Abstract
Background:
Propofol and midazolam are widely used for the sedation of bronchoscopy. This systematic review and meta-analysis is conducted to compare the efficacy of propofol and midazolam for bronchoscopy.
Methods:
The databases including PubMed, EMbase, Web of science, EBSCO, and Cochrane library databases are systematically searched for collecting the randomized controlled trials (RCTs) regarding the efficacy of propofol and midazolam for bronchoscopy.
Results:
This meta-analysis has included 4 RCTs. Compared with midazolam intervention in patients undergoing bronchoscopy, propofol intervention is associated with remarkably reduced recovery time [standard mean difference (SMD) = −0.74; 95% confidence interval (95% CI) = −1.04 to −0.45; P < .00001], but demonstrates no significant impact on operation time (SMD = −0.01; 95% CI = −0.16 to 0.13; P = .87), induction time (SMD = −0.58; 95% CI = −1.19 to 0.03; P = .06), lowest oxyhemoglobin saturation (SpO2, SMD = 0.24; 95% CI = −0.09 to 0.58; P = .15), SpO2 <90% [risk ratio (RR) = 1.02; 95% CI = 0.82–1.25; P = .88), and major arrhythmias (RR = 0.56; 95% CI = 0.26–1.19; P = .13).
Conclusion:
Propofol sedation is able to reduce recovery time and shows similar safety compared with midazolam sedation during bronchoscopy.
Keywords: bronchoscopy, meta-analysis, midazolam, propofol, randomized controlled trials
1. Introduction
Bronchoscopy can cause various procedure-related symptoms and discomfort [1–3] and midazolam and an opioid is the most common combination used to improve patient tolerance and satisfaction.[4–6] Incremental midazolam sedation is recommended for patients undergoing bronchoscopy, and a bolus of midazolam is often administered when suffering from procedure-related discomfort during bronchoscopic procedures.[7–10] However, midazolam administration is limited by the delayed recovery.[11,12]
Various sedative protocols have been recently investigated for bronchoscopy. Intermittent propofol (2,6-diisopropylphenol) bolus has demonstrated good tolerance and fast recovery in patients undergoing bronchoscopy.[13–16] Propofol can reach peak concentration in a short time (2 minutes), and demonstrates fast redistribution and clearance so that it is available to maintain steady plasma concentrations with continuous infusion.[17–19] In addition, propofol is reported to provide a higher quality of sedation in terms of neuropsychometric recovery and patient tolerance during bronchoscopy than midazolam.[13]
However, propofol and opioids combination may result in oversedation and cardiopulmonary depression.[20,21] Considering these inconsistent effects, we therefore conduct a systematic review and meta-analysis of randomized controlled trials (RCTs) to compare the effectiveness of propofol versus midazolam in patients undergoing bronchoscopy.
2. Materials and methods
Preferred Reporting Items for Systematic Reviews and Meta-analysis statement[22] and the Cochrane Handbook for Systematic Reviews of Interventions[23] are used to guide the performance of this systematic review and meta-analysis. Two investigators have independently searched articles, extracted data, and assessed the quality of included studies.
2.1. Literature search and selection criteria
Several databases, including PubMed, EMbase, Web of science, EBSCO, and the Cochrane library, are systematically searched using the keywords propofol, and midazolam, and bronchoscopy. The time in publishing the studies is from inception to October 28, 2017. The inclusion criteria are as follows: study design is RCT, study population are patients undergoing bronchoscopy, and intervention treatments are propofol versus midazolam.
2.2. Data extraction and outcome measures
Some information is collected for summarizing the baseline characteristics of patients in the included RCTs, and they include first author, publication year, sample size, baseline characteristics of patients, propofol, and midazolam. The primary outcome is recovery time. Secondary outcomes include operation time, induction time, lowest oxyhemoglobin saturation (SpO2), SpO2 <90%, and major arrhythmias.
2.3. Quality assessment in individual studies
The methodological quality of included RCTs is evaluated using the Jadad Scale, which is composed of 3 evaluation elements, including randomization (0–2 points), blinding (0–2 points), dropouts, and withdrawals (0–1 points).[24] One point would be allocated to each element on the basis of the description, randomization, and/or blinding of the included RCTs. The score of Jadad Scale has a range from 0 to 5 points, and 1 study with Jadad score ≥3 is thought to have the high quality.[25]
2.4. Statistical analysis
Review Manager Version 5.3 (The Cochrane Collaboration, Software Update, Oxford, UK) is used for the all statistical analyses. We have calculated the SMD with 95% confidence interval (95% CI) for continuous outcomes (recovery time, operation time, induction time, and lowest SpO2), and RR with 95% CIs for dichotomous outcomes (SpO2 <90% and major arrhythmias). Heterogeneity is quantified with the I2 statistic, and an I2 value greater than 50% represents the significant heterogeneity. The random-effect model with DerSimonian and Laird weights is applied for all the meta-analyses regardless of the heterogeneity. When the significant heterogeneity presents, sensitivity analysis is conducted to detect the influence of a single study on the overall estimate or perform the subgroup analysis. Publication bias is not evaluated because of the limited number (<10). P < .05 is thought to be statistically significant.
3. Results
3.1. Literature search, study characteristics, and quality assessment
Figure 1 demonstrates the flow chart for the selection process and detailed identification. Six hundred fifty-nine publications are searched after the initial search of databases. One hundred eighty-seven duplicates and 465 papers after checking the titles/abstracts are excluded. Three studies are removed because of the study design and 4 RCTs are ultimately included in the meta-analysis.[13,26–28]
Figure 1.
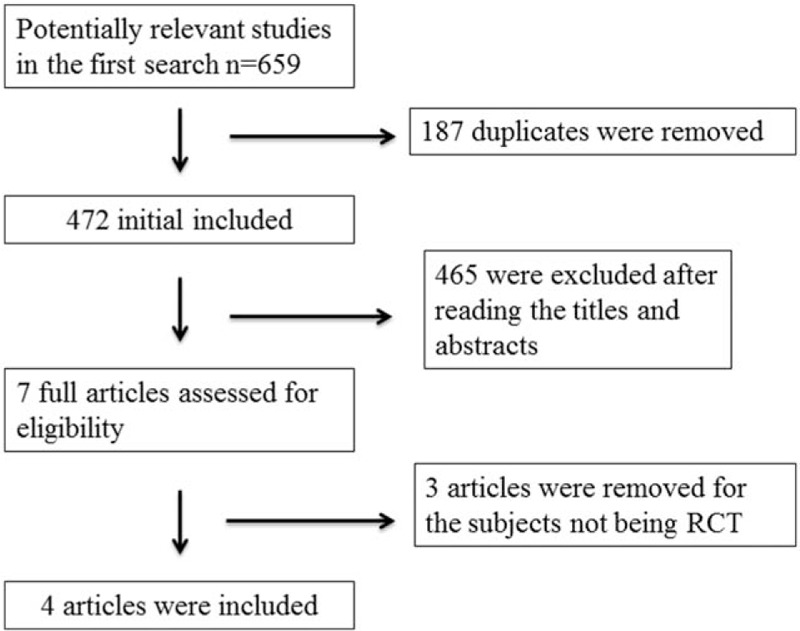
Flow diagram of study searching and selection process.
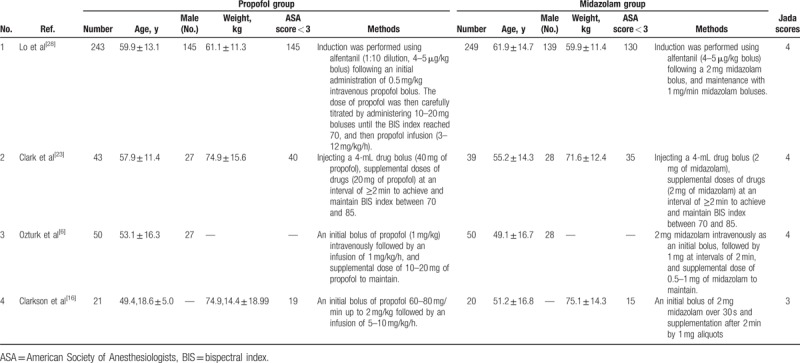
Table 1 summarizes the baseline characteristics of 4 eligible RCTs. [6,16,23,28] The 4 studies are published between 1993 and 2011, and the total sample size is 715. The detail methods of propofol and midazolam for bronchoscopy are summarized in Table 1. Among the 4 RCTs, 3 studies report the recovery time,[13,26,27] 4 studies report the operation time,[13,26,27,28] 3 studies report the induction time,[13,26,27] 2 studies report the lowest SpO2,[26,28] 3 studies report the SpO2 < 90%,[26–28] and 2 studies report the major arhythmias.[26,28] Jadad scores of the 4 eligible studies vary from 3 to 4, and thus, this quality assessment confirms these studies with high quality.
Table 1.
Characteristics of included studies.
3.2. Primary outcome: recovery time
The random-effect model is used for the analysis of recovery time, and 3 included RCTs report this index. Propofol intervention results in a significantly shorter recovery time (SMD = −0.74; 95% CI = −1.04 to −0.45; P < .00001) than midazolam intervention for bronchoscopy, with low heterogeneity among the studies (I2 = 44%, heterogeneity P = .17, Fig. 2).
Figure 2.
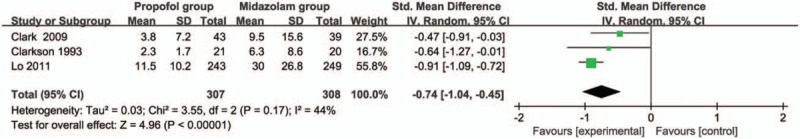
Forest plot for the meta-analysis of recovery time (min).
3.3. Sensitivity analysis
The meta-analysis of recovery time has the low heterogeneity among the included studies, and thus, we do not perform sensitivity analysis by omitting 1 study in each turn or conduct the subgroup analysis.
3.4. Secondary outcomes
Compared with midazolam intervention for bronchoscopy, propofol intervention shows no remarkable influence on operation time (SMD = -0.01; 95% CI = -0.16 to 0.13; P = .87; Fig. 3), induction time (SMD = -0.58; 95% CI = -1.19 to 0.03; P = .06; Fig. 4), lowest SpO2 (SMD = 0.24; 95% CI = -0.09 to 0.58; P = .15; Fig. 5), SpO2 < 90% (RR = 1.02; 95% CI = 0.82–1.25; P = .88; Fig. 6), and major arrhythmias (RR = 0.56; 95% CI = 0.26–1.19; P = .13; Fig. 7).
Figure 3.
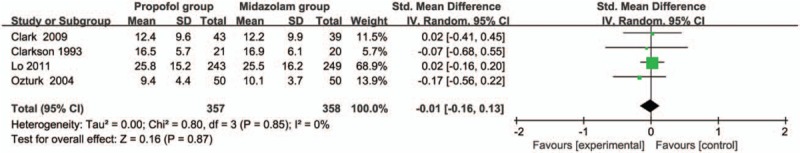
Forest plot for the meta-analysis of operation time (min).
Figure 4.
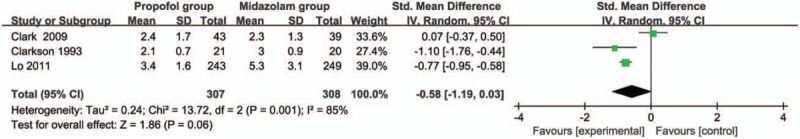
Forest plot for the meta-analysis of induction time (min).
Figure 5.
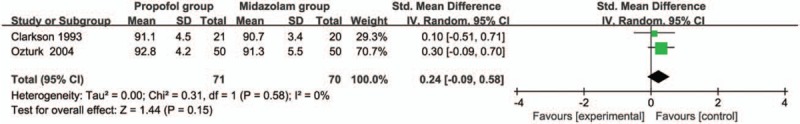
Forest plot for the meta-analysis of lowest SpO2 (%).
Figure 6.
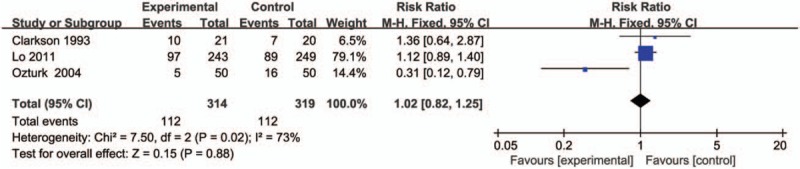
Forest plot for the meta-analysis of SpO2 < 90%.
Figure 7.
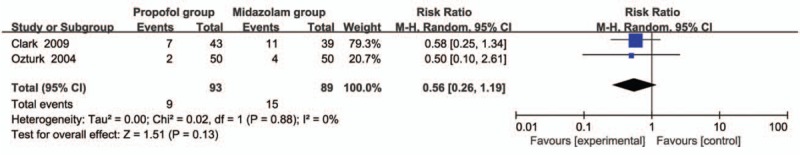
Forest plot for the meta-analysis of major arrhythmias.
4. Discussion
Propofol sedation is reported to provide faster induction, less procedural interference for bronchoscopists, better tolerance, and faster recovery for patients undergoing bronchoscopy than midazolam infusion.[27,29] Our meta-analysis suggests that compared with midazolam infusion during bronchoscopy, propofol sedation treatment can substantially decrease recovery time, but has no significant influence on the operation time and induction time.
Bispectral index (BIS) is known as a noninvasive and objective indicator of the depth of anesthesia. Good correlations are revealed between propofol drug concentration, sedative score, and BIS level.[30,31] BIS index between 70 and 85 can be maintained via BIS-guided propofol bolus during simple bronchoscopy procedures.[13] BIS level of 65 to 75 is recommended for bronchoscopy sedation, and a BIS level of 70 is set for induction in this protocol to achieve patients who are amnesic but still with reflex responsiveness to noxious stimulation.[30,32]
Patients receiving propofol in bronchoscopy show better global tolerance, but have no influence on the perception of coughing, bronchoscopists’ assessment compared with patients using midazolam.[13] The discomfort score and safety profiles of patients with propofol are similar to those with midazolam sedation.[16] One included RCT has reported that BIS-guided propofol infusion is as safe as the current standard method of clinically judged midazolam sedation based on the number of patients experiencing hypoxemia and hypotension.[27]
Patients with propofol sedation demonstrate similar lowest SpO2, the number of SpO2 < 90%, and major arrhythmias compared with midazolam infusion during bronchoscopy based on the results of our meta-analysis. BIS-guided propofol infusion with alfentanil administration is revealed to provide additional benefits for the bronchoscopists (less procedural interference) and patients (less discomfort from scope insertion, dyspnea, and cough), and these may be explained by that adding alfentanil can modify the pharmacokinetic property of propofol and provide a more steady plasma concentration in order to reduce the required dose of propofol and recovery time with less cardiovascular depression.[17,33,34]
There are still several limitations. First, only 4 RCTs are included in this meta-analysis, and 2 of them have a relatively small sample size (n < 100). These may lead to overestimation of the treatment effect in smaller trials. Although there is low heterogeneity among the included studies, different methods of propofol and midazolam in each included RCT may affect the pooled results. Finally, the plasma concentration of drug is not tested in the included RCT. The optimal dose and method of esmolol treatment remains elusive.
5. Conclusion
Propofol sedation can provide the shorter recovery time during bronchoscopy than midazolam sedation. Propofol sedation is recommended to be administered for bronchoscopy with caution, and more studies are needed to confirm this issue.
Author contributions
Conceptualization: Tianyang Dai.
Data curation: Zhizhen Wang.
Methodology: Zhizhen Wang, Tianyang Dai.
Visualization: Zhi Hu.
Writing – original draft: Zhi Hu.
Writing – review & editing: Zhi Hu.
Footnotes
Abbreviations: BIS = bispectral index, CI = confidence interval, RCTs = randomized controlled trials, SMD = standard mean difference, SpO2 = oxyhemoglobin saturation.
The authors declare no conflict of interest.
References
- [1].Diette GB, White P, Jr, Terry P, et al. Quality assessment through patient self-report of symptoms prefiberoptic and postfiberoptic bronchoscopy. Chest 1998;114:1446–53. [DOI] [PubMed] [Google Scholar]
- [2].Haga T, Fukuoka M, Morita M, et al. A prospective analysis of the efficacy and complications associated with deep sedation with midazolam during fiberoptic bronchoscopy. J Bronchology Interv Pulmonol 2016;23:106–11. [DOI] [PubMed] [Google Scholar]
- [3].Minami D, Takigawa N, Watanabe H, et al. Safety and discomfort during bronchoscopy performed under sedation with fentanyl and midazolam: a prospective study. Jpn J Clin Oncol 2016;46:871–4. [DOI] [PubMed] [Google Scholar]
- [4].Matot I, Kramer MR. Sedation in outpatient bronchoscopy. Respir Med 2000;94:1145–53. [DOI] [PubMed] [Google Scholar]
- [5].Matsumoto T, Otsuka K, Kato R, et al. Evaluation of discomfort and tolerability to bronchoscopy according to different sedation procedures with midazolam. Exp Ther Med 2015;10:659–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Soroceanu A, Burton DC, Oren JH, et al. Medical complications after adult spinal deformity surgery: incidence, risk factors, and clinical impact. Spine (Phila Pa 1976) 2016;41:1718–23. [DOI] [PubMed] [Google Scholar]
- [7].British Thoracic Society Bronchoscopy Guidelines Committee aSoSoCCoBTS. British Thoracic Society guidelines on diagnostic flexible bronchoscopy. Thorax 2001; 56 suppl 1:i1--21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Chen XK, Zhou YP, Zhang X, et al. Conscious sedation with midazolam and dezocine for diagnostic flexible bronchoscopy. Eur Rev Med Pharmacol Sci 2015;19:3688–92. [PubMed] [Google Scholar]
- [9].Du Rand IA, Barber PV, Goldring J, et al. Summary of the British Thoracic Society guidelines for advanced diagnostic and therapeutic flexible bronchoscopy in adults. Thorax 2011;66:1014–5. [DOI] [PubMed] [Google Scholar]
- [10].Szczeklik W, Andrychiewicz A, Gorka K, et al. Flexible bronchoscopy under conscious sedation with midazolam and fentanyl can be safely performed by nonanesthesiologists. Pol Arch Med Wewn 2015;125:869–71. [DOI] [PubMed] [Google Scholar]
- [11].Goneppanavar U, Magazine R, Periyadka Janardhana B, et al. Intravenous dexmedetomidine provides superior patient comfort and tolerance compared to intravenous midazolam in patients undergoing flexible bronchoscopy. Pulm Med 2015;2015:727530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Williams TJ, Bowie PE. Midazolam sedation to produce complete amnesia for bronchoscopy: 2 years’ experience at a district general hospital. Respir Med 1999;93:361–5. [DOI] [PubMed] [Google Scholar]
- [13].Clark G, Licker M, Younossian AB, et al. Titrated sedation with propofol or midazolam for flexible bronchoscopy: a randomised trial. Eur Respir J 2009;34:1277–83. [DOI] [PubMed] [Google Scholar]
- [14].Franzen D, Bratton DJ, Clarenbach CF, et al. Target-controlled versus fractionated propofol sedation in flexible bronchoscopy: a randomized noninferiority trial. Respirology 2016;21:1445–51. [DOI] [PubMed] [Google Scholar]
- [15].Ozturk T, Acikel A, Yilmaz O, et al. Effects of low-dose propofol vs ketamine on emergence cough in children undergoing flexible bronchoscopy with sevoflurane-remifentanil anesthesia: a randomized, double-blind, placebo-controlled trial. J Clin Anesth 2016;35:90–5. [DOI] [PubMed] [Google Scholar]
- [16].Stolz D, Kurer G, Meyer A, et al. Propofol versus combined sedation in flexible bronchoscopy: a randomised non-inferiority trial. Eur Respir J 2009;34:1024–30. [DOI] [PubMed] [Google Scholar]
- [17].Lichtenbelt BJ, Mertens M, Vuyk J. Strategies to optimise propofol-opioid anaesthesia. Clin Pharmacokinet 2004;43:577–93. [DOI] [PubMed] [Google Scholar]
- [18].von Ungern-Sternberg BS, Trachsel D, Zhang G, et al. Topical lidocaine does not exaggerate laryngomalacia in infants during flexible bronchoscopy under propofol anesthesia. J Bronchol Interv Pulmonol 2016;23:215–9. [DOI] [PubMed] [Google Scholar]
- [19].Yuan F, Fu H, Yang P, et al. Dexmedetomidine-fentanyl versus propofol-fentanyl in flexible bronchoscopy: a randomized study. Exp Ther Med 2016;12:506–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Graber RG. Propofol in the endoscopy suite: an anesthesiologist's perspective. Gastrointest Endosc 1999;49:803–6. [DOI] [PubMed] [Google Scholar]
- [21].Yoon HI, Kim JH, Lee JH, et al. Comparison of propofol and the combination of propofol and alfentanil during bronchoscopy: a randomized study. Acta Anaesthesiol Scand 2011;55:104–9. [DOI] [PubMed] [Google Scholar]
- [22].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Higgins JPT GS. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. 2011. Available at: www.cochrane-handbook.org. [Google Scholar]
- [24].Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17:1–2. [DOI] [PubMed] [Google Scholar]
- [25].Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta-analyses. Ann Intern Med 2001;135:982–9. [DOI] [PubMed] [Google Scholar]
- [26].Clarkson K, Power CK, O’Connell F, et al. A comparative evaluation of propofol and midazolam as sedative agents in fiberoptic bronchoscopy. Chest 1993;104:1029–31. [DOI] [PubMed] [Google Scholar]
- [27].Lo YL, Lin TY, Fang YF, et al. Feasibility of bispectral index-guided propofol infusion for flexible bronchoscopy sedation: a randomized controlled trial. PLoS One 2011;6:e27769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ozturk T, Cakan A, Gulerce G, et al. Sedation for fiberoptic bronchoscopy: fewer adverse cardiovascular effects with propofol than with midazolam. Anasthesiol Intensivmed Notfallmed Schmerzther 2004;39:597–602. [DOI] [PubMed] [Google Scholar]
- [29].Chrissian AA, Bedi H. Bronchoscopist-directed continuous propofol infusion for targeting moderate sedation during endobronchial ultrasound bronchoscopy: a practical and effective protocol. J Bronchology Interv Pulmonol 2015;22:226–36. [DOI] [PubMed] [Google Scholar]
- [30].Bower AL, Ripepi A, Dilger J, et al. Bispectral index monitoring of sedation during endoscopy. Gastrointest Endosc 2000;52:192–6. [DOI] [PubMed] [Google Scholar]
- [31].Miner JR, Biros MH, Seigel T, et al. The utility of the bispectral index in procedural sedation with propofol in the emergency department. Acad Emerg Med 2005;12:190–6. [DOI] [PubMed] [Google Scholar]
- [32].Vernon JM, Lang E, Sebel PS, et al. Prediction of movement using bispectral electroencephalographic analysis during propofol/alfentanil or isoflurane/alfentanil anesthesia. Anesth Analg 1995;80:780–5. [DOI] [PubMed] [Google Scholar]
- [33].Gan TJ, Glass PS, Windsor A, et al. Bispectral index monitoring allows faster emergence and improved recovery from propofol, alfentanil, and nitrous oxide anesthesia. BIS Utility Study Group. Anesthesiology 1997;87:808–15. [DOI] [PubMed] [Google Scholar]
- [34].Lysakowski C, Dumont L, Pellegrini M, et al. Effects of fentanyl, alfentanil, remifentanil and sufentanil on loss of consciousness and bispectral index during propofol induction of anaesthesia. Br J Anaesth 2001;86:523–7. [DOI] [PubMed] [Google Scholar]


