Abstract
Background:
Blood management after arthroplasties has become a serious problem. The objective is to perform a meta-analysis to compare the efficacy and safety between oral tranexamic acid (TXA) and intravenous TXA for blood management in total knee and hip arthroplasty.
Methods:
We systematically searched randomized controlled trials (RCTs) from Medline, Embase, and the Cochrane Central Register of Controlled Trials (CENTRAL), Web of Science and Google scholar. Eligibility criteria: Patients: adult patients with end-stage joint osteoarthritis, rheumatoid arthritis, and osteonecrosis of the femoral head, who prepared for TJA; Interventions: The experiential group received the intravenous form of TXA; Comparisons: Oral form of TXA; Outcomes: Total blood loss, hemoglobin reduction, transfusion requirements, duration of hospitalization, and thrombotic complications including deep vein thrombosis (DVT) and pulmonary embolism (PE); Study design: Randomized control trials (RCTs) and non-RCT. Meta-analysis results were collected and analyzed by the software STATA 11.0. After testing for heterogeneity between studies, data were aggregated for random-effects models when necessary.
Results:
Four RCTs and 2 non-RCTs were included in the meta-analysis. The present meta-analysis revealed that there were no significant differences regarding total blood loss (WMD = −25.013, 95% CI: −51.002 to 0.977, P = .059), postoperative hemoglobin decline (WMD = −0.090, 95% CI: −0.205 to 0.024, P = .122), or transfusion rate (RD = −0.039, 95% CI: −0.080 to 0.002, P = .062) between the 2 groups.
Conclusion:
Oral TXA shows comparable efficacy to that of the intravenous forms after total knee and hip arthroplasty. Due to the limited quality of evidence currently available, higher quality RCTs is necessary.
Keywords: blood loss, meta-analysis, total hip arthroplasty, total knee arthroplasty, tranexamic acid
1. Introduction
Total knee and hip arthroplasty (TKA and THA) has shown well-recognized efficacy in improving functional outcomes as well as in relieving pain for patients with joint degeneration. With the aging population, the number of TKAs is increasing. However, arthroplasties were associated with substantial bleeding.[1,2] Thus, a considerable number of patients require blood transfusions in order to treat anemia. As known, allogenic blood transfusion was associated with numerous adverse reactions including anaphylactic reactions, infectious diseases, as well as metabolic disorders prolonging the length of hospital stays, and resulting in serious medical costs.
Numerous methods have been implemented to minimize perioperative blood loss including drug intervention, autologous donation, perioperative hemodilution, minimally invasive surgery, blood salvage, and reinfusion.[3,4] However, high incidence of blood transfusion remains a major concern.
Tranexamic acid (TXA), a 4-aminomethyl cyclohexane-carbolic acid, is a synthetic amino acid derivative of lysine where the drug binds to the lysine site of the plasminogen in order to promote clot stabilization, thereby inhibiting clot degradation.[5] A majority of orthopedists focused on intravenous and topical administration of TXA. Published articles have indicated that both intravenous and topical application of TXA were associated with improved outcomes in total joint arthroplasties (TJAs).[6,7] Oral TXA was also effective in reductions in blood loss, and transfusion requirements and it was estimated to be more cost effective than the intravenous application.[8]
Despite these apparent advantages, whether oral TXA would be equivalent to intravenously in reducing blood loss in TJA remains unclear. Thus, we conducted a systemic review and meta-analysis to compare the efficacy and safety between oral and intravenous TXA in patients undergoing joint arthroplasties.
2. Methods
This systematic review was reported according to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines. No ethical approval was required because this paper was based on the previous articles.
2.1. Search methodology
Studies were retrieved from the databases of Pubmed (1966–2018.06), Embase (1980–2018.06), and the Cochrane Central Register of Controlled Trials (1980–2018.06), Web of Science (1966–2018.06), and Google scholar (1966–2018.06). Reference lists of relevant articles were manually searched to identify additional trials. No restrictions were imposed on language. A structured search was performed using the following search string: “Total knee replacement or arthroplasty,” “Total hip replacement or arthroplasty,” “tranexamic acid,” “intravenous,” and “oral.”
2.2. Inclusion criteria
-
(1)
Patients: adult patients with end-stage joint osteoarthritis, rheumatoid arthritis, and osteonecrosis of the femoral head, who prepared for TJA.
-
(2)
Interventions: The experiential group received the intravenous form of TXA.
-
(3)
Comparisons: Oral form of TXA.
-
(4)
Outcomes: Total blood loss, hemoglobin reduction, transfusion requirements, duration of hospitalization, and thrombotic complications including deep vein thrombosis (DVT) and pulmonary embolism (PE).
-
(5)
Study design: Randomized control trials (RCTs) and non-RCT.
2.3. Selection criteria
All relevant studies were collected and duplicate literatures were excluded. Then, 2 researchers independently excluded studies by reading titles and abstracts. At last, the irrelevant studies were removed following inclusion criteria. If no consensus was reached, a third investigator was consulted.
2.4. Date extraction
The available data were extracted independently from the included studies by 2 reviewers. The information included author name, publishing year, age, sample size, gender, intervention procedure, transfusion trigger and follow-up. We send emails to authors to obtain incomplete outcome data.
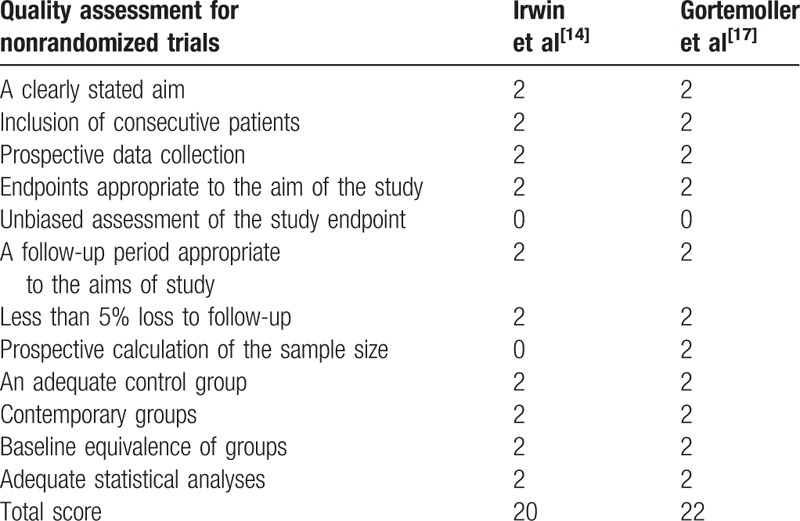
2.5. Quality assessment
The methodological qualities of included studies were assessed independently by the 2 reviewers described by the Cochrane Collaboration for Systematic Reviews. We conducted a “risk of bias” table including the following key points: random sequence generation, allocation concealment, blinding, incomplete outcome data, free of selective reporting and other bias and each item was recorded by “Yes”- “No”- or “Unclear”. The Methodological Index for Non-Randomized Studies (MINORS) scale[9] was applied to evaluate non-RCTs with scores ranging 0 to 24.
2.6. Statistical methods
Stata 11.0 software was utilized for the meta-analysis. Continuous various outcomes, such as the total blood loss, hemoglobin decline, and length of stay were all expressed as the weighted mean difference (WMD) with a 95% confidence intervals (CIs). The results of dichotomous outcomes (transfusion rate, DVT, PE) were expressed as a risk difference (RD) with 95% confidence intervals (CIs). The statistical heterogeneity was determined by the Chi-squared test in accordance with the value of P and I2. If I2 > 50%, P < .05, statistical was considered to be heterogeneous, we used a random-effects model to analysis the data. Otherwise, the fixed-effects model was performed to conduct meta-analysis.
3. Results
3.1. Search result
Four hundred twenty-five studies were identified from databases. According to the inclusion criteria, 419 studies were excluded. No gray paper was included. Finally, 4 RCTs[10–13] and 2 non-RCT[14,15] which published from 2004 to 2017 were included in our study and includes 621 participates in the TXA groups and 2963 patients in the control groups. The search process was performed as presented in Fig. 1.
Figure 1.
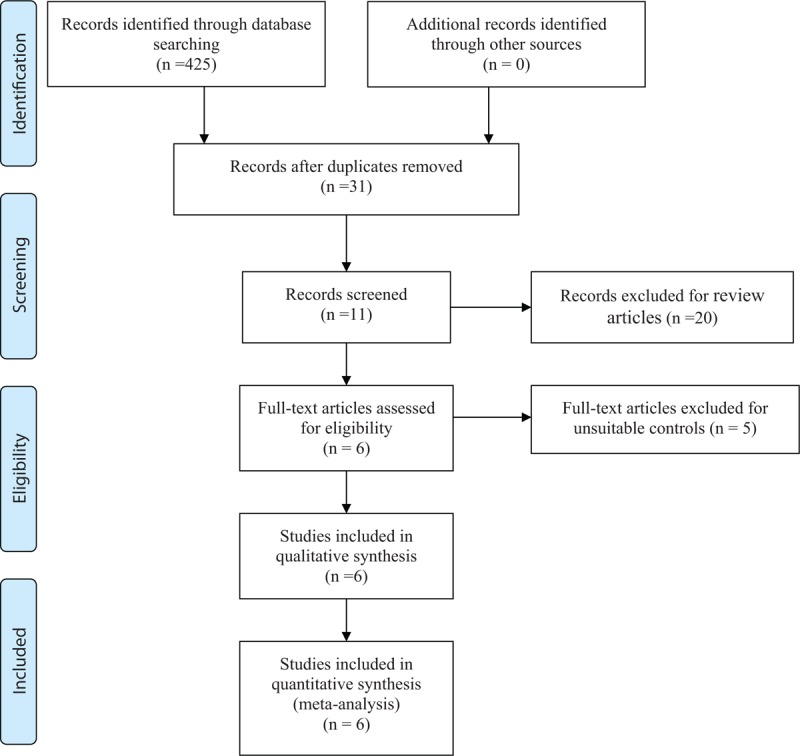
Search results and the selection procedure.
3.2. Study characteristics
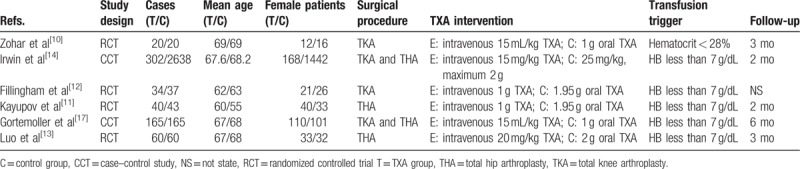
The basic information of the included articles are concluded in Table 1. The included articles were published from 2004 to 2017. The sample size ranged from 40 to 2940 and mean age of patients ranged from 55 to 69 years. Duration follow-up ranged from 2 to 6 months.
Table 1.
Cohort characteristics.
3.3. Risk of bias assessment
Cochrane Collaboration's tool is applied to evaluate the risk of bias (Tables 2 and 3). Randomization was performed in all RCTs[10–13] which mentioned that the list of random numbers were generated from computers. All articles[11–13] used sealed envelopes for allocation concealment. Double blinding was shown in 3 RCTs. It was not clear whether assessors were blinded. Two non-RCTs were appraised by the MINORS[9] (Table 4).
Table 2.
Methodological quality of the randomized controlled trials.
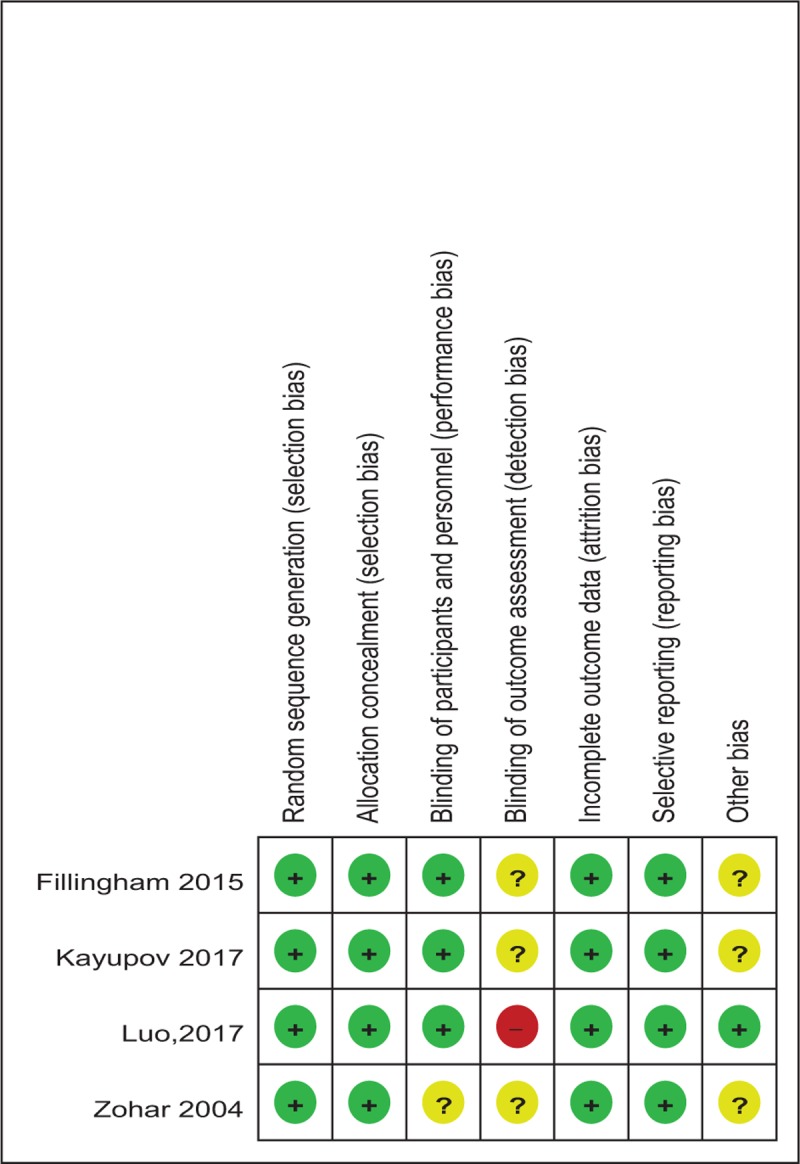
Table 3.
Risk of bias.
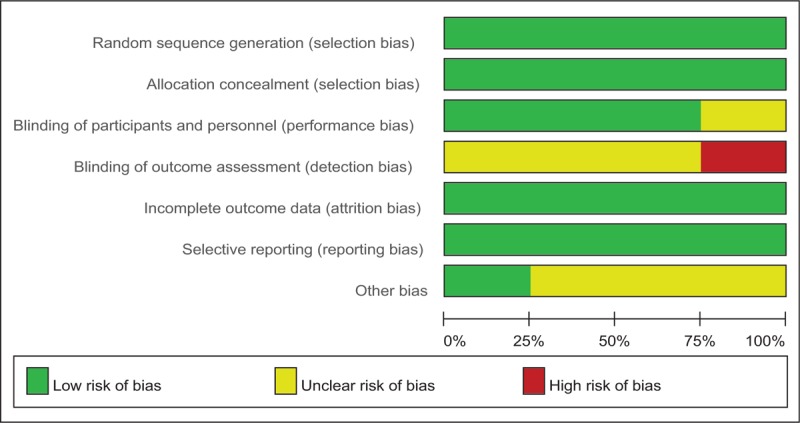
Table 4.
Methodological quality of the nonrandomized controlled trials.
3.4. Meta-analysis results
3.4.1. Total blood loss
All studies[10–15] provided the total blood loss after TJAs. No significant statistical heterogeneity was found (χ2 = 1.03, df = 5, I2 = 0.0%, P = .960) and a fixed-effects model was adopted. Our study revealed that there was no significant difference in terms of total blood loss (WMD = −25.013, 95% CI: −51.002 to 0.977, P = .059; Fig. 2).
Figure 2.
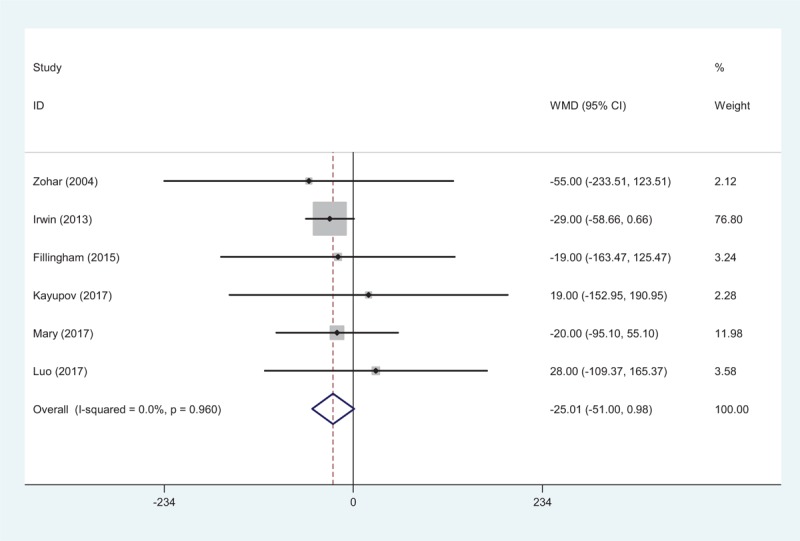
Forest plot showing the meta-analysis of total blood loss between the 2 groups.
3.4.2. Hemoglobin decline
Six studies[10–15] showed the postoperative hemoglobin decline after TJA. There was no significant heterogeneity (χ2 = 0.25, df = 5, I2 = 0.0%, P = .998). Hemoglobin decline did not show significant differences between groups (WMD = −0.090, 95% CI: −0.205 to 0.024, P = .122; Fig. 3).
Figure 3.
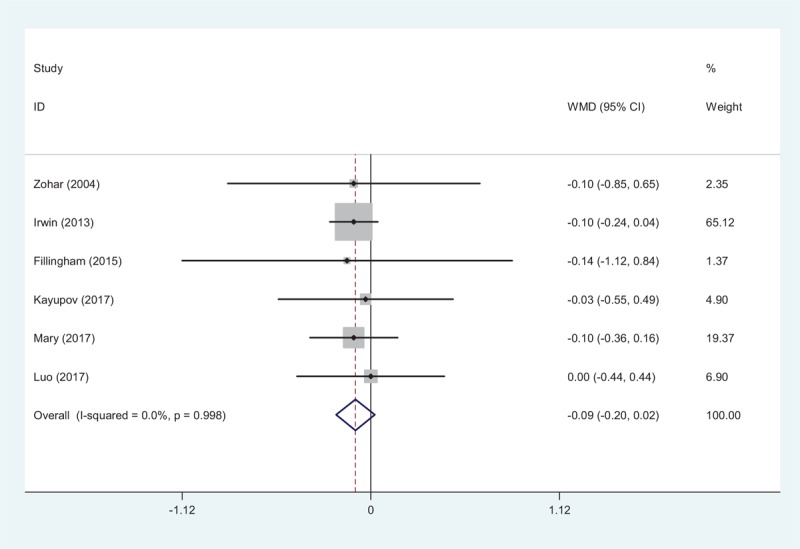
Forest plot showing the meta-analysis of hemoglobin decline between the 2 groups.
3.4.3. Transfusion rates
Transfusion requirements were shown in six articles.[10–15] We found no significant heterogeneity (χ2 = 3.78, df = 5, I2 = 0.0%, P = .581); a fixed-effects model was adopted. There was no significant difference between groups regarding transfusion rates (RD = −0.039, 95% CI: −0.080 to 0.002, P = .062; Fig. 4).
Figure 4.
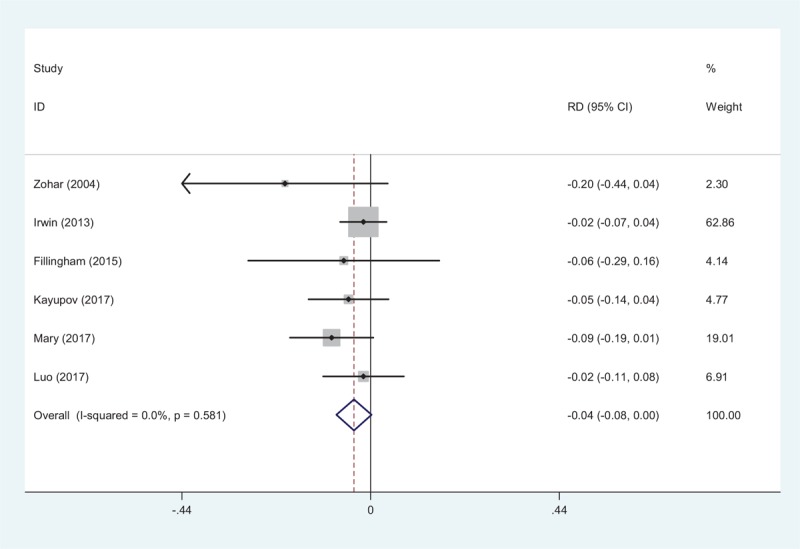
Forest plot showing the meta-analysis of transfusion rate between the 2 groups.
3.4.4. Duration of hospitalization
All RCTs provided the duration of hospitalization.[10–15] There was no significant heterogeneity among articles (χ2 = 2.80, df = 5, I2 = 0.0%, P = .731). No significant difference in the duration of hospitalization was observed (WMD = −0.093, 95% CI: −0.280 to 0.094, P = .331; Fig. 5).
Figure 5.
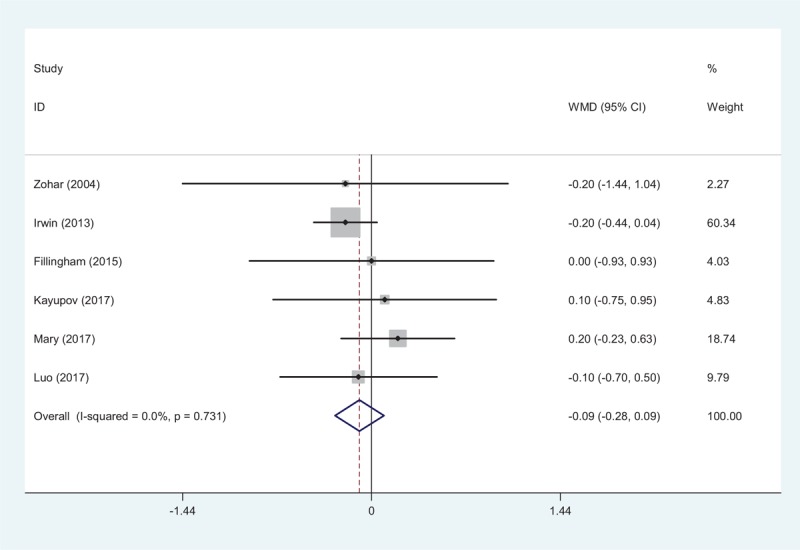
Forest plot showing the meta-analysis of length of hospital stay between the 2 groups.
3.4.5. Deep vein thrombosis
Six studies[10–15] showed the thrombotic complications of DVT. No significant statistical heterogeneity was found, and a fixed-effects model was used (χ2 = 0.14, df = 5, I2 = 0.0%, P = 1.000). Similar incidence of the risk of DVT was identified between groups (RD = −0.002, 95% CI: −0.010 to 0.006, P = .597; Fig. 6).
Figure 6.
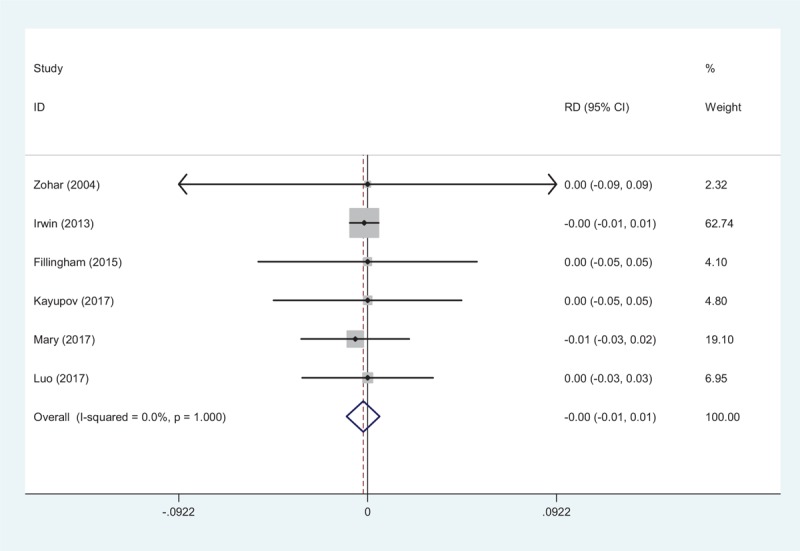
Forest plot showing the meta-analysis of the incidence of DVT between the 2 groups.
3.4.6. Pulmonary embolism
Six articles reported the thrombotic complications of PE following TJA[10–15]. A fixed-effects model was adopted (χ2 = 0.14, df = 5, I2 = 0.0%, P = 1.000). No significant difference was found in the PE incidence between the 2 groups (RD = −0.002, 95% CI: −0.012 to 0.008, P = .722; Fig. 7).
Figure 7.
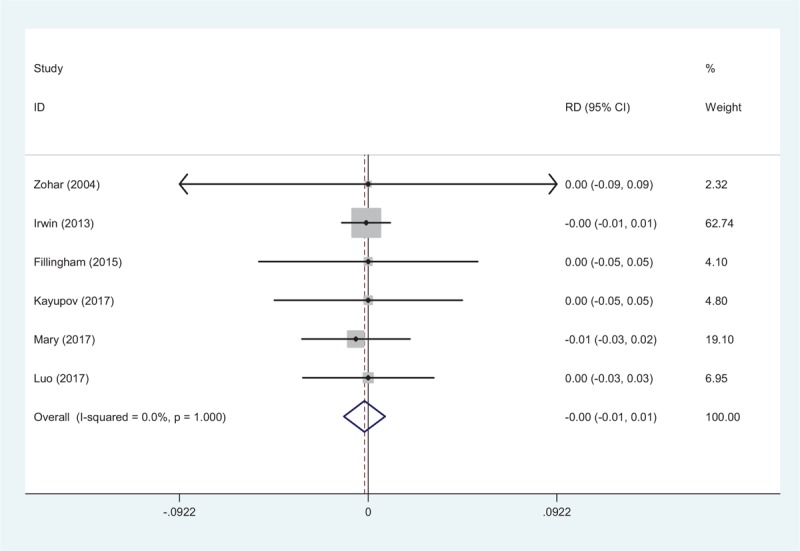
Forest plot showing the meta-analysis of the incidence of PE between the 2 groups.
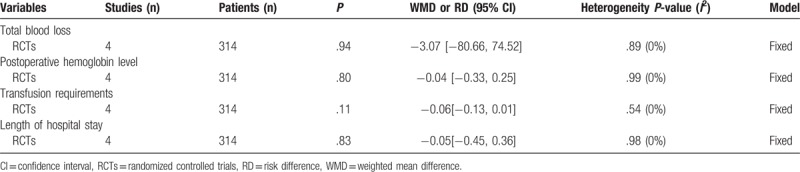
3.4.7. Subgroup analysis
Subgroup analysis was conducted for the outcome of blood loss, hemoglobin reduction, transfusion rates and duration of hospitalization (Table 5). The overall results demonstrated there was no significant difference between groups.
Table 5.
Subgroup analysis.
4. Discussion
To the best of our knowledge, this is the first systemic review to assess the efficacy between intravenous and oral forms of TXA application in TKA and THA. We found that oral TXA shows comparable efficacy to that of the intravenous forms after TKA and THA.
TXA, which acts as antifibrinolytic agent is famous for proven success in reducing peri- and postoperative blood loss and widely used in surgical procedure.[16–18] TXA could be applied by various routes including intravenous, intraarticular, oral and intramuscular.
TXA is administered for approximately 2 hours via the oral, 30 minutes via the intramuscular, and 5 to 15 minutes via the intravenous routes[19] in order to maintain the effective plasma concentration. Konig at al[20] reported that topical administration of TXA for patients undergoing primary THA was effective in reducing blood loss. Fu et al[21] performed a meta-analysis from 22 RCTs and showed that TXA is beneficial for patients undergoing TKA, which could significantly minimize total blood loss. However, oral routine of TXA in the setting of TJA have been limited due to the seldom published studies. Lee et al[22] reported that oral TXA showed improved outcomes in blood sparing for hemoglobin decline and total blood loss in THA. Currently, whether oral administration of TXA was superior to intravenous routes in these patients was unknown. Our study revealed that oral TXA showed comparable efficacy to that of the intravenous forms after TKA and THA.
It was reported that TKA without antifibrinolytics was associated with massive blood loss ranging from 761 to 1784 mL[23–26] and allogenic blood transfusion was frequently performed to relieve anemia. Potential side effects might occur, for instance, transmission of infection, possible hemolytic transfusion reactions, etc. Substantial high-quality RCTs and meta-analysis have been published to confirm that both intravenous and topical administration of TXA could diminish the need for allogeneic blood transfusions in patients undergoing TJA. Seol et al[27] observed a 24% decrease in the requirement for transfusion in the intravenous TXA group compared control group after TKA. Whether oral administration of TXA was superior to intravenous TXA for reducing transfusion rate in TJA remained controversial. Meta-analysis is performed as major statistical method in the present study. It could strengthen statistical power and enlarger sample size by pooling results of published articles that could point out stronger evidence. Current meta-analysis revealed that the difference of transfusion requirements between oral and intravenous groups was not significant.
DVT is considered as a common postoperative complication, which may develop into PE and even result in death following total joint arthroplasty.[28] Published articles have suggest a potential higher risk of thrombotic complication when TXA was used. This result might be due to the tendency of TXA, which is an antifibrinolytic agent, to promote the risk of clotting. Though the intravenous administration of TXA might be more likely to result in the formation of a thrombus because of the higher TXA level of blood concentration. There was no significant difference between both groups in terms of the risk of DVT or PE in the present analysis. However, further study was still necessary.
The limitations of this study were as follows: The small sample size might have affected the results of the meta-analysis; Functional outcome was not reported and could not be included in the meta-analysis; A large confidence interval for the results would influence the data interpretation and large sample size studies were required; All studies lacked long-term follow-up; Only English publications were included in our meta-analysis and publication bias was unavoidable.
5. Conclusion
Oral TXA shows comparable efficacy to that of the intravenous forms after total knee and hip arthroplasty. Due to the limited quality of evidence currently available, higher quality RCTs is necessary.
Author contributions
Dongxu Zhao conceived the design of the study. Kun-Chi Zhao and Ming-Ming Zhao performed and collected the data and contributed to the design of the study. Fei Wang finished the manuscript. All authors read and approved the final manuscript.
Conceptualization: Dong-Xu Zhao.
Data curation: Kun-Chi Zhao, Dong-Xu Zhao.
Formal analysis: Ming-Ming Zhao.
Writing – original draft: Fei Wang.
Writing – review & editing: Fei Wang.
Footnotes
Abbreviations: DVT = deep vein thrombosis, PE = pulmonary embolism, RCTs = randomized controlled trials, THA = total hip arthroplasty, TKA = total knee arthroplasty, TXA = tranexamic acid.
FW and K-CZ contributed equally to this work.
The authors have no conflicts of interest to disclose.
References
- [1].Sehat KR, Evans R, Newman JH. How much blood is really lost in total knee arthroplasty?. Correct blood loss management should take hidden loss into account. Knee 2000;7:151–5. [DOI] [PubMed] [Google Scholar]
- [2].Browne JA, Adib F, Brown TE, et al. Transfusion rates are increasing following total hip arthroplasty: risk factors and outcomes. J Arthroplasty 2013;28(8 suppl):34–7. [DOI] [PubMed] [Google Scholar]
- [3].Harris RN, Moskal JT, Capps SG. Does tranexamic acid reduce blood transfusion cost for primary total hip arthroplasty? A case-control study. J Arthroplasty 2015;30:192–5. [DOI] [PubMed] [Google Scholar]
- [4].Bierbaum BE, Callaghan JJ, Galante JO, et al. An analysis of blood management in patients having a total hip or knee arthroplasty. J Bone Joint Surg Am 1999;81:2–10. [DOI] [PubMed] [Google Scholar]
- [5].Benoni G, Lethagen S, Fredin H. The effect of tranexamic acid on local and plasma fibrinolysis during total knee arthroplasty. Thromb Res 1997;85:195–206. [DOI] [PubMed] [Google Scholar]
- [6].Akgul T, Buget M, Salduz A, et al. Efficacy of preoperative administration of single high dose intravenous tranexamic acid in reducing blood loss in total knee arthroplasty: a prospective clinical study. Acta Orthop Traumatol Turc 2016;50:429–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Poeran J, Rasul R, Suzuki S, et al. Tranexamic acid use and postoperative outcomes in patients undergoing total hip or knee arthroplasty in the United States: retrospective analysis of effectiveness and safety. BMJ 2014;349:g4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lee QJ, Ching WY, Wong YC. Blood sparing efficacy of oral tranexamic acid in primary total knee arthroplasty: a randomized controlled trial. Knee Surg Relat Res 2017;29:57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Dent O. Methodological index for non-randomized studies. ANZ J Surg 2003;73:675–6. [DOI] [PubMed] [Google Scholar]
- [10].Zohar E, Ellis M, Ifrach N, et al. The postoperative blood-sparing efficacy of oral versus intravenous tranexamic acid after total knee replacement. Anesth Analg 2004;99:1679–83. [DOI] [PubMed] [Google Scholar]
- [11].Kayupov E, Fillingham YA, Okroj K, et al. Oral and intravenous tranexamic acid are equivalent at reducing blood loss following total hip arthroplasty: a randomized controlled trial. J Bone Joint Surg Am 2017;99:373–8. [DOI] [PubMed] [Google Scholar]
- [12].Fillingham YA, Kayupov E, Plummer DR, et al. Rand Young Investigator's Award: a randomized controlled trial of oral and intravenous tranexamic acid in total knee arthroplasty: the same efficacy at lower cost? J Arthroplasty 2016;31(9 suppl):26–30. [DOI] [PubMed] [Google Scholar]
- [13].Luo ZY, Wang HY, Wang D, et al. Oral vs intravenous vs topical tranexamic acid in primary hip arthroplasty: a prospective, randomized, double-blind, controlled study. J Arthroplasty 2018;33:786–93. [DOI] [PubMed] [Google Scholar]
- [14].Irwin A, Khan SK, Jameson SS, et al. Oral versus intravenous tranexamic acid in enhanced-recovery primary total hip and knee replacement: results of 3000 procedures. Bone Joint J 2013;95-B:1556–61. [DOI] [PubMed] [Google Scholar]
- [15].Gortemoller MA, Allen B, Forsyth R, et al. Comparison of oral and intravenous tranexamic acid for prevention of perioperative blood loss in total knee and total hip arthroplasty. Ann Pharmacother 2017;52:246–50. [DOI] [PubMed] [Google Scholar]
- [16].Shaaban MM, Ahmed MR, Farhan RE, et al. Efficacy of tranexamic acid on myomectomy-associated blood loss in patients with multiple myomas: a randomized controlled clinical trial. Reproduct Sci 2016;23:908–12. [DOI] [PubMed] [Google Scholar]
- [17].Prokopchuk-Gauk O, Rosin MW, Mycyk TR, et al. Dual-route tranexamic acid to reduce blood loss in coronary artery bypass graft surgery: a randomized controlled trial. Can J Anaesth 2016;63:1110–1. [DOI] [PubMed] [Google Scholar]
- [18].Massicotte L, Denault AY, Beaulieu D, et al. Aprotinin versus tranexamic acid during liver transplantation: impact on blood product requirements and survival. Transplantation 2011;91:1273–8. [DOI] [PubMed] [Google Scholar]
- [19].Ronday HK, Te Koppele JM, Greenwald RA, et al. Tranexamic acid, an inhibitor of plasminogen activation, reduces urinary collagen cross-link excretion in both experimental and rheumatoid arthritis. Br J Rheumatol 1998;37:34–8. [DOI] [PubMed] [Google Scholar]
- [20].Konig G, Hamlin BR, Waters JH. Topical tranexamic acid reduces blood loss and transfusion rates in total hip and total knee arthroplasty. J Arthroplasty 2013;28:1473–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Fu DJ, Chen C, Guo L, et al. Use of intravenous tranexamic acid in total knee arthroplasty: a meta-analysis of randomized controlled trials. Chin J Traumatol 2013;16:67–76. [PubMed] [Google Scholar]
- [22].Lee QJ, Chang WY, Wong YC. Blood-sparing efficacy of oral tranexamic acid in primary total hip arthroplasty. J Arthroplasty 2017;32:139–42. [DOI] [PubMed] [Google Scholar]
- [23].Veien M, Sorensen JV, Madsen F, et al. Tranexamic acid given intraoperatively reduces blood loss after total knee replacement: a randomized, controlled study. Acta Anaesthesiol Scand 2002;46:1206–11. [DOI] [PubMed] [Google Scholar]
- [24].Hiippala S, Strid L, Wennerstrand M, et al. Tranexamic acid (Cyklokapron) reduces perioperative blood loss associated with total knee arthroplasty. Br J Anaesth 1995;74:534–7. [DOI] [PubMed] [Google Scholar]
- [25].Camarasa MA, Olle G, Serra-Prat M, et al. Efficacy of aminocaproic, tranexamic acids in the control of bleeding during total knee replacement: a randomized clinical trial. Br J Anaesth 2006;96:576–82. [DOI] [PubMed] [Google Scholar]
- [26].Benoni G, Fredin H. Fibrinolytic inhibition with tranexamic acid reduces blood loss and blood transfusion after knee arthroplasty: a prospective, randomised, double-blind study of 86 patients. J Bone Joint Surg Br 1996;78:434–40. [PubMed] [Google Scholar]
- [27].Seol YJ, Seon JK, Lee SH, et al. Effect of tranexamic acid on blood loss and blood transfusion reduction after total knee arthroplasty. Knee Surg Relat Res 2016;28:188–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Wang C, Han Z, Zhang T, et al. The efficacy of a thrombin-based hemostatic agent in primary total knee arthroplasty: a meta-analysis. J Orthop Surg Res 2014;9:90. [DOI] [PMC free article] [PubMed] [Google Scholar]


