Abstract
The treatment of advanced triple-negative breast cancer, which failed in first-line or second-line therapy, is a significant challenge. We conducted this retrospective study to explore the efficacy and safety of apatinib and capecitabine as the third-line treatment for advanced triple-negative breast cancer.
This retrospective study involved 44 advanced triple-negative breast cancer patients who failed in first-line or second-line therapy in Tangshan People's Hospital from January 2016 to February 2017. Twenty-two patients received apatinib and capecitabine, while 22 patients were treated with capecitabine monotherapy as third-line therapy. The progression-free survival (PFS), objective response rate (ORR), disease control rate (DCR), and adverse events were compared between 2 groups.
The apatinib and capecitabine group exhibited a higher PFS than capecitabine group (P = .001). Meanwhile, ORR and DCR in apatinib and capecitabine group were better than in capecitabine group (P = .042; .016). The 2 groups showed no significant difference in adverse events except degree I-II bleeding (P = .021). Both the apatinib and capecitabine and the capecitabine regimens revealed good tolerability.
The apatinib and capecitabine regimen can achieve a better efficacy and similar serious adverse events compared with capecitabine regimen as the third-line treatment for advanced triple-negative breast cancer.
Keywords: advanced triple-negative breast cancer, apatinib, capecitabine, third-line therapy
1. Introduction
Breast cancer is one of the most commonly diagnosed cancers and the leading cause of cancer death among females worldwide.[1] Triple-negative breast cancer (TNBC) is a special type of breast cancer, which lacks expression of estrogen receptor (ER), progesterone receptor (PR), and HER2 gene. TNBC accounts for 15% to 20% of newly diagnosed BC cases.[2] Because of lacking recognized therapeutic molecular biology targets, the treatment of TNBC is particularly challenging. Unfortunately, TNBC is highly proliferative and aggressiveness, rapid disease progression, high risk for recurrence, and poor prognosis.[3] Chemotherapy is the mainstay of TNBC's treatment at present,[4] and novel strategies and drugs are urgently needed.
Angiogenesis is one of the hallmarks of cancer,[5] which is vital for tumor growth, development, and metastasis. Anti-angiogenesis is an important anticancer strategy.[6] Vascular endothelial growth factor (VEGF) signaling plays an important role in angiogenesis via activation of VEGF receptor (VEGFR).[6] The VEGFR family involves 3 molecular subtypes (VEGFR-1, VEGFR-2, and VEGFR-3), which are type-II transmembrane proteins characterized by a tyrosine kinase (TK) activity.[7] Among them, VEGFR-2 is the majorly implicated in the pathological overformation of blood vessels in the context of several solid tumors.[8]
Apatinib is a novel small molecule receptor TK inhibitor selectively targeting VEGFR 2 (VEGFR-2).[9] Recently, apatinib has demonstrated its satisfying efficacy on various types of cancers such as gastric cancer,[10] breast cancer,[11] and nasopharyngeal carcinoma.[12] At the same time, it also has shown acceptable toxicities.
Capecitabine (Xeloda) is an oral fluoropyrimidine carbamate that undergoes sequential conversion to 5-fluorouracil (5-FU).[13] The conversion to 5-FU occurs in several steps, and the final enzyme in the pathway is thymidine phosphorylase, which is located at much higher concentrations in tumor tissue than in normal tissues, the active form of the drug is mainly present at the tumor site.[14] In addition, capecitabine typically lacks cumulative toxicity with prolonged use, so is suitable for long-term administration. A number of studies have indicated that capecitabine is effective for the treatment of metastatic breast cancer as a single agent or as part of a combination regimen.[15,16] However, there is no report on the efficacy and safety of combination therapy of apatinib and capecitabine in the treatment of advanced TNBC as the third-line treatment.
In this retrospective study, we aimed to explore the efficacy and safety of apatinib and capecitabine as the third-line treatment for advanced TNBC.
2. Patients and methods
2.1. Patients
This study was a retrospective analysis, which included 44 advanced TNBC patients who failed in first-line or second-line therapy in Tangshan People's Hospital from January 2016 to February 2017. Among them, 22 patients received apatinib and capecitabine as third-line therapy, while another 22 patients treated with capecitabine monotherapy served as control. The 2 groups were matched in age, Karnofsky performance score (KPS), metastatic site, and history of chemotherapy. The inclusion criteria were as follows: age ≥18 years; definite pathological diagnosis; failure of first-line or second-line; KPS≥80; and life expectancy ≥3 months.
2.2. Drug administration
In apatinib and capecitabine group, apatinib, 500 mg, was orally administered daily on days 1 through 28 of each 4-week cycle. Capecitabine, at 12,500 mg/m2, was orally taken twice daily for 14 days followed by a 7-day rest period until disease progression. In capecitabine group, an oral dose of capecitabine 1250 mg/m2 was taken twice daily for 14 days followed by a 7-day rest period until disease progression.
2.3. Efficacy and safety assessments
The primary end point of our study was PFS, defined as the time from enrollment to documented tumor progression with computed tomography (CT)/magnetic resonance imaging (MRI) scan or death as a result of any cause, whichever occurred first. The secondary end points were ORR (CR +PR), DCR (CR+PR+SD), and toxicity.
Treatment efficacy was evaluated in accordance with the Response Evaluation Criteria in Solid Tumors, which were classified into complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). Adverse events were assessed and graded according to the National Cancer Institute Common Toxicity Criteria version 3.0 and classified as degree 0∼ IV.
2.4. Ethical statement
The study was approved by the institutional ethics committee of Tangshan People's Hospital. All procedures performed in this study were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
2.5. Statistical analysis
Pearson x2 test or Fisher exact test was used to analyze the baseline characteristics, treatment efficacy, and adverse events. The t test was used to compare the clinical parameters between both groups. Survival analysis was done according to Kaplan–Meier method. Data were expressed as the mean ± standard deviation and P < .05 was considered statistically significant. The SPSS (Statistical Package for the Social Science, SPSS Inc., Chicago, IL) version 16.0 or GraphPad Prism (GraphPad Software, San Diego, CA) version 5.0 was used for all statistical analyses.
3. Results
3.1. Baseline characteristics
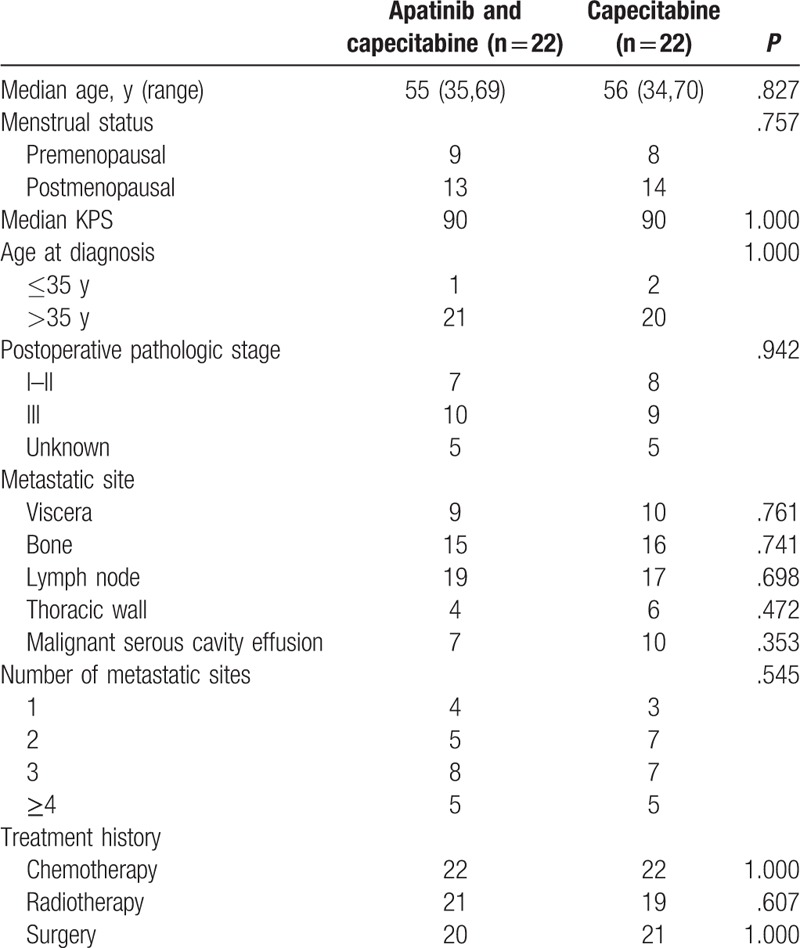
This retrospective cohort involved 44 advanced TNBC patients fulfilling the inclusion criteria during the period from January 2016 till February 2017. Among them, 22 patients received apatinib and capecitabine, while 22 patients received capecitabine monotherapy. The characteristics of the patients are summarized in Table 1. There was no significant difference in age, KPS, and disease characteristics between the apatinib and capecitabine group and the capecitabine group.
Table 1.
Characteristics of patients with advanced triple-negative breast cancer patients.
3.2. Treatment efficacy
Median follow-up time was 5 months (range, 2–11 months). The median PFS time of patients in apatinib and capecitabine group was 5.5 months, and for the capecitabine group, was 3.5 months (Fig. 1). The apatinib and capecitabine group exhibited a higher PFS than capecitabine group [P = .001, hazard ratio (HR) = 0.2583, 95% confidence interval (95% CI) 0.1150–0.5803].
Figure 1.
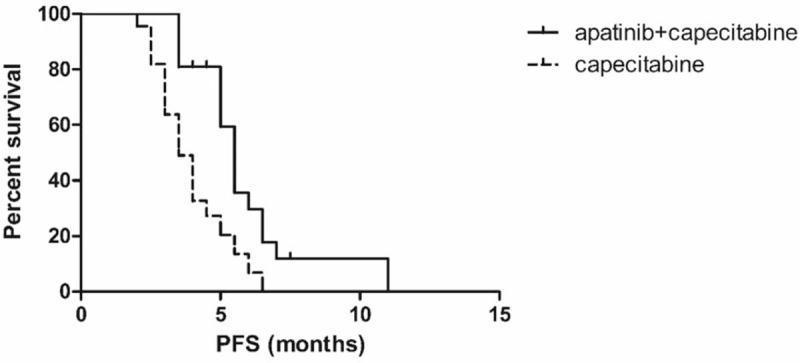
Kaplan–Meier curves of progression-free survival (PFS). The median PFS time of patients in apatinib and capecitabine group was 5.5 months, and for the capecitabine group was 3.5 months (P = .001).
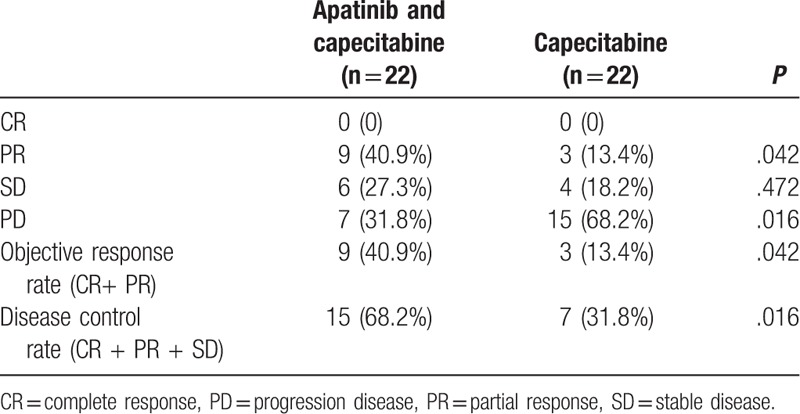
Of the total 44 patients, no patient was rated as having CR. In apatinib and capecitabine group, 9 patients (40.9%) were rated as having a PR, 6 patients (27.3%) were rated as having a SD, and 7 patients (31.8%) had a PD. ORR was 40.9%, and DCR was 68.2%. In capecitabine group, 3 patients (13.4%) were rated as having a PR, 4 patients (18.2%) were rated as having a SD, and 15 patients (68.2%) had a PD. ORR was 13.4%, and DCR was 31.8%. The ORR and DCR in apatinib and capecitabine group were better than in capecitabine group (P = .042; .016). There was a significant difference between the 2 groups. The apatinib and capecitabine regimen had a better efficacy than capecitabine regimen. The clinical response rates are listed in the Table 2.
Table 2.
Comparison of efficacy between the apatinib and capecitabine group and the capecitabine group (n, %).
3.3. Adverse events
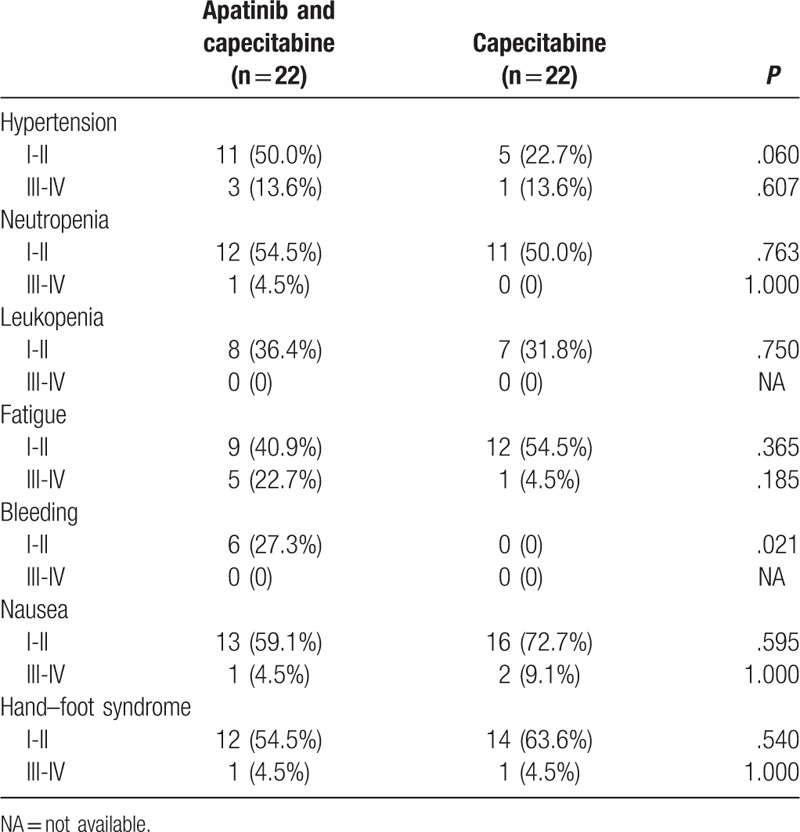
Previous studies have reported that the main toxicities of apatinib were hypertension, proteinuria, liver dysfunction, hand–foot syndrome, thrombocytopenia, leukopenia, and neutropenia, which generally manageable.[11,17] As presented in Table 3, the most common adverse events in the patients were hypertension, neutropenia, leukopenia, fatigue, bleeding, nausea, and hand–foot syndrome. Among the 2 groups, most of adverse events were degree I-II, which were not serious. There was no significant difference in the adverse events such as hypertension, neutropenia, leukopenia, fatigue, nausea, hand–foot syndrome, or degree III-IV adverse event of bleeding between the 2 groups. Although the apatinib and capecitabine group showed higher incidence of degree I-II bleeding than the capecitabine group (P = .021), this degree I-II adverse event was manageable and not serious, which had no clinical significance.
Table 3.
Comparison of adverse events between the apatinib and capecitabine group and the capecitabine group (n, %).
4. Discussion
TNBC is a heterogeneous disease and associated with a higher risk of early relapse with visceral metastasis.[18] Because of uncommonness, aggressiveness, and impressive heterogeneity, the treatment of TNBC remains a major clinical challenge,[19] especially for the patients who failed in first-line or second-line therapy. Although a great deal of therapies have been developed to specific molecular targets, such as poly-(ADP-ribose)-polymerase inhibitors, epidermal growth factor receptor inhibitors, Src TK inhibitors, and mTOR inhibitors,[20] chemotherapy is still the mainstay of treatment.
Capecitabine has been approved for the treatment of breast cancer,[21] and it is widely used in metastatic breast cancer. It has been proved effective for the treatment of metastatic breast cancer in a number of Phase I, Phase II, and Phase III trials.[22–24] A retrospective study including 363 patients with TNBC treated with capecitabine demonstrated that capecitabine is a treatment option for patients with TNBC in advanced disease including first-line and second/third-line.[25]
Apatinib is an oral, highly potent TK inhibitor targeting VEGFR2. It Antitumor activity has been demonstrated across a broad range of malignancies, including gastric, colorectal, and breast cancer, and good tolerability in phase I study conducted in former studies.[26,27] A nonclinical trial has confirmed the encouraging efficacy and manageable safety of apatinib on pretreated metastatic breast cancer patients.[17] In a multicenter phase II study, apatinib has also been evaluated the efficacy and safety in heavily pretreated patients with metastatic TNBC.[11]
In this study, the advanced TNBC patients who failed in first-line or second-line therapy received apatinib and capecitabine or capecitabine monotherapy as the third-line treatment. The apatinib and capecitabine group exhibited a higher PFS than the capecitabine group (P = .001). A retrospective study with 363 TNBC patients showed that capecitabine monotherapy achieved a ORR of 21% and a DCR of 33%[25]; our study had a similar efficacy with it and the ORR in capecitabine group was 13.4%, and the DCR in capecitabine group was 31.8%. Although in apatinib and capecitabine group, it was 40.9% and 68.2%. The ORR and DCR in apatinib and capecitabine group were higher than that in the capecitabine group (P = .042; .016), which demonstrated that advanced TNBC patients with apatinib and capecitabine had a better efficacy than those with capecitabine monotherapy.
Between the 2 groups, the most common adverse events were nausea, hand–foot syndrome, fatigue, neutropenia, and leukopenia. We found no significant difference in the above adverse events, while the capecitabine group showed significantly lower incidence of degree I-II bleeding than the apatinib and capecitabine group (P = .021). However, the minor bleeding events (degree I-II) were manageable; as a result, there was no clinical significance. At the same time, no serious bleeding (degree III-IV) occurred in both groups. Overall, compared with capecitabine monotherapy, apatinib and capecitabine did not increase serious adverse events. Both the apatinib and capecitabine and the capecitabine regimens showed good tolerability.
In summary, the results of our present study, although retrospective, support that third-line treatment of advanced TNBC with the apatinib and capecitabine regimen showed better efficacy and similar serious adverse events compared with capecitabine regimen. Given the limited treatment options for advanced TNBC patients who failed in first-line or second-line therapy, apatinib and capecitabine regimen may be one of the more effective treatments. However, our study is a single-center retrospective study with a small sample size, and more prospective studies with a large sample size are needed to verify the results of our study.
Acknowledgments
We greatly thank the patients, investigators, and participating institutions.
Author contributions
Conceptualization: Yi Hui Li, Jian Gong Wang, Xiao Hong Wang.
Data curation: Yang Zhou, Yu Wei Wang, Jian Gong Wang, Xiao Hong Wang.
Formal analysis: Yang Zhou, Yu Wei Wang, Jian Gong Wang, Xiao Hong Wang.
Investigation: Yi Hui Li, Yu Wei Wang, Ling Tong, Run Xue Jiang, Jing Qi Feng.
Methodology: Yang Zhou, Lei Xiao.
Resources: Yi Hui Li, Yu Wei Wang, Ling Tong, Run Xue Jiang, Lei Xiao, Shu Shan Xing.
Software: Shu Shan Xing, Jing Qi Feng.
Supervision: Ling Tong, Guang Ju Zhang, Fang Qian.
Validation: Fang Qian, Ya Ling Zhao.
Visualization: Guang Ju Zhang, Ya Ling Zhao.
Writing – original draft: Yi Hui Li, Yang Zhou.
Writing – review & editing: Jian Gong Wang, Xiao Hong Wang.
Footnotes
Abbreviations: 5-FU = 5-fluorouracil, CR = complete response, KPS = Karnofsky performance score, PD = progressive disease, PFS = progression free survival, PR = partial response, SD = stable disease, TNBC = triple-negative breast cancer.
Y-HL, YZ and Y-WW contributed equally to this work.
Funding/support: The research was supported by Science and Technology Program of Hebei, China, 1627777232.
The authors declare no conflict of interest.
References
- [1].2018;Siegel RL, Miller KD, Jemal A. Cancer statisticsJT CA Cancer J Clin. 68:7–30. [DOI] [PubMed] [Google Scholar]
- [2].Hudis CA, Gianni L. Triple-negative breast cancer: an unmet medical need. Oncologist 2011;16(suppl 1):1–1. [DOI] [PubMed] [Google Scholar]
- [3].Kassam F, Enright K, Dent R, et al. Survival outcomes for patients with metastatic triple-negative breast cancer: implications for clinical practice and trial design. Clin Breast Cancer 2009;9:29–33. [DOI] [PubMed] [Google Scholar]
- [4].Chacón RD, Costanzo MV. Triple-negative breast cancer 2010;12(suppl 2):S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646–74. [DOI] [PubMed] [Google Scholar]
- [6].Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol 2005;23:1011–27. [DOI] [PubMed] [Google Scholar]
- [7].Young HS, Summers AM, Bhushan M, et al. Single-nucleotide polymorphisms of vascular endothelial growth factor in psoriasis of early onset. J Invest Dermatol 2004;122:209–15. [DOI] [PubMed] [Google Scholar]
- [8].Roviello G, Ravelli A, Polom K, et al. Apatinib: a novel receptor tyrosine kinase inhibitor for the treatment of gastric cancer. Cancer Lett 2016;372:187–91. [DOI] [PubMed] [Google Scholar]
- [9].Tian S, Quan H, Xie C, et al. YN968D1 is a novel and selective inhibitor of vascular endothelial growth factor receptor-2 tyrosine kinase with potent activity in vitro and in vivo. Cancer Sci 2011;102:1374–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Li J, Qin S, Xu J, et al. Apatinib for chemotherapy-refractory advanced metastatic gastric cancer: results from a randomized, placebo-controlled, parallel-arm, phase II trial. J Clin Oncol 2013;31:3219–25. [DOI] [PubMed] [Google Scholar]
- [11].Hu X, Zhang J, Xu B, et al. Multicenter phase II study of apatinib, a novel VEGFR inhibitor in heavily pretreated patients with metastatic triple-negative breast cancer. Int J Cancer 2014;135:1961–9. [DOI] [PubMed] [Google Scholar]
- [12].Peng QX, Han YW, Zhang YL, et al. Apatinib inhibits VEGFR-2 and angiogenesis in an in vivo murine model of nasopharyngeal carcinoma. Oncotarget 2017;8:52813–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kaufman PA, Awada A, Twelves C, et al. Phase III open-label randomized study of eribulin mesylate versus capecitabine in patients with locally advanced or metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol 2015;33:594–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ishikawa T, Utoh M, Sawada N, et al. Tumor selective delivery of 5-fluorouracil by capecitabine, a new oral fluoropyrimidine carbamate, in human cancer xenografts. Biochem Pharmacol 1998;55:1091–7. [DOI] [PubMed] [Google Scholar]
- [15].Kusama M, Nomizu T, Aogi K, et al. Phase II study of 4-weekly capecitabine monotherapy in advanced/metastatic breast cancer. Breast Cancer 2010;17:233–40. [DOI] [PubMed] [Google Scholar]
- [16].Chan A, Verrill M. Capecitabine and vinorelbine in metastatic breast cancer. Eur J Cancer 2009;45:2253–65. [DOI] [PubMed] [Google Scholar]
- [17].Lin Y, Wu Z, Zhang J, et al. Apatinib for metastatic breast cancer in non-clinical trial setting: satisfying efficacy regardless of previous anti-angiogenic treatment. Tumor Biol 2017;39:1010428317711033. [DOI] [PubMed] [Google Scholar]
- [18].Khosravi-Shahi P, Cabezón-Gutiérrez L, Custodio-Cabello S. Metastatic triple negative breast cancer: optimizing treatment options, new and emerging targeted therapies. Asia Pac J Clin Oncol 2018;14:32–9. [DOI] [PubMed] [Google Scholar]
- [19].Zhu GQ, Shi KQ, You J, et al. Systematic review with network meta-analysis: adjuvant therapy for resected biliary tract cancer. Aliment Pharmacol Ther 2014;40:759–70. [DOI] [PubMed] [Google Scholar]
- [20].Khosravi-Shahi P, Cabezon-Gutierrez L, Custodio-Cabello S. Metastatic triple negative breast cancer: optimizing treatment options, new and emerging targeted therapies. Asia Pac J Clin Oncol 2018;14:32–9. [DOI] [PubMed] [Google Scholar]
- [21].Kaklamani VG, Gradishar WJ. Role of capecitabine (Xeloda) in breast cancer. Expert Rev Anticancer Ther 2003;3:137–44. [DOI] [PubMed] [Google Scholar]
- [22].Oshaughnessy JA, Blum J, Moiseyenko V, et al. Randomized, open-label, phase II trial of oral capecitabine (Xeloda) vs. a reference arm of intravenous CMF (cyclophosphamide, methotrexate and 5-fluorouracil) as first-line therapy for advanced/metastatic breast cancer. Ann Oncol 2001;12:1247–54. [DOI] [PubMed] [Google Scholar]
- [23].Cassidy J, Dirix L, Bissett D, et al. A Phase I study of capecitabine in combination with oral leucovorin in patients with intractable solid tumors. Clin Cancer Res 1998;4:2755–61. [PubMed] [Google Scholar]
- [24].Sjostrom J, Blomqvist C, Mouridsen H, et al. Docetaxel compared with sequential methotrexate and 5-fluorouracil in patients with advanced breast cancer after anthracycline failure: a randomised phase III study with crossover on progression by the Scandinavian Breast Group. Eur J Cancer 1999;35:1194–201. [DOI] [PubMed] [Google Scholar]
- [25].Kotsori AA, Dolly S, Sheri A, et al. Is capecitabine efficacious in triple negative metastatic breast cancer? Oncology (Basel) 2010;79:331–6. [DOI] [PubMed] [Google Scholar]
- [26].Li J, Zhao X, Chen L, et al. Safety and pharmacokinetics of novel selective vascular endothelial growth factor receptor-2 inhibitor YN968D1 in patients with advanced malignancies. BMC Cancer 2010;10:529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Xichun H, Jun C, Wenwei H, et al. Multicenter phase II study of Apatinib in non-triple-negative metastatic breast cancer. BMC Cancer 2014;14:820. [DOI] [PMC free article] [PubMed] [Google Scholar]


