Abstract
Background:
Diabetic retinopathy (DR) was considered to be a common complication of diabetes. The purpose of the current study was to investigate the potential association between obesity and DR risk by conducting a meta-analysis of prospective studies.
Methods:
A consummate literature search of PubMed, EMBASE, and web of science was conducted until July 2016. A total of 13 prospective cohort studies were included in this meta-analysis.
Results:
On meta-analysis of all the studies assessing DR risk, obesity was associated with a significant increase in DR incidence (relative risk [RR], 1.20; 95% confidence interval [CI], 1.01–1.43; I2 = 59.6%). When only proliferative DR (PDR) was considered, no significant association between obesity and risk of PDR was detected. Significant harmful effect was detected in type 2 diabetes mellitus (T2DM) group (RR, 1.40; 95% CI, 1.05–1.87; I2 = 67.6%) but not mixed group (RR, 1.04; 95% CI, 0.97–1.18; I2 = 0.00%). No significant publication bias was detected in the selected 13 studies.
Conclusion:
Obesity was a risk factor for non-proliferative DR. However additional well-designed and well-conducted epidemiologic studies were required to deepen our understanding of the relation between obesity and DR.
Keywords: body mass index, diabetic retinopathy, meta-analysis, obesity, risk factor
1. Introduction
Both type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM) were considered as important burdens on public health system.[1] Diabetic retinopathy (DR), which was a common complication of diabetes, remained to be one of the leading preventable causes of visual impairment in the whole world.[2] Through intense glucose control, retinal laser photocoagulation and vitrectomy, the incidence of blindness caused by DR was significantly reduced. However, visual disability caused by DR would continue to be important issue in coming decades. To gain the most effective management of DR, early diagnosis and careful management would provide fundamental contribution. The detection of modifiable risk factors of DR had an important value on public health and clinical management. Epidemiological study could provide better knowledge on risk factors of DR and previous national population-based studies reported common risk factors for DR.[3] Longer diabetes duration, worse glucose control, hypertension, and tobacco smoking were considered generally established risk factors for DR.[4] Early management and intense treatment for patients with higher DR risks would provide better prognoses.
The impacts of obesity on carcinomas, cardiovascular, and metabolic systems disorders have been widespread and obese was regarded as a harmful factor in most diseases.[5,6] Considering that there was significant relation between obesity and diabetes risk, it was natural to consider the potential effect of obesity on the incidence of DR. Through a population-based study involving 6499 individuals with a follow-up of 11.1 years, it was found that obesity was associated with an increased risk of diabetes.[7] Besides, obesity was an established risk factor for several systemic diseases including hypertension, stroke, dyslipidemia, and sleep apnea,[8] and these diseases were reported as potential risk factors of DR.[9,10] The association between obesity and DR risk was reported in several previous observational studies, however, no accordant conclusions were obtained. Data of a hospital-based study with 156 diabetic persons showed that obesity may be considered as a risk factor for DR in T2DM patients.[11] While in a cross-sectional study using clinical data in Saudi National Diabetes Registry data with a cohort of 50,464 Saudi patients, overweight and obesity were reported to be associated with an inversed risk of DR after adjustments of age and sex.[12] Besides, a database analyses in UK demonstrated the body mass index (BMI) was not associated with retinopathy status in T2DM patients through multivariate logistic regression.[13] Meta-analysis is a useful statistical tool to gain a more powerful conclusion through pooling consubstantiate but independent studies together.[14] The purpose of this current meta-analysis was to investigate the relationship between obesity and DR risk of prospective cohorts. Besides, detailed analyses in this study would also provide certain suggestions for improvement of following study designs.
2. Methods
This systematic review and meta-analysis was conducted according to the Meta-analysis of Observational Studies in Epidemiology (MOOSE)[15] and reported following preferred reporting items for systematic reviews and meta-analyses (PRISMA)[16] guidelines. No ethics committee or institutional review board approvement was required in this study.
2.1. Search strategy
PubMed, EMBASE, and web of science were searched using selected key words regarding obesity and DR (last search update July 206). The key word group for obesity was composed by obesity, overweight, adiposity, body mass index, BMI, metabolic syndrome, intra-abdominal fat, waist–hip ratio, and waist circumference. No restrictions of language or publication data in literature search were applied. Besides, the reference lists of the selected articles were reviewed to identify additional eligible publications. When supplemental data were required, the contacts with corresponding authors of related studies were conducted if necessary.
2.2. Inclusion and exclusion criteria
For inclusion, the studies would be included if they met the following criteria: the association between obesity and DR was evaluated; a prospective cohort study design was adopted; the odds ratios (OR), relative risk (RR) with 95% confidence intervals (CI) or sufficient data to calculate them were reported. The exclusion criteria included: not cohort design was adopted; no available data in suitable format was reported.
2.3. Data extraction and quality scale
Two investigators (WZ and YW) extracted the data from each included publication independently. The extracted data included first author of each publication, country, age of participants, study period, publication year, diagnosis and deification of DR, exposure definition, adjustments or matched factors, RR value in each study. If both adjusted and non-adjusted data were provided, only the adjusted value was used. When stratified data were provided in the original article, only the highest/lowest group data were extracted and used in the calculation. If only OR was provided, the following formula was used to the calculate RR:
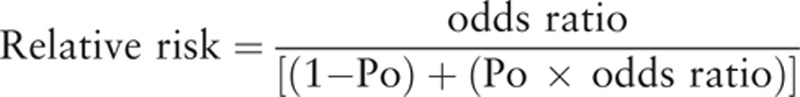 |
In the formula, Po was the incidence rate of DR in the nonexposed group. If only the primary data were reported, we converted it into RR value.
Considering the only prospective cohort studies were included in this study, Newcastle-Ottawa Scale (NOS) was obtained in the quality scale for each included study.[17] NOS was a quality assessment scale of methodological quality for both case-control and cohort studies. In this study, the selection, comparability, and exposure of each study were scored and the studies with NOS score over 6 stars were considered as relatively high quality. Any disagreements in data extraction, data reforming, and quality scale were resolved by discussion with the third reviewer (YFM).
3. Statistical analysis
Considering the observational design nature of the included studies, a random-effects model was used for the quantitative synthesis. As all the included studies were prospective cohort study, RR values were used to evaluate the associations between obesity and the risk of DR.
The test of heterogeneity in quantitative calculation across studies was carried out using both χ2 and I2 test. Because tests for heterogeneity lack power, an I2 value >50% or P < .1 in χ2 test was considered to show substantial heterogeneity. In this current meta-analysis, subgroup analyses by stratifying study characteristics (such as DM type, study site, and adjusting status) were conducted to detect the sources of heterogeneity. Potential publication bias would be detected by both the funnel plot analysis and Egger test.[18,19] All the statistical analyses were performed using STATA Version 12 (StataCorp Stata Statistical Software: release 12.0, College Station, TX).
4. Results
4.1. Literature search
A total of 4172 articles were detected from the 3 databases (1631 in PubMed, 2669 in EMBASE, and 412 in web of science). Besides, 22 additional records were detected through reviewing the reference lists of relevant articles. After the exclusion of 3707 articles with unrelated topics, the abstracts of 937 publications were reviewed for potential inclusion. Of the 937 remaining articles, 710 papers were excluded because they were case reports, reviews, and overlapped articles. Besides, the papers in which DR was not involved were also dropped through screening the abstracts. After reviewing the full text of the 227 remaining articles, a total of 13 prospective cohort studies were included in the final quantitative synthesis. Among the 214 excluded studies, 44 studies didn’t reported study designs equivocally, 166 studies didn’t report outcomes of interest with raw data, and 4 cohort studies were previously reported. In total, 13 publications between 1993 and 2016 were included in this current meta-analysis.[20–32] The flow diagram for the literature search identifying the relevant studies was present in Fig. 1.
Figure 1.
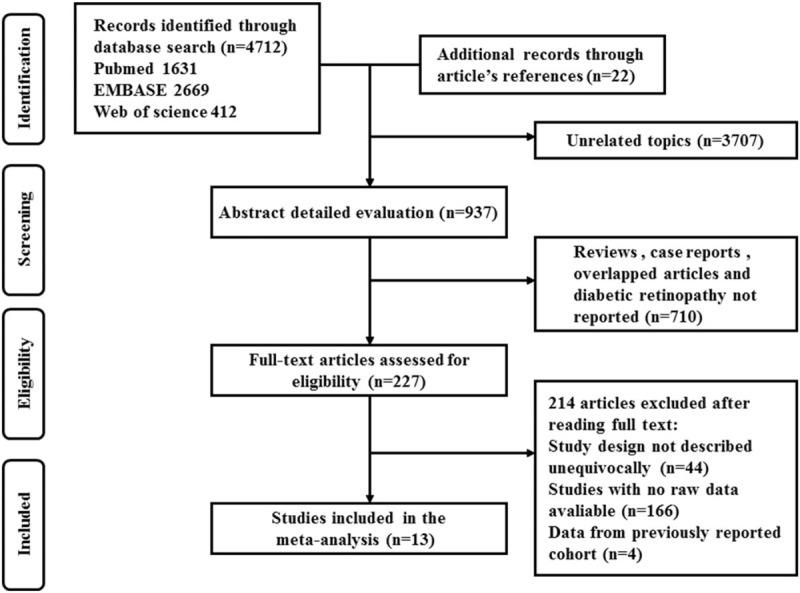
PRISMA diagram for identification of relevant studies. PRISMA = preferred reporting items for systematic reviews and meta-analyses.
4.2. Study characteristics
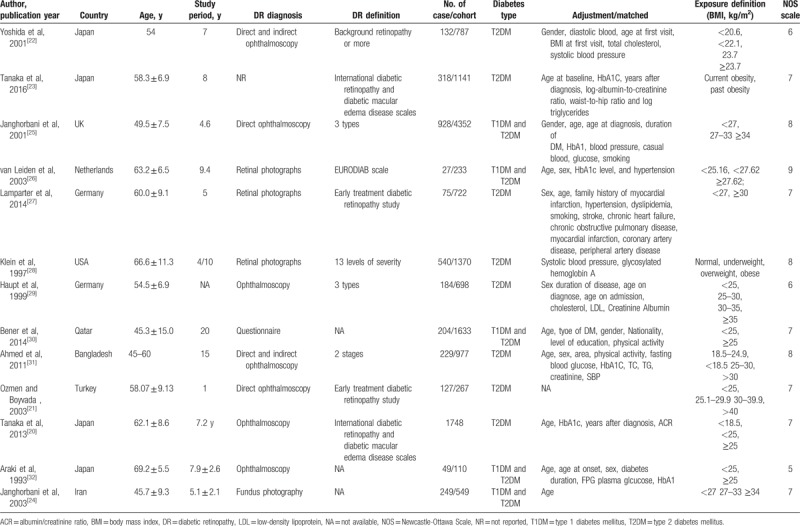
The main characteristics of all the included studies in this present analysis were showed in Table 1. In the all the included studies, a total of 14,575 participants were included in this meta-analysis. The range of publication date was between 1993 and 2016. When the diabetes type was considered, both T2DM and mixed types were included in the analysis. The follow-up duration among all the studies ranged from 1 year to 20 years. The sites where the studies had been carried out were as follows: 7 in Asia, 5 in Europe, and 1 in Americas. The methodological quality of each included study was assessed by NOS. NOS was designed for the quality assessment of observational studies and the maximum was 9 stars. The NOS scales for all the included studies ranged from 5 to 8 and the average score was 7.15 stars. The rate of high-quality (>6 stars) were present in most included studies (12 in 13).
Table 1.
Characteristics of studies of obesity and diabetic retinopathy risk included in the final analysis (n = 13).
4.3. Obesity and the risk of DR
Figure 2 demonstrated the effect of obesity on DR risk through pooling all the included studies in this meta-analysis. The analyses of the 13 included studies showed that obesity was a risk factor for the incidence of DR (RR, 1.20; 95% CI, 1.01–1.43; I2 = 59.6%).
Figure 2.
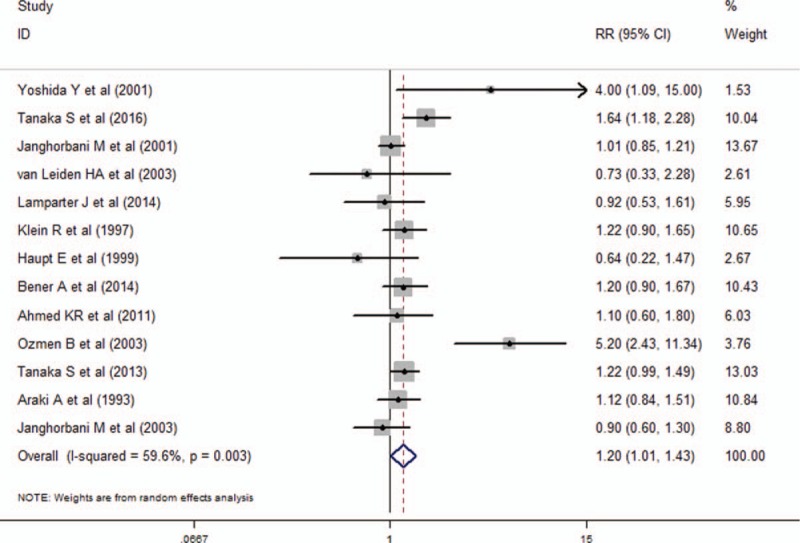
Forest plot for the association between obesity and DR risk. DR = diabetic retinopathy.
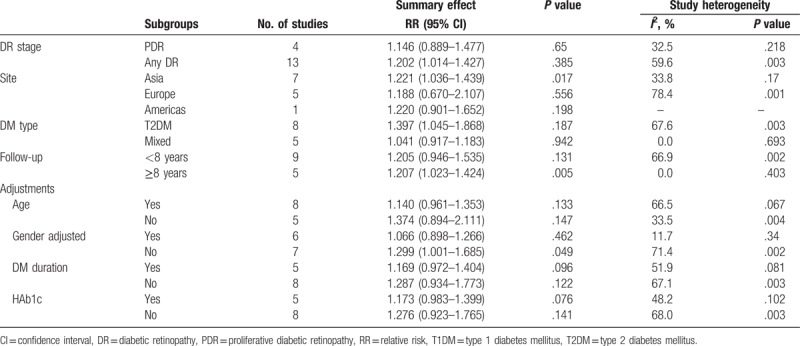
To deepen the understanding on the relationship between obesity and DR, the subgroup analyses by study characters and adjusting status were conducted (Table 2). When only proliferative DR (PDR) was considered, no significant association between obesity and risk of PDR was detected (RR, 1.15; 95% CI, 0.89–1.48; I2 = 32.5%). Considering that different subtypes of diabetes demonstrated diverse clinical manifestations, significant harmful effect was detected in T2DM group (RR, 1.40; 95% CI, 1.05–1.87; I2 = 67.6%) but not mix group (RR, 1.04; 95% CI, 0.97–1.18; I2 = 0.00%). Subgroup analysis by study sites showed that the studies in Asia demonstrated significant relationship between obesity and DR risk (RR, 1.22; 95% CI, 1.04–1.44; I2 = 33.8%). However, the studies conducted in neither Europe nor America demonstrated statistically significant association. Besides, it was in longer follow-up group (>8 years) but not shorter follow-up group (<8 years) demonstrated significant association. When the adjustment status was considered and no significant association was detected in subgroup meta-analysis by the adjustments of age, sex, diabetes duration, and HAb1c.
Table 2.
Summary relative risk (RR) and 95% confidence interval (CI) for subgroup meta-analysis by study designs and adjusting status.
4.4. Sensitivity analysis and publication bias
To assess the robustness of the conclusion in this study, we conducted a sensitivity analyses through dropping the studies with lower methodological quality (<6 stars in NOS). After excluding 1 study from the meta-analysis and it was found that obesity was a significant harmful factor for DR (RR, 1.22; 95% CI, 1.00–1.47; I2 = 62.9%).
To assess the publication bias, both visual inspection of funnel plots and Egger test were used. No significant publication bias was found in the selected 13 studies (Begg test, P = .180; Eegg test, P = .377). The funnel plot was symmetrical and presented in Fig. 3.
Figure 3.
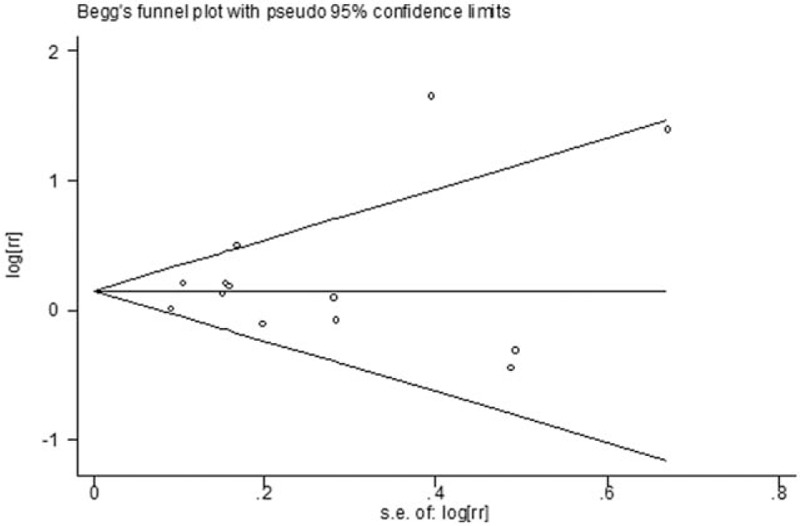
Funnel plot for assessment of publication bias.
5. Discussion
This meta-analysis of 13 prospective cohort studies on obesity for DR risk demonstrated the existence of a significant harmful effect for DR incidence. In general the conclusion was robust and no publication bias was detected. However, advanced analyses by study designs or adjusting statuses demonstrated no significant associations.
Obesity, which was a major public health problem, was reported to be associated with development of different diseases[33,34] and important cause of mortality in the whole world.[6,35] It also indicated that the relation between obesity and diabetes risk was significant.[36] However, inconsistent conclusions on the association between obesity and DR were detected in previous epidemiological studies. In a cross-sectional study including 501 adults with T1DM, it was found that obesity (BMI > 30 kg/m2) was the predominant risk factor for retinopathy.[37] While through multinomial logistic regression analyses in a cross-sectional clinic-based study, it was found that BMI was inversely associated with mild-moderate and severe DR. Thus, a higher BMI appeared to confer a protective effect on DR risk in Asian patients with T2DM.[38] Insignificant association between BMI and DR was also reported in previous cross-sectional studies.[39,40] Besides, case control studies were also conducted to detect the effect of obesity on DR. However, previous case-control studies also indicated inconsistent conclusions.[41,42] Cohort study design demonstrated stronger power in the detection of the risk factors. Besides, meta-analysis was a useful tool in epidemiological study. In this current meta-analysis, only prospective cohort studies were included and robust conclusion was gained. Through pooling 13 independent studies together, slight but significant harmful effect of obesity on the DR incidence was detected. Thus, the results in this study provided high level of evidence for the existence of the relationship between obesity and DR.
However, no significant association was detected in several advanced subgroup analyses and thus more detailed researches were required. When the diabetes types were considered, it was found significant association between obesity and DR in T2DM. While no previous prospective cohort study was conducted to detect the effect of obesity on the incidence of DR in T1DM cases. Even no stratified results on obesity and DR risk were reported, however, the relation between BMI and retinopathy incidence in T1DM cases was reported. A population-based cohort of 727 T1DM patients with 25 years follow-up, it was found that BMI was associated with DR in risk age and sex adjusted multivariate models.[43] A retrospective cohort consisting of 989 T1DM patients who were followed up for a mean of 10.1 years showed that BMI at baseline was not associated with the development of DR.[44] When time-dependent obesity along the follow-up duration was considered, BMI was associated with DR incidence. Besides, the occurrence of DR has been related to high BMI in the T1DM cases in a study in Sweden.[45] When diabetes of both subtypes were considered, it was found that obesity was associated with high risk of DR incidence in this meta-analysis. However, further advanced cohort analyses were required to gain more knowledge in the effect of obesity on the risk of DR.
In this study, the definition of obesity was based on the BMI. A previous cross-sectional clinic-based study showed that BMI was inversely associated with DR incidence while waist-to-hip ratio (WHR) was positively associated with retinopathy risk.[38] The interesting results of that study indicated that more clinical trials were required to determine whether WHR is a more clinically relevant risk marker than BMI for individuals with T2DM. The data from Sankara Nethralaya Diabetic Retinopathy Epidemiology and Molecular Genetics Study (SN-DREAMS-I) reported that increased WHR in women was associated with DR; higher BMI group had a protective role for any DR in the overall group through logistic regression analyses.[46] Among the 13 included prospective study, 2 studies reported the results based WHR while no significant association was detected (RR, 2.60; 95% CI, 0.332–20.390; I2 = 85.9%). In the other hand, WHR abnormality was a sign of metabolism syndrome and several previous studies indicated the relationship between metabolism syndrome and DR incidence. A case-control study with 2551 Chinese participants, it was found that metabolism syndrome was a strong and independent indicator of DR even to the same extent as glycemic control.[42] Thus, more advanced study should be conducted to detect the potential association.
The main strength of this study lied in the detailed literature search. Systematical literature strategy and a huge amount of full-text articles (over 200 publications) were reviewed in the literature searching progress. Besides, considering the higher evidence of cohort study design, only prospective cohort studies were included in this meta-analysis and selection bias could be ignored. However, there were also some limitations should be acknowledged in this meta-analysis. First, even a systematical literature was conducted in this study, the number of the included studies was small. The relatively small amount of included study might be influence the strength of the conclusion in this study. First, the conclusion of this study might be influenced by deficient criteria of obesity or different BMI stratifications among the included studies. In the meta-analysis, we adopted the highest versus lowest data in quantitative synthesis. Even no statistical heterogeneity was detected, the variability in the study design might influence the conclusion of this study. Second, we attempted to detect the dose–response relationship between obesity and DR risk. However, even significant association was detected, no sufficient data (including BMI stratification, pervasion-years data, and RR value in each group) could be extracted from most included studies. More well-designed studies with detailed data were required in the future. Third, the pooled risk estimate may be affected by 1 individual study and thus more related studies were required.
6. Conclusions
In conclusion, the findings in this current meta-analysis of prospective cohort studies suggest that obesity was a risk factor for non-proliferative DR. However, the significance could not be detected in several subgroup analysis and thus additional well-designed and well-conducted epidemiologic studies were required to deepen our understanding of the relation between obesity and DR risk. Advanced studies with more data on T1DM cases and different obesity definitions were urgent required.
Acknowledgments
The authors have none to acknowledge in this study.
Author contributions
Conceptualization: Jiong Lu.
Data curation: Wei Zhu, Yan Wu, Jiong Lu.
Formal analysis: Qian Xing.
Funding acquisition: Wei Zhu, Jian-Jun Tao.
Investigation: Qian Xing.
Methodology: Wei Zhu, Yan Wu, Yi-Fang Meng, Jian-Jun Tao, Jiong Lu.
Project administration: Wei Zhu.
Resources: Qian Xing, Jian-Jun Tao.
Software: Wei Zhu, Yi-Fang Meng.
Supervision: Yan Wu.
Validation: Wei Zhu, Yan Wu.
Writing – original draft: Wei Zhu, Yan Wu, Yi-Fang Meng, Qian Xing, Jian-Jun Tao, Jiong Lu.
Writing – review and editing: Qian Xing.
Footnotes
Abbreviations: BMI = body mass index, CI = confidence intervals, DR = diabetic retinopathy, MOOSE = Meta-analysis of Observational Studies in Epidemiology, NOS = Newcastle-Ottawa Scale, OR = odds ratios, PDR = proliferative diabetic retinopathy, PRISMA = preferred reporting items for systematic reviews and meta-analyses, RR = relative risk, T1DM = type 1 diabetes mellitus, T2DM = type 2 diabetes mellitus, WHR = waist-to-hip ratio.
WZ and YW have contributed equally to this work.
Funding: This work was supported in whole or in part, by the Project supported by the National Science Foundation for Young Scientists of China (Grant No. 81700804), Foundation for Young Scholars of Suzhou, China (Grant No. kjxw2015044), and Foundation for Young Medical Talents of Jiangsu Province, 2016 (Grant No. QNRC2016211). The sponsor or funding organization had no role in the design or conduct of this research.
The authors declare that they have no competing interests.
References
- [1].Macdonald GC, Campbell LV. Mental illness: the forgotten burden on diabetes populations? Lancet 2016;388:561. [DOI] [PubMed] [Google Scholar]
- [2].Burdon KP, Fogarty RD, Shen W, et al. Genome-wide association study for sight-threatening diabetic retinopathy reveals association with genetic variation near the GRB2 gene. Diabetologia 2015;58:2288–97. [DOI] [PubMed] [Google Scholar]
- [3].Buch H, Vinding T, La Cour M, et al. Prevalence and causes of visual impairment and blindness among 9980 Scandinavian adults: the Copenhagen City Eye Study. Ophthalmology 2004;111:53–61. [DOI] [PubMed] [Google Scholar]
- [4].Sabanayagam C, Yip W, Ting DS, et al. Ten emerging trends in the epidemiology of diabetic retinopathy. Ophthalmic Epidemiol 2016;23:209–22. [DOI] [PubMed] [Google Scholar]
- [5].Chen GC, Chen SJ, Zhang R, et al. Central obesity and risks of pre- and postmenopausal breast cancer: a dose-response meta-analysis of prospective studies. Obes Rev 2016;17:1167–77. [DOI] [PubMed] [Google Scholar]
- [6].Yang HK, Han K, Kwon HS, et al. Obesity, metabolic health, and mortality in adults: a nationwide population-based study in Korea. Sci Rep 2016;6:30329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Dhana K, Nano J, Ligthart S, et al. Obesity and life expectancy with and without diabetes in adults aged 55 years and older in the Netherlands: a prospective cohort study. PLoS Med 2016;13:e1002086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Rush T, LeardMann CA, Crum-Cianflone NF. Obesity and associated adverse health outcomes among US military members and veterans: findings from the millennium cohort study. Obesity (Silver Spring) 2016;24:1582–9. [DOI] [PubMed] [Google Scholar]
- [9].Zheng Y, Lamoureux EL, Lavanya R, et al. Prevalence and risk factors of diabetic retinopathy in migrant Indians in an urbanized society in Asia: the Singapore Indian eye study. Ophthalmology 2012;119:2119–24. [DOI] [PubMed] [Google Scholar]
- [10].Leong WB, Jadhakhan F, Taheri S, et al. Effect of obstructive sleep apnoea on diabetic retinopathy and maculopathy: a systematic review and meta-analysis. Diabet Med 2016;33:158–68. [DOI] [PubMed] [Google Scholar]
- [11].Katusic D, Tomic M, Jukic T, et al. Obesity--a risk factor for diabetic retinopathy in type 2 diabetes? Coll Antropol 2005;29(suppl):47–50. [PubMed] [Google Scholar]
- [12].Al-Rubeaan K, Abu El-Asrar AM, Youssef AM, et al. Diabetic retinopathy and its risk factors in a society with a type 2 diabetes epidemic: a Saudi National Diabetes Registry-based study. Acta Ophthalmol 2015;93:e140–7. [DOI] [PubMed] [Google Scholar]
- [13].Kostev K, Rathmann W. Diabetic retinopathy at diagnosis of type 2 diabetes in the UK: a database analysis. Diabetologia 2013;56:109–11. [DOI] [PubMed] [Google Scholar]
- [14].Zhu W, Wu Y, Xu M, et al. Antiphospholipid antibody and risk of retinal vein occlusion: a systematic review and meta-analysis. PLoS One 2014;10:e0122814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008–12. [DOI] [PubMed] [Google Scholar]
- [16].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. Open Med 2009;3:e123–30. [PMC free article] [PubMed] [Google Scholar]
- [17].Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- [18].Stuck AE, Rubenstein LZ, Wieland D. Bias in meta-analysis detected by a simple, graphical test. Asymmetry detected in funnel plot was probably due to true heterogeneity. BMJ 1998;316:469author reply 470-1. [PMC free article] [PubMed] [Google Scholar]
- [19].Stanley TD, Massey S. Evidence of nicotine replacement's effectiveness dissolves when meta-regression accommodates multiple sources of bias. J Clin Epidemiol 2016;79:41–5. [DOI] [PubMed] [Google Scholar]
- [20].Tanaka S, Tanaka S, Iimuro S, et al. Predicting macro- and microvascular complications in type 2 diabetes: the Japan Diabetes Complications Study/the Japanese Elderly Diabetes Intervention Trial risk engine. Diabetes Care 2013;36:1193–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ozmen B, Boyvada S. The relationship between self-monitoring of blood glucose control and glycosylated haemoglobin in patients with type 2 diabetes with and without diabetic retinopathy. J Diabetes Complications 2003;17:128–34. [DOI] [PubMed] [Google Scholar]
- [22].Yoshida Y, Hagura R, Hara Y, et al. Risk factors for the development of diabetic retinopathy in Japanese type 2 diabetic patients. Diabetes Res Clin Pract 2001;51:195–203. [DOI] [PubMed] [Google Scholar]
- [23].Tanaka S, Tanaka S, Iimuro S, et al. Maximum BMI and microvascular complications in a cohort of Japanese patients with type 2 diabetes: the Japan Diabetes Complications Study. J Diabetes Complications 2016;30:790–7. [DOI] [PubMed] [Google Scholar]
- [24].Janghorbani M, Amini M, Ghanbari H, et al. Incidence of and risk factors for diabetic retinopathy in Isfahan, Iran. Ophthalmic Epidemiol 2003;10:81–95. [DOI] [PubMed] [Google Scholar]
- [25].Janghorbani M, Jones RB, Murray KJ, et al. Incidence of and risk factors for diabetic retinopathy in diabetic clinic attenders. Ophthalmic Epidemiol 2001;8:309–25. [DOI] [PubMed] [Google Scholar]
- [26].van Leiden HA, Dekker JM, Moll AC, et al. Risk factors for incident retinopathy in a diabetic and nondiabetic population: the Hoorn study. Arch Ophthalmol 2003;121:245–51. [DOI] [PubMed] [Google Scholar]
- [27].Lamparter J, Raum P, Pfeiffer N, et al. Prevalence and associations of diabetic retinopathy in a large cohort of prediabetic subjects: the Gutenberg Health Study. J Diabetes Complications 2014;28:482–7. [DOI] [PubMed] [Google Scholar]
- [28].Klein R, Klein BE, Moss SE. Is obesity related to microvascular and macrovascular complications in diabetes? The Wisconsin Epidemiologic Study of Diabetic Retinopathy. Arch Intern Med 1997;157:650–6. [PubMed] [Google Scholar]
- [29].Haupt E, Benecke A, Haupt A, et al. The KID Study VI: diabetic complications and associated diseases in younger type 2 diabetics still performing a profession. Prevalence and correlation with duration of diabetic state, BMI and C-peptide. Exp Clin Endocrinol Diabetes 1999;107:435–41. [DOI] [PubMed] [Google Scholar]
- [30].Bener A, Al-Laftah F, Al-Hamaq AO, et al. A study of diabetes complications in an endogamous population: an emerging public health burden. Diabetes Metab Syndr 2014;8:108–14. [DOI] [PubMed] [Google Scholar]
- [31].Ahmed KR, Karim MN, Bukht MS, et al. Risk factors of diabetic retinopathy in Bangladeshi type 2 diabetic patients. Diabetes Metab Syndr 2011;5:196–200. [DOI] [PubMed] [Google Scholar]
- [32].Araki A, Ito H, Hattori A, et al. Risk factors for development of retinopathy in elderly Japanese patients with diabetes mellitus. Diabetes Care 1993;16:1184–6. [DOI] [PubMed] [Google Scholar]
- [33].Valent AM, Hall ES, DeFranco EA. The influence of obesity on perinatal outcomes in pregnancies achieved with assisted reproductive technology: a population-based retrospective cohort study. Obstet Med 2016;9:34–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Masumoto S, Terao A, Yamamoto Y, et al. Non-absorbable apple procyanidins prevent obesity associated with gut microbial and metabolomic changes. Sci Rep 2016;6:31208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Sun H, Ren X, Chen Z, et al. Association between body mass index and mortality in a prospective cohort of Chinese adults. Medicine (Baltimore) 2016;95:e4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Zeng Q, Dong SY, Wang ML, et al. Obesity and novel cardiovascular markers in a population without diabetes and cardiovascular disease in China. Prev Med 2016;91:62–9. [DOI] [PubMed] [Google Scholar]
- [37].Price SA, Gorelik A, Fourlanos S, et al. Obesity is associated with retinopathy and macrovascular disease in type 1 diabetes. Obes Res Clin Pract 2014;8:e178–82. [DOI] [PubMed] [Google Scholar]
- [38].Man RE, Sabanayagam C, Chiang PP, et al. Differential association of generalized and abdominal obesity with diabetic retinopathy in Asian patients with type 2 diabetes. JAMA Ophthalmol 2016;134:251–7. [DOI] [PubMed] [Google Scholar]
- [39].Maghbooli Z, Pasalar P, Keshtkar A, et al. Predictive factors of diabetic complications: a possible link between family history of diabetes and diabetic retinopathy. J Diabetes Metab Disord 2014;13:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Wang J, Chen H, Zhang H, et al. The performance of a diabetic retinopathy risk score for screening for diabetic retinopathy in Chinese overweight/obese patients with type 2 diabetes mellitus. Ann Med 2014;46:417–23. [DOI] [PubMed] [Google Scholar]
- [41].Khamseh ME, Safarnejad B, Baradaran HR. The effect of captopril on progression of retinopathy in type 2 diabetes. Diabetes Technol Ther 2009;11:711–5. [DOI] [PubMed] [Google Scholar]
- [42].Gao L, Xin Z, Yuan MX, et al. High prevalence of diabetic retinopathy in diabetic patients concomitant with metabolic syndrome. PLoS One 2016;11:e0145293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Grauslund J, Green A, Sjolie AK. Prevalence and 25 year incidence of proliferative retinopathy among Danish type 1 diabetic patients. Diabetologia 2009;52:1829–35. [DOI] [PubMed] [Google Scholar]
- [44].Forga L, Goni MJ, Ibanez B, et al. Influence of age at diagnosis and time-dependent risk factors on the development of diabetic retinopathy in patients with Type 1 diabetes. J Diabetes Res 2016;2016:9898309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Henricsson M, Nystrom L, Blohme G, et al. The incidence of retinopathy 10 years after diagnosis in young adult people with diabetes: results from the nationwide population-based Diabetes Incidence Study in Sweden (DISS). Diabetes Care 2003;26:349–54. [DOI] [PubMed] [Google Scholar]
- [46].Raman R, Rani PK, Gnanamoorthy P, et al. Association of obesity with diabetic retinopathy: Sankara Nethralaya Diabetic Retinopathy Epidemiology and Molecular Genetics Study (SN-DREAMS Report no. 8). Acta Diabetol 2010;47:209–15. [DOI] [PubMed] [Google Scholar]


