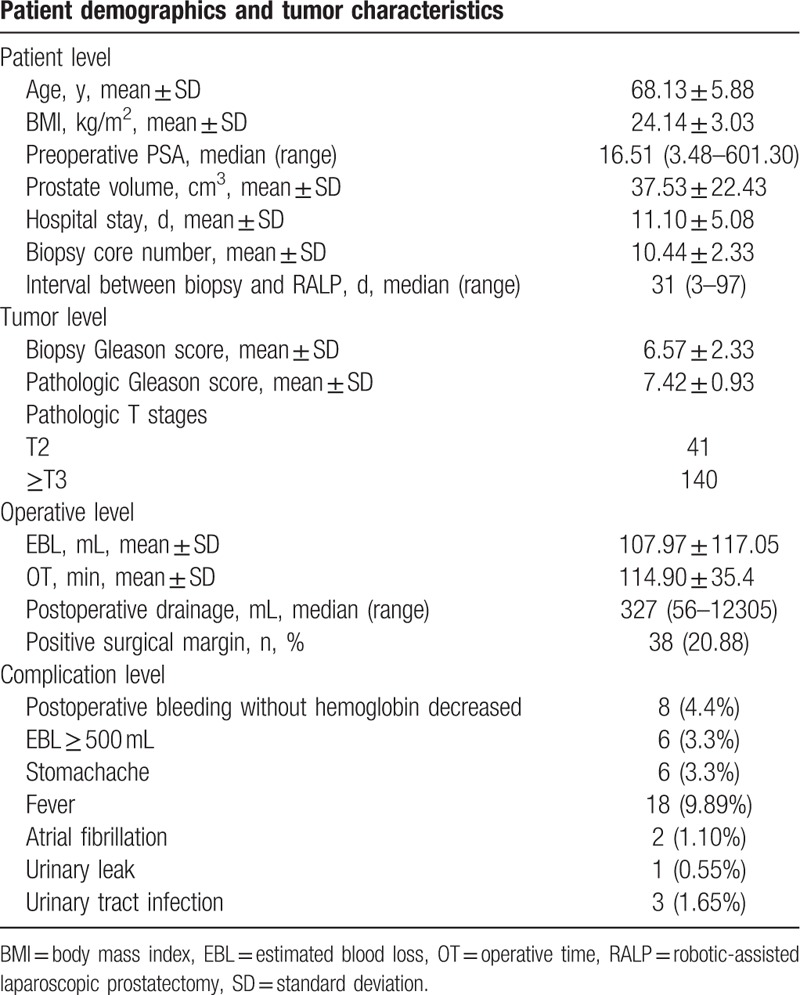
A total of 181 patients underwent RALP enrolled into our study. Patient demographics are listed in Table 2. The median IBBP was 31 days, the mean core numbers of prostate biopsy was 10.44. The positive margin rate was 20.88%. A total of 44 complications were recorded, including postoperative bleeding without hemoglobin decreased (n = 8, 4.4%), EBL ≥ 500 mL (n = 6, 3.3%), stomachache (n = 6, 3.3%), fever (n = 18, 9.89%), atrial fibrillation (n = 2, 1.10%), urinary leak (n = 1, 0.55%), urinary tract infection (n = 3, 1.65%), and no patient required transfusion (Table 2). According to the Clavien–Dindo system, these complications included 38 grade 1 and 6 grade 2. Because we took the standard procedure of TP and the oral quinolones was routinely administered prior to biopsy as EAU guideline recommend, only 2 certain cases of hematuria were reported. Thus, we could not divide it into groups according to its categories.
Table 2.
Patient demographics and tumor characteristics.
3.2. Comparison of perioperative outcomes in different groups
Patients were divided into 4 groups according to the IBBP: ≤2 weeks, 3 to 4 weeks, 5 to 6 weeks, and >6 weeks. There were no significant differences of perioperative outcomes between 4 groups (all P > .05, Table 1). Besides, the Bonferroni adjusted pairwise comparisons indicated that there were no significant differences of perioperative outcomes between any 2 groups of the 4 (all P > .05, Table 1). Patients were also divided into 2 groups according to the biopsy core numbers: <12 and ≥12 cores. There were no significant differences of perioperative outcomes between 2 groups except the EBL was significantly higher in ≥12 cores group (83.34 vs 138.92 mL, P = .001).
3.3. Spearman correlation and binary logistic regression
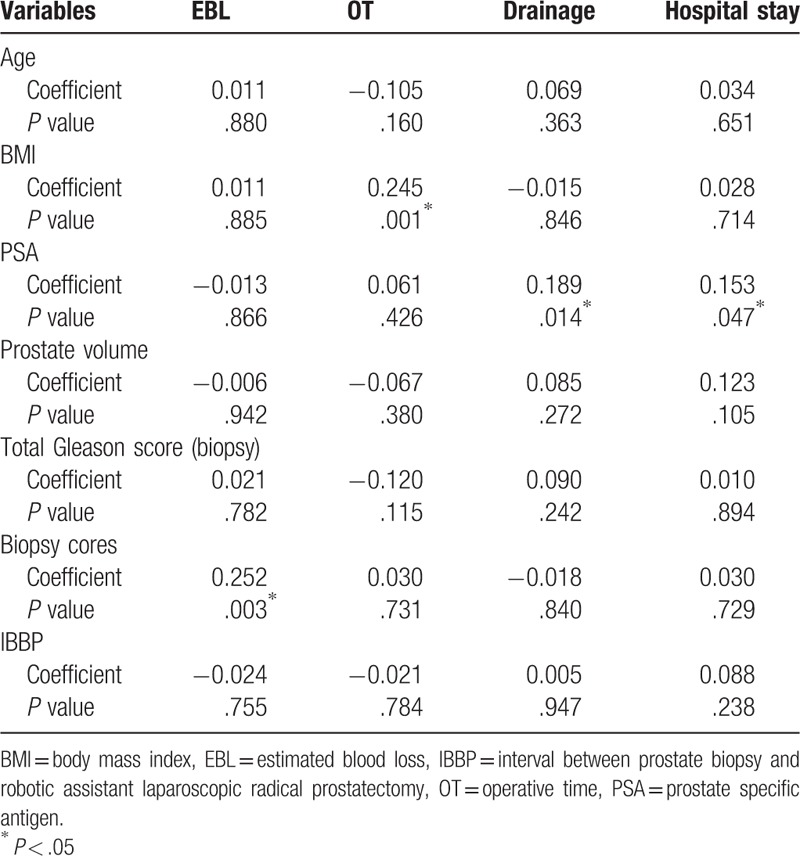
Spearman correlation analysis showed that IBBP has not significant correlation with any perioperative outcomes. The biopsy cores were significantly correlated with EBL (r = 0.252, P = .003). Higher BMI was significantly correlated with longer OT (r = 0.245, P = .001), and higher PSA was significantly correlated with higher postoperative drainage (r = 0.189, P = .014) and longer hospital stay (r = 0.153, P = .047, Table 3). As expected, longer OT was significantly associated with higher EBL and postoperative drainage (data not shown).
Table 3.
Spearman correlation between preoperative variables and clinical outcomes.
In univariate logistic regression analysis, neither IBBP nor biopsy cores were significantly associated with any perioperative outcomes, including perioperative complications, EBL ≥ 500 mL, OT > 3 hours, and positive surgical margins whether as a continuous variable or a categorical variable. Interestingly, patients with higher BMI may have higher risk of EBL ≥ 500 mL (OR = 1.354, 95%CI = 1.043–1.758, P = .023) and OT > 3 hours (OR = 1.232, 95%CI = 1.078–1.409, P = .002); lower volume has a significant association with OT > 3 hours (OR = 0.973, 95%CI = 0.947–1.000, P = .046), higher PSA before surgery (OR = 1.007, 95%CI = 1.000–1.013, P = .04), and higher pathological Gleason scores (OR = 1.547, 95%CI = 1.013–2.362, P = .043) significantly predicted a higher risk of perioperative complications; and higher pathological T stage significantly predicted a higher risk of positive margin (OR = 3.632, 95%CI = 1.049–12.578, P = .042, Table 4).
Table 4.
Univariate analysis and multivariate analysis of factors with an impact on clinical outcomes.
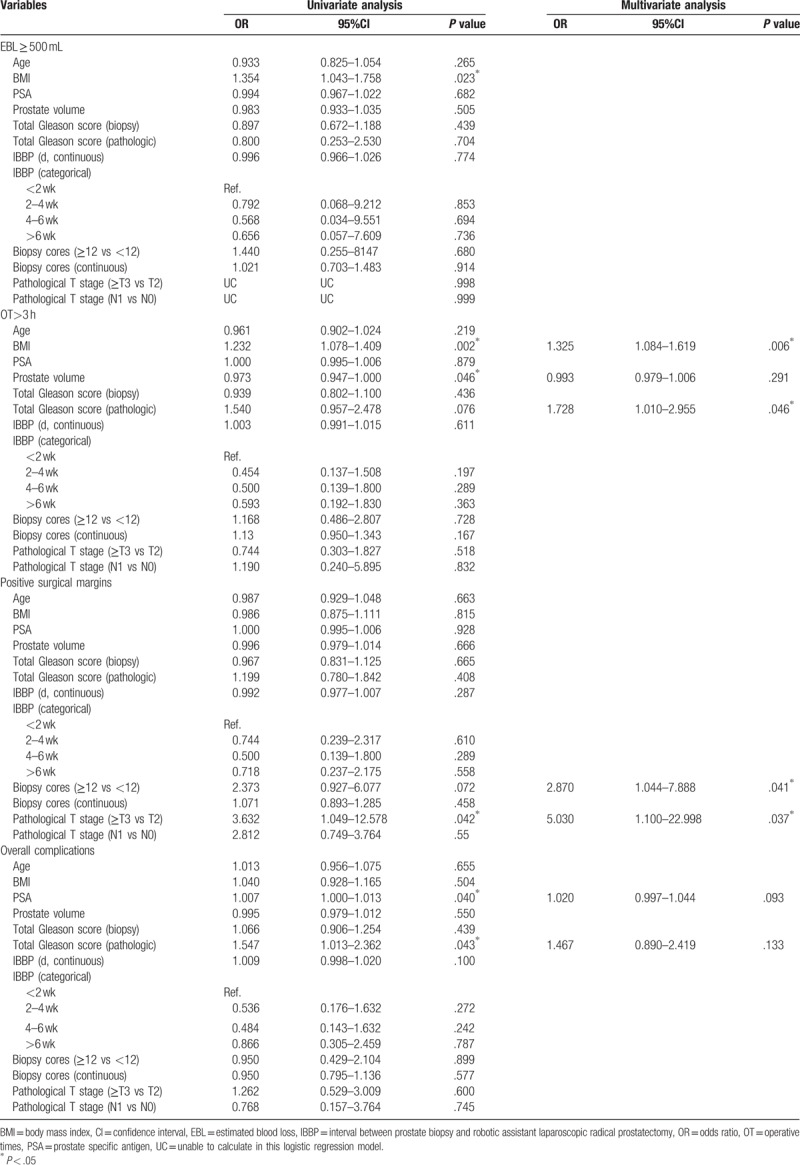
In multivariate logistic regression analysis, the BMI (OR = 1.325, 95%CI = 1.084–1.619, P = .006) and pathologic Gleason scores (OR = 1.728, 95%CI = 1.010–2.955, P = .046) were still significantly associated with OT > 3 hours. The PSA level and pathologic Gleason scores showed a trend to associate with the overall complications but not reach the significance. Interestingly, the biopsy cores (OR = 2.870, 95%CI = 1.044–7.888, P = .041) and pathologic stage (OR = 5.030, 95%CI = 1.100–22.998, P = .037) have significant association with the positive surgical margin (Table 4).
4. Discussion
Nowadays, most cases of prostate cancer were diagnosed by the prostate biopsy. Traditionally, it was recommended that the radical prostatectomy should be performed at least 6 to 12 weeks after the biopsy[4] because that the inflammation or hematoma caused by biopsy might eliminate the surgical planes of dissection and resulting in a detrimental effect on perioperative outcomes.[19] However, this recommendation may no longer apply in the era of RALP,[7] the technological advances provided surgeons several benefits in precise dissection, 3-dimensional vision, reliable hemostasis, and a magnified surgical field,[13] which made the prostatectomy more feasible and decreased the perioperative morbidity.[20] In this study, we showed that the interval between biopsy and RALP was not significantly associated with any perioperative complications. Indeed, the only significant effect of biopsy on RALP is that a higher biopsy cores may increase the EBL and the risk of positive surgical margins. Thus, delay of the surgery may inapplicable in era of RALP.
A prospective study showed that performing the RALP in 2 weeks after the prostate biopsy in feasible and safe even though the EBL may be slightly increased.[9] Park et al also reported that biopsy-to-surgery interval did not affect the perioperative outcomes, including the surgical margin, OT or EBL in open, laparoscopic, and robotic approach.[10] Another large retrospective study carried out by Jo et al has produced similar findings,[7] they have even found that surgery late after biopsy (after 6 weeks) associated with an increased OT[7] which may result from the processed inflammation. In fact, most studies supported the opinion that the interval between the biopsy and surgery was not related to the perioperative outcomes even in open prostatectomy.[21–23] Furthermore, undergoing the prostatectomy as soon as possible may be beneficial for patients in high risk group (higher PSA level or Gleason scores).[24]
According to the recent guidelines, at least 10 to 12 biopsy cores were recommended for initial diagnosis of prostate cancer. But the effect of biopsy cores numbers on perioperative outcomes remains unclear. In this study, we found that a higher number of biopsy cores were associated with higher EBL and higher risk of positive surgical margin which may result from increased dissection difficulties caused by increased biopsy core numbers. Choi et al also reported a negligible increasing of OT in patients with more than 10 biopsy cores.[13] Thus, further studies should focus on how to improve the diagnostic efficiency of prostate cancer without increasing the incidence of surgical complications.
In present study, we also analyzed other parameters may related to the perioperative outcomes, we found that higher BMI was associated with longer OT which consistent with previous studies,[10,25,26] higher PSA level before surgery was associated with higher drainage and longer hospital stay. Our experience suggested that this reflects the higher complexity of RALP in patients with higher BMI or PSA level.
To our knowledge, this study is the first study to focus on the effect of both a short interval of biopsy to RALP (<2 weeks) and the biopsy core numbers on perioperative outcomes. We admitted that there were some limitations in our study. First, it was a single center and retrospective study which may lead to selection and information bias. Second, because of a relative short following-up period, we did not analyze the effect of interval between biopsy and RALP on the potency, continence, and biochemical recurrence. Third, not all of the biopsies were performed in our institution. Despite these limitations, this study demonstrated that the interval of biopsy to RALP was not significantly related to any perioperative outcomes. There was no reason to delay surgery after prostate biopsy in the RALP era.
5. Conclusion
In our study, a short interval of biopsy to RALP did not render any adverse effects on the perioperative outcomes. Surgeons could be more freely in selecting the time of RALP. But higher biopsy core numbers may result in higher EBL and risk of positive surgical margins, further studies should focus on how to improve the diagnostic efficiency of prostate cancer without increasing the incidence of surgical complications.
Acknowledgment
The authors thank Dong Pan for his valuable works in data collection.
Author contributions
Jianming Guo had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Jianming Guo, Hang Wang.
Data collection: Yaohui Li, Minke He, Yeqing Xu, Yanjun Zhu, Xiaoyi Hu, and Shuai Jiang.
Analysis and interpretation of data: Yaohui Li, and Minke He.
Drafting of the manuscript: Yaohui Li, and Minke He
Statistical analysis: Yaohui Li, and Minke He
Administrative, technical, or material support: None.
Other: None.
Data curation: Yaohui Li, Zhuoyi Xiang, Li-an Sun, Yanjun Zhu, Xiaoyi Hu, Jianming Guo, Hang Wang.
Formal analysis: Jianming Guo.
Software: Minke He, Yaohui Li.
Writing – original draft: Minke He, Yaohui Li.
Supplementary Material
Footnotes
Abbreviations: BMI = body mass index, CI = confidence interval, EBL = estimated blood loss, IBBP = interval between the prostate biopsy and RALP, OR = odds ratio, OT = operative time, PSA = prostate special antigen, RALP = robotic-assisted laparoscopic prostatectomy, RP = radical prostatectomy, TP = transperineal biopsy.
MH and YL contributed equally to this work.
The authors have no funding and conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115–32. [DOI] [PubMed] [Google Scholar]
- [2].Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7–30. [DOI] [PubMed] [Google Scholar]
- [3].Mottet N, Bellmunt J, Bolla M, et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol 2017;71:618–29. [DOI] [PubMed] [Google Scholar]
- [4].Wein A, Kavoussi L, Partin A, et al. Campbell-Walsh Urology. 2015;Amsterdam: Elsevier, 11th ed. [Google Scholar]
- [5].Lim SK, Kim KH, Shin T, et al. Current status of robot-assisted laparoscopic radical prostatectomy: how does it compare with other surgical approaches? Int J Urol 2013;20:271–84. [DOI] [PubMed] [Google Scholar]
- [6].Trinh Q, Sammon J, Sun M, et al. Perioperative outcomes of robot-assisted radical prostatectomy compared with open radical prostatectomy: results from the nationwide inpatient sample. Eur Urol 2012;61:679–85. [DOI] [PubMed] [Google Scholar]
- [7].Jo JK, Oh JJ, Lee S, et al. Can robot-assisted laparoscopic radical prostatectomy (RALP) be performed very soon after biopsy? World J Urol 2017;35:605–12. [DOI] [PubMed] [Google Scholar]
- [8].White S, Hricak H, Forstner R, et al. Prostate cancer: effect of postbiopsy hemorrhage on interpretation of MR images. Radiology 1995;195:385–90. [DOI] [PubMed] [Google Scholar]
- [9].Yip SK. Does performance of robot-assisted laparoscopic radical prostatectomy within 2 weeks after biopsy affect the outcome? Int J Urol 2011;18:146–7. [DOI] [PubMed] [Google Scholar]
- [10].Park B, Choo SH, Jeon HG, et al. Interval from prostate biopsy to radical prostatectomy does not affect immediate operative outcomes for open or minimally invasive approach. J Korean Med Sci 2014;29:1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Carneiro A, Sivaraman A, Sanchez-Salas R, et al. Higher number of transrectal ultrasound guided prostate biopsy cores is associated with higher blood loss and perioperative complications in robot assisted radical prostatectomy. Actas Urol Esp 2017;41:155–61. [DOI] [PubMed] [Google Scholar]
- [12].Wadhwa K, Patruno G, Patterson A, et al. Robotic assisted laparoscopic radical prostatectomy following transrectal compared to transperineal prostate biopsy: surgical, oncological and functional outcomes. Minerva Urologica e Nefrologica = Italian J Urol Nephrol 2017;69:85–92. [DOI] [PubMed] [Google Scholar]
- [13].Choi H, Ko YH, Kang SG, et al. Biopsy related prostate status does not affect on the clinicopathological outcome of robotic assisted laparoscopic radical prostatectomy. Cancer Res Treat 2009;41:205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Xue J, Qin Z, Cai H, et al. Comparison between transrectal and transperineal prostate biopsy for detection of prostate cancer: a meta-analysis and trial sequential analysis. Oncotarget 2017;8:23322–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Faul F, Erdfelder E, Lang AG, et al. G∗Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 2007;39:175–91. [DOI] [PubMed] [Google Scholar]
- [16].Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Patel VR. Robotic Urologic Surgery. 1st ed.London: Springer; 2007. [Google Scholar]
- [18].R Core Team (2016) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available at: https://www.R-project.org/. 2016. Accessed November 9, 2016. [Google Scholar]
- [19].Martin GL, Nunez RN, Humphreys MD, et al. Interval from prostate biopsy to robot-assisted radical prostatectomy: effects on perioperative outcomes. BJU Int 2009;104:1734–7. [DOI] [PubMed] [Google Scholar]
- [20].Tewari A, Sooriakumaran P, Bloch DA, et al. Positive surgical margin and perioperative complication rates of primary surgical treatments for prostate cancer: a systematic review and meta-analysis comparing retropubic, laparoscopic, and robotic prostatectomy. Eur Urol 2012;62:1–5. [DOI] [PubMed] [Google Scholar]
- [21].Eggener SE, Yossepowitch O, Serio AM, et al. Radical prostatectomy shortly after prostate biopsy does not affect operative difficulty or efficacy. Urology 2007;69:1128–33. [DOI] [PubMed] [Google Scholar]
- [22].Lee DK, Allareddy V, O’donnell MA, et al. Does the interval between prostate biopsy and radical prostatectomy affect the immediate postoperative outcome? BJU Int 2006;97:48–50. [DOI] [PubMed] [Google Scholar]
- [23].Adiyat KT, Murugesan M, Katkoori D, et al. Total prostatectomy within 6 weeks of a prostate biopsy: is it safe? Int Braz J Urol 2010;36:177–81. 182. [DOI] [PubMed] [Google Scholar]
- [24].Berg WT, Danzig MR, Pak JS, et al. Delay from biopsy to radical prostatectomy influences the rate of adverse pathologic outcomes. Prostate 2015;75:1085–91. [DOI] [PubMed] [Google Scholar]
- [25].Ahlering TE, Eichel L, Edwards R, et al. Impact of obesity on clinical outcomes in robotic prostatectomy. Urology 2005;65:740–4. [DOI] [PubMed] [Google Scholar]
- [26].Castle EP, Atug F, Woods M, et al. Impact of body mass index on outcomes after robot assisted radical prostatectomy. World J Urol 2008;26:91–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


