Abstract
Objective
To investigate the association of enlarged perivascular spaces (ePVS) with cognition in elderly without dementia.
Methods
We included 5 studies from the Uniform Neuro-Imaging of Virchow-Robin Space Enlargement (UNIVRSE) consortium, namely the Austrian Stroke Prevention Family Study, Study of Health in Pomerania, Rotterdam Study, Epidemiology of Dementia in Singapore study, and Risk Index for Subclinical Brain Lesions in Hong Kong study. ePVS were counted in 4 regions (mesencephalon, hippocampus, basal ganglia, and centrum semiovale) with harmonized rating across studies. Mini-Mental State Examination (MMSE) and general fluid cognitive ability factor (G-factor) were used to assess cognitive function. For each study, a linear regression model was performed to estimate the effect of ePVS on MMSE and G-factor. Estimates were pooled across studies with the use of inverse variance meta-analysis with fixed- or random-effect models when appropriate.
Results
The final sample size consisted of 3,575 persons (age range 63.4–73.2 years, 50.6% women). Total ePVS counts were not significantly associated with MMSE score (mean difference per ePVS score increase 0.001, 95% confidence interval [CI] −0.007 to 0.008, p = 0.885) or G-factor (mean difference per ePVS score increase 0.002, 95% CI −0.001 to 0.006, p = 0.148) in age-, sex-, and education-adjusted models. Adjustments for cardiovascular risk factors and MRI markers did not change the results. Repeating the analyses with region-specific ePVS rendered similar results.
Conclusions
In this study, we found that ePVS counts were not associated with cognitive dysfunction in the general population. Future studies with longitudinal designs are warranted to examine whether ePVS contribute to cognitive decline.
Cerebral small vessel disease (SVD) is implicated as a major cause of stroke and cognitive impairment.1 The neuroimaging signature of SVD includes lacunes, white matter hyperintensities (WMH), and cerebral microbleeds. Accumulating evidence suggests that enlarged perivascular spaces (ePVS) may be another feature of SVD.2,3 ePVS are reported to be the extensions of the subarachnoid space surrounding the penetrating arteries.4 Initially, pathologic studies proposed that ePVS seldom reflect damage in the brain parenchyma and hence are regarded as devoid of clinical significance.1 However, recently, ePVS were linked to lower cognitive performance in a healthy elderly population and in individuals with cerebral SVD.5 Moreover, these lesions were associated with cognitive decline and vascular dementia in a large population-based setting.6 In contrast, a hospital-based study reported no association of ePVS with cognitive dysfunction.7 These negative results were confirmed in another study in which ePVS in hippocampus were not related to cognitive performance at baseline and risk of dementia.8 Nevertheless, comparison among these studies is hampered by small sample sizes and heterogeneous methods to assess ePVS. Moreover, the association of ePVS in different regions of the brain, i.e., the mesencephalon, hippocampus, basal ganglia, and centrum semiovale, with cognition has not been examined previously.
Hence, we performed a meta-analysis across 5 population-based studies, with a harmonized grading of ePVS, to determine the effects of ePVS on cognition. We also investigated the association of region-specific ePVS burden on cognitive testing, adjusting for vascular risk factors and other MRI markers of SVD and neurodegeneration.
Methods
Study population
We initiated this study as part of the Uniform Neuro-Imaging of Virchow-Robin Spaces Enlargement (UNIVRSE) consortium, a collaboration among several population-based studies from Europe, North America, and Asia.9 The present analysis is restricted to 5 studies because of availability of cognition data. The 5 studies included the Austrian Stroke Prevention Family Study (ASPS-Fam), Study of Health in Pomerania (SHIP) study, Rotterdam Study cohorts I and II (RS-I and RS-II), Epidemiology of Dementia in Singapore (EDIS) study, and Chinese University of Hong Kong–Risk Index for Subclinical Brain Lesions in Hong Kong (CU-RISK).
ASPS-Fam study
ASPS-Fam is a prospective, single-center, community-based study of the cerebral effects of vascular risk factors in the normal aged population of the city of Graz, Austria.10,11 ASPS-Fam represents an extension of the Austrian Stroke Prevention Study, which was established in 1991.12,13 Between 2006 and 2013, study participants of the ASPS and their first-degree relatives were invited to enter ASPS-Fam. Inclusion criteria were no history of previous stroke or dementia and a normal neurologic examination. A total of 419 individuals from 176 families were included in the study. The number of members per family ranged from 2 to 6. The entire cohort underwent a thorough diagnostic workup, including clinical history, laboratory evaluation, cognitive testing, extended vascular risk factor assessment, and brain MRI. The MRI scans were assessed for quality and incidental findings by 4 imaging experts, with 1 assessor having 20 years and other 3 assessors with >1 year of experience. After the exclusion of individuals with no ePVS and cognition data (absence of both detailed cognitive tests and Mini-Mental State Examination [MMSE]) (n = 144), the final sample size consisted of 275 participants.
SHIP study
SHIP is a prospective cohort with recruited individuals from the general population of the State Mecklenburg Western Pomerania in Germany. The Institute for Community Medicine at the University Medicine Greifswald led SHIP. It started at baseline with SHIP-0 between 1997 and 2001. After ≈5 years, all participants were reinvited for a follow-up visit, i.e., SHIP-1. From 2008 to 2012, the second follow-up examination, SHIP-2, was carried out. SHIP-2 started to include whole-body MRI scans. Trained certified radiologists, each with >5 years' experience in MRI interpretation, visually inspected head MRI scans for artifacts and clinical findings. SHIP-2 had 1,140 individuals who completed neuroimaging scanning. While SHIP-2 has a wide age range (30–90 years), MMSE testing has been conducted only in persons >60 years of age (n = 478). The final SHIP-2 sample included in this study was 400 individuals after considering those without incidental findings, complete MRI quality control record, ePVS assessment, and MMSE data.
RS study
RS is a population-based cohort study among inhabitants of a district of Rotterdam (Ommoord), the Netherlands, that aims to examine the determinants of disease and health in the elderly with a focus on neurogeriatric, cardiovascular, bone, and eye disease. In 1990 to 1993, 7,983 persons participated and were reexamined every 3 to 4 years (RS-I). In 2000 to 2001, the cohort was expanded by 3,011 persons who had not yet been part of the RS-II.14 MRI was incorporated into the core study protocol beginning in 2005. Trained study investigators with 2 years of experience visually checked the MRI scans for incidental findings and ePVS. Of the 2,182 eligible persons, 67 had an ungradable/incomplete MRI scan. Hence, the total number of individuals with available scans was 2,115. After the exclusion of persons with no ePVS gradings and all cognition tests (both detailed cognitive testing and MMSE), the final sample size consisted of 1,973 individuals.
EDIS study
EDIS drew participants from the population-based study of Chinese, Malay, and Indian cohorts ≥40 years of age who participated in the Singapore Epidemiology of Eye Disease (SEED). In the first phase of the EDIS study, participants from SEED ≥60 years of age (n = 1,538 Chinese and n = 1,014 Malay) were screened with the 10-point Abbreviated Mental Test and a self-report of progressive forgetfulness. Screen positives were defined as Abbreviated Mental Test score ≤6 among those with ≤6 years of formal education or ≤8 among those with >6 years of formal education or if the participant or caregiver reported progressive forgetfulness (yes/no). A total of 300 Chinese and 323 Malay screen-positive participants agreed to take part in the second phase of this study, which included an extensive neuropsychological test battery and brain MRI.15 All MRI scans were visually assessed for incidental findings and ePVS by trained imaging investigators with 5 years of experience. The present analysis was restricted to Chinese and Malay because ePVS data were available only for these 2 ethnicities. Individuals with incomplete MRI sequences required for ePVS grading and no cognition data were excluded, leaving a final sample size of 535 for the analysis.
CU-RISK study
CU-RISK drew functionally independent, stroke- and dementia-free persons ≥65 years of age through the use of stratified sampling from housing estates and community centers. Inclusion criteria of the study included age ≥65 years, community dwelling, sufficient Cantonese competency for cognitive testing, and written informed consent given. Screened individuals (n = 910) were invited to neurology research facilities at the Prince of Wales Hospital, where 851 participants underwent clinical and cognitive assessments followed by brain MRI.16 A subsample of 401 individuals were assessed for ePVS by a trained neuroradiologist with >5 years of experience. Data were available for 392 persons after the removal of individuals with poor-quality scans; hence, they were included in the analysis.
Standard protocol approvals, registrations, and patient consents
For ASPS-Fam, the ethics committee of the Medical University of Graz, Austria, approved the study protocol, and written informed consent was obtained from all participants. The SHIP study was approved by the ethics committee of the University of Greifswald and complies with the Declaration of Helsinki. All study participants gave written informed consent. The RS was approved by the medical ethics committee according to the Population Study Act Rotterdam Study and by the Ministry of Health, Welfare and Sports of the Netherlands. Written informed consent was obtained from all participants before study recruitment. Ethics approval for the EDIS study was obtained from the Singapore Eye Research Institute and National Healthcare Group Domain-Specific Review Board. Written informed consent was obtained by bilingual study coordinators. Ethics approval for CU-RISK study was obtained from the Joint Chinese University of Hong Kong–New Territories East Cluster Clinical Research Ethics Committee, and all study participants gave written informed consent.
Neuroimaging
The MRI scanners and protocols used in the participating studies have been described in detail previously.9,10,16,17 Briefly, 3T MRI scans were performed in ASPS-Fam, EDIS, and CU-RISK studies, whereas SHIP-2 and RS used 1.5T scanners. All the studies had common T1-weighted, T2-weighted, and fluid-attenuated inversion recovery (FLAIR) sequences except for SHIP-2, which did not have T2-weighted images.
Rating of ePVS
ePVS were defined as ovoid or linear lesions visible as hypointense on T1-weighted and hyperintense on T2-weighted images and considered dilated if the size exceeded ≥1mm. ePVS were visually counted in 4 regions of the brain—the mesencephalon, hippocampus, basal ganglia, and centrum semiovale—with the Rotterdam-Graz Dilated Virchow-Robin Spaces,18 which has been validated in all 5 studies. ePVS in the mesencephalon and hippocampus were graded in all the slices, whereas for the basal ganglia, the grading was restricted to the height of the anterior commissure, and for centrum semiovale, it was 10 mm above the lateral ventricle.18 The primary rating sequence was T2-weighted images in all studies except for SHIP-2, for which T1-weighted images were used to grade ePVS. Because ePVS may be underestimated on T1-weighted images, we performed a reliability test with T2-weighted images as described previously.9 Briefly, 25 scans from the RS with both good-quality T1- and T2-weighted sequences were rated twice using each sequence separately. This yielded good reliability between the 2 sequences (mean intraclass correlation coefficient 0.8).9 ePVS were differentiated from lacunes by the absence of a hyperintense rim on FLAIR and the center after orientation of the penetrating vessel.
Assessment of covariates
Details on demographics and vascular risk factors were assessed in all studies. Education was categorized as <8, 8 to 10, and >10 years of formal education. Information on ethnicity was available only in the EDIS study and was used in study-specific analysis. Systolic blood pressure, diastolic blood pressure, and cholesterol were measured at all sites. Type 2 diabetes mellitus was defined on the basis of history and medications verified by medical records. Lacunes were defined as lesions involving the subcortical regions, 3 to 15 mm in diameter, with low signal on T1-weighted images and FLAIR, a high signal on T2-weighted images, and a hyperintense rim with a center after CSF intensity. Brain tissue and WMH volumes were quantified with automatic segmentation. Intracranial, gray matter, and white matter volumes were quantified on T1- and T2-weighted images, whereas the WMH volume was segmented with the FLAIR sequence.19–21
Cognitive assessment
A brief cognitive test, i.e., MMSE, and an extensive neuropsychological battery were used to assess cognition. MMSE score was available in all participating studies, whereas an extensive neuropsychological battery was available in all except SHIP-2 and CU-RISK. General fluid cognitive ability factor (G-factor) was calculated from the extensive neuropsychological battery present in ASPS-Fam, EDIS, and RS.
General fluid cognitive ability factor
In view of heterogeneity in cognitive assessment, a G-factor was constructed from a number of cognitive tasks, each assessing different cognitive domains. Principal component analysis was applied to the cognitive task scores to derive a measure of G-factor. In ASPS-Fam, scores on the following cognitive ability tests were used to create G-factor: Alterskonzentrations Test (concentration test–time in seconds), Lern- und Gedächtnistest (LGT) (figural memory, total number of correct answers of 2 figural subtests; and verbal memory, total number of correct answers of 3 verbal subtests), complex reaction time task (computerized task; reaction time in milliseconds), Digit Span Backward (length of highest correctly repeated digit list), Purdue Pegboard Test (visuo-practical skills; total number of correct elements in most difficult condition [assembly]), and Trail Making Test B (time in seconds). The Pearson correlations among the 7 tests in ASPS-Fam ranged from 0.13 to 0.53 (mean 0.33). Principal component analysis was applied to these 7 tests. The first unrotated principal component (FUPC) accounted for 42.9% of the total test variance. Loadings on the FUPC were as follows: Alterskonzentrations Test = −0.54 figural memory (LGT) = 0.65, verbal memory (LGT) = 0.73, complex reaction time task = −0.54, Digit Span = 0.59, Purdue Pegboard Test = 0.72, and Trail Making Test B = −0.77.22 In RS, scores on the 15-Word Learning Test (delayed [once] recall), Stroop Card 3 (time needed to complete the card), Verbal Fluency (number of animals named within 1 minute), and Letter-Digit Substitution Task (number correctly coded) were used to create G-factor. The absolute Pearson correlations among the 4 tests in RS ranged from 0.14 to 0.44 (mean 0.37). Principal component analysis was applied to these 4 tests. The FUPC accounted for 52.7% of the total test variance. Loadings on the FUPC were as follows: Stroop Card 3 = −0.71, 15-Word Learning Test = 0.69, Verbal Fluency = 0.71, and Letter-Digit Substitution Task score = 0.79.23 In EDIS, scores of the Frontal Assessment Battery (similarities between objects, serial motor activities, tapping on consecutive numbers), Maze Task (finding the route from start to exit in the maze in seconds), Digit Span, Visual Memory Span and Auditory Detection (correct numbers identified and total number of items recall), Boston Naming Test and Verbal Fluency (total number of animal and food items named within 1 minute), Symbol Digit Modality Test and Digit Cancellation (numbers correctly paired with symbols/numbers in minutes), Wechsler Memory Scale (WMS), Visual Reproduction Copy task (drawing from memory recall), Clock Drawing (drawing face of the clock in seconds) and Wechsler Adult Intelligence Scale (building design/patterns from colored boxes), Word List Recall (number of items recalled in seconds), and Story Recall (recalling story in minutes for immediate and delayed recall) were used to create G-factor. The absolute Pearson correlations among the 28 tests in EDIS ranged from 0.09 to 0.86 (mean 0.42). Principal component analysis was applied to these 28 tests. The FUPC accounted for 45.4% of the total test variance. Loadings on the FUPC were as follows: Frontal Assessment Battery = 0.22, Digit Span Forward = 0.14, Digit Span Backward = 0.19, Visual Memory Span Forward = 0.18, Visual Memory Span Backward = 0.21, Auditory Detection Test = 0.15, Modified Boston Naming = 0.18, Animal Naming = 0.17, Food Naming = 0.14, Word List Immediate Recall = 0.18, Word List Delayed Recall = 0.17, Word List Delayed Recognition = 0.16, Story Recall A (immediate recall) = 0.22, Story Recall A (delayed recall) = 0.22, Story Recall B (immediate) = 0.22, Story Recall B (delayed) = 0.22, Picture Recall (immediate) = 0.11, Picture Recall (delayed) = 0.12, Picture Recall (delayed recognition) = 0.11, WMS (immediate recall) = 0.24, WMS (delayed recall) = 0.23, WMS (recognition) = 0.17, WMS Visual Reproduction Copy = 0.2, Clock Drawing Test = 0.21, Block Design = 0.21, Digit Cancellation Task = 0.22, Symbol Digit Modalities Task = 0.25, and Maze Task = −0.16.
Statistical analysis
Analysis within the studies
To avoid reducing the number of observations in the analysis because of missing data, multiple imputation using chained equations was performed in each cohort to impute missing covariates or the G-factor with plausible values. Predictive mean matching, logistic regression, and multinomial logit models were considered to impute missing values for continuous, binary, and categorical variables before considering random sample from the observed data. Random sampling was used to impute missing values for all studies because it was the only feasible approach for one of the studies. The number of imputations for each cohort was equal to the percentage of incomplete cases. G-factor was imputed only for RS (n = 419) and EDIS (n = 1) among participants with MMSE data. Multiple imputation using chained equations was performed with the mice package in R for imputation.24
After imputation was performed, the association between ePVS counts and cognition was determined with linear regression to obtain effect estimates (i.e., adjusted mean difference) and standard errors. We chose to present the results with ePVS counts in this study because the numbers of participants with absent ePVS were too few in the binary category. Adjustments for covariates were then constructed initially for age, sex, and education and subsequently for vascular risk factors (systolic blood pressure, diastolic blood pressure, cholesterol, and type 2 diabetes mellitus) and other MRI markers (lacunes, WMH, intracranial volume, gray matter volume, and white matter volume).
Meta-analysis
The overall pooled effect estimates were based on the cohort-specific effects from imputation (as obtained in the previous steps), and the fixed-effect model was used to pool the effect estimates if there was no evidence of heterogeneity between studies (i.e., p < 0.1 from Cochran Q statistic and a value >50% from Cochran I2 statistic). In case of heterogeneity between studies, the random-effect model was considered. The metafor package in R was used to perform the meta-analysis.25 Associations with values of p < 0.05 were considered statistically significant. We did not perform individual-level meta-analysis because legal and informed consent restrictions in participating studies precluded the sharing of data with other institutions.
Analyses of cognitive domains within studies
In view of the similarity between cognitive tests in RS and EDIS, we additionally constructed cognitive domains in both studies. Thus, in RS, cognitive domains were constructed with tests for executive function (Stroop Test 3, Letter Digit Substitution Test, Word Fluency Test), processing speed (Stroop Test 1, Stroop Test 2, Letter Digit Substitution Test), memory (Word List Recall immediate, delayed, and recognition), and motor speed (Purdue Pegboard Test). In EDIS, the cognitive domains were constructed with tests for executive function (Frontal Assessment Battery), visuoconstruction (WMS–Revised Visual Reproduction Copy task, Clock Drawing, Wechsler Adult Intelligence Scale–Revised subtest of Block Design), visuomotor speed (Symbol Digit Modality Test, Digit Cancellation), and memory (Word List Recall, Story Recall, Picture Recall, and WMS–Revised Visual Reproduction). Linear regression models were used to determine the association between ePVS counts and cognitive domains separately for RS and EDIS. All the models were adjusted in a manner similar to that described above. A meta-analysis was then conducted on the results obtained from the fully adjusted models in the linear regression using inverse-variance weighting with fixed effects. Correction for multiple comparison (among 4 cognitive domains) in the meta-analyzed data was done using the Sidak method with a significance level set at p = ≈0.0127.
Data availability
Studies participating in this meta-analysis have separate and specific data request and approval policies, depending on local, national, and international laws and regulations. Because of restrictions based on such privacy laws and regulations and informed consent of the participants, data cannot be made freely available in a public repository for any of the participating studies. Requests for information on procedures and formal data requests can be submitted to investigators from the respective studies (M.A.I., H.J.G., V.M., C.C, and R.S.).
Results
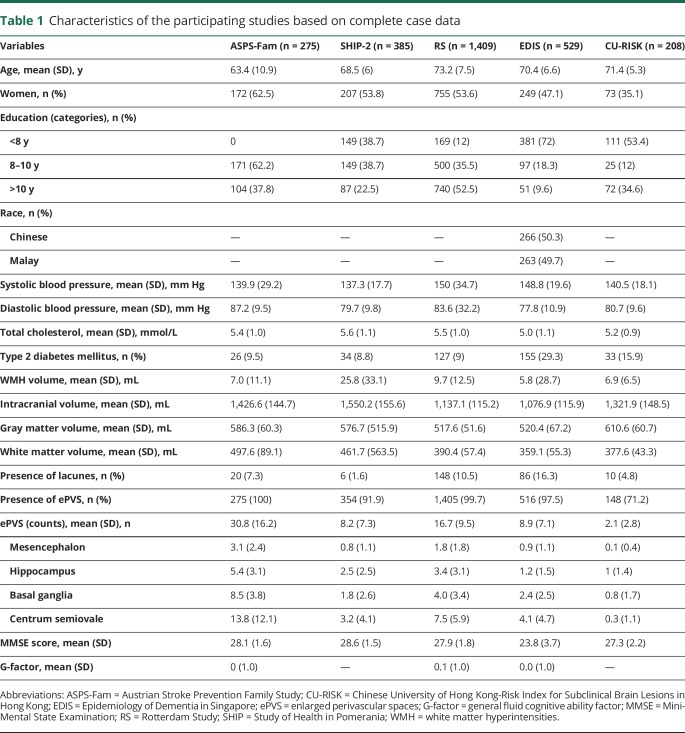
The characteristics of the 5 participating studies based on complete-case data (defined by the presence of all the variables, including ePVS, cognition and all the relevant covariates data: age, sex, ethnicity, education, systolic blood pressure, diastolic blood pressure, total cholesterol, type 2 diabetes mellitus, lacunes, WMH, intracranial volume, gray matter volume and white matter volume) are presented in table 1. The percentage of incomplete cases for ASPS-Fam, EDIS, RS, SHIP-2 and CU-RISK was 0%, 1.1%, 28.6%, 3.8%, and 46.9%, respectively. In brief, participants from RS were older and more educated compared to the other studies. There were more women in ASPS-Fam followed by RS. A higher systolic blood pressure was observed in RS and EDIS, whereas diabetes mellitus was more prevalent in EDIS. The prevalence of ePVS was comparable among 4 studies: ASPS-Fam, SHIP-2, RS, and EDIS (range 91.9%–100%); in CU-RISK, the prevalence of ePVS was 71.2%. The region-specific ePVS counts were highest in ASPS followed by RS, while the numbers were almost similar in SHIP, EDIS, and CU-RISK. Participants from EDIS had lower MMSE scores (mean MMSE score 23.8) compared to the other 4 sites.
Table 1.
Characteristics of the participating studies based on complete case data
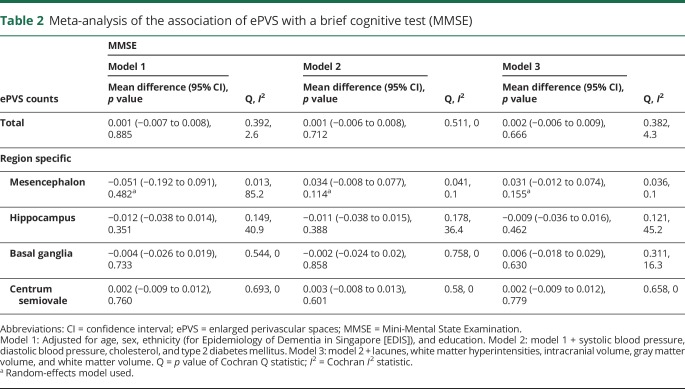
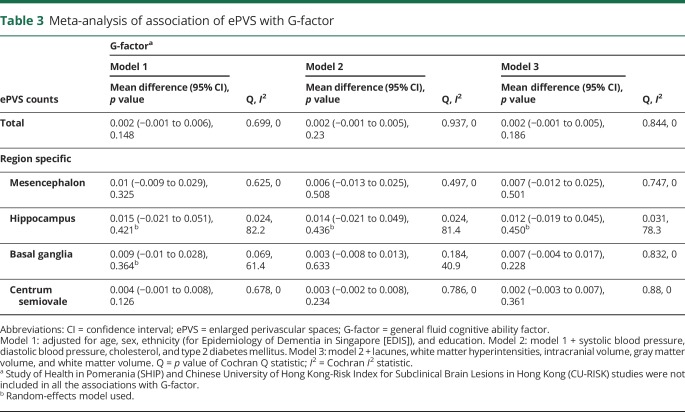
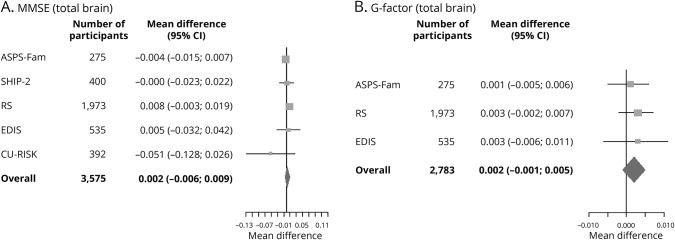
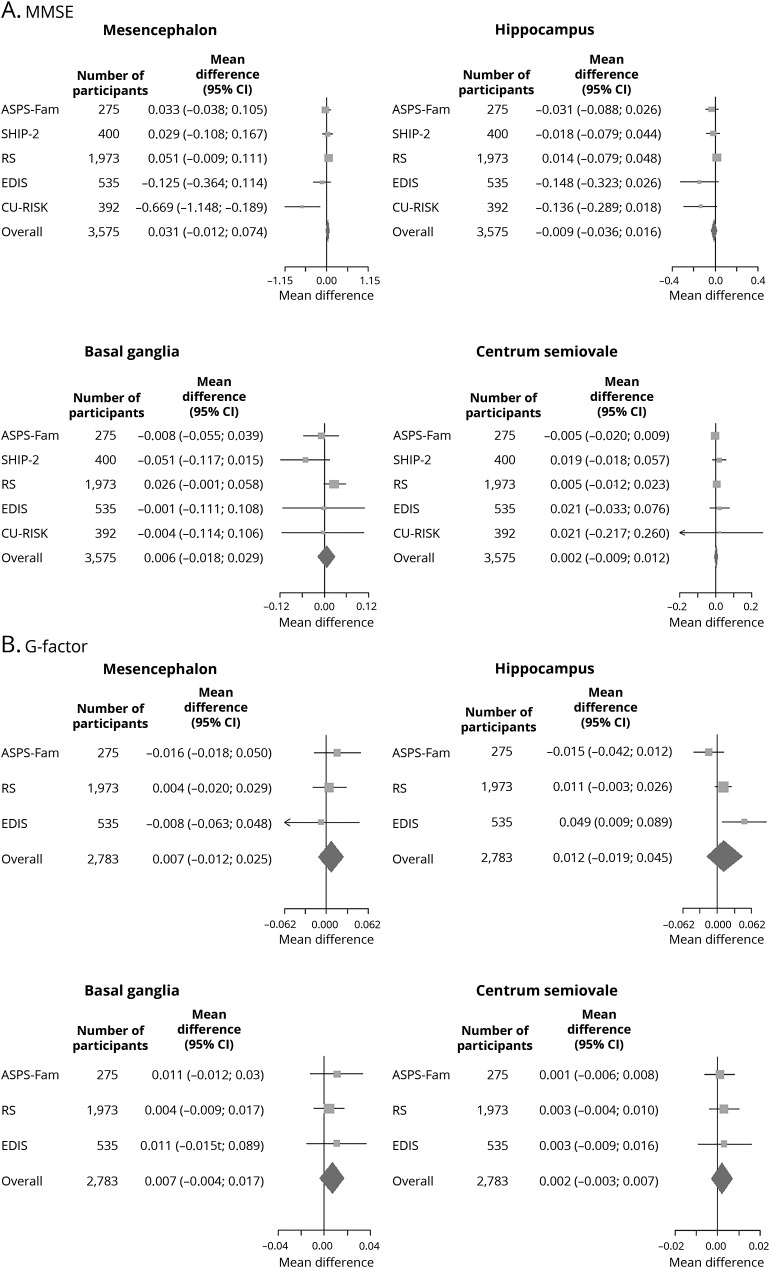
Meta-analysis of the association between ePVS and cognition showed that higher ePVS counts were not associated with MMSE (mean difference in score per ePVS increase 0.001, 95% confidence interval [CI] −0.007 to 0.008, p = 0.885) (table 2) or G-factor (mean difference in score per ePVS increase 0.002, 95% CI −0.001 to 0.006, p = 0.148) in age-, sex-, and education-adjusted models (table 3). Adjustment for systolic blood pressure, diastolic blood pressure, cholesterol, diabetes mellitus, and MRI markers did not alter these associations. Region-specific analyses of ePVS counts with cognitive function rendered similar results (tables 2 and 3). Forest plots of the association of total brain ePVS counts with MMSE and G-factor are shown in figure 1. Forest plots of region-specific ePVS counts with MMSE and G-factor are shown in figure 2. Similar results were obtained with predictive mean matching, logistic regression, and multinomial logit model to impute missing values for continuous, binary, and categorical variables for the studies in which these approaches were feasible.
Table 2.
Meta-analysis of the association of ePVS with a brief cognitive test (MMSE)
Table 3.
Meta-analysis of association of ePVS with G-factor
Figure 1. Forest plots of the association of total brain ePVS counts with (A) MMSE score and (B) G-factor.
Effect estimates adjusted for age, sex, ethnicity, education, systolic blood pressure, diastolic blood pressure, total cholesterol, type 2 diabetes mellitus, lacunes, white matter hyperintensities, intracranial volume, gray matter volume, and white matter volume for the association between total enlarged perivascular spaces (ePVS) counts and Mini-Mental State Examination (MMSE) and general fluid cognitive ability factor (G-factor). ASPS-Fam = Austrian Stroke Prevention Family Study; CI = confidence interval; CU-RISK = Chinese University of Hong Kong–Risk Index for Subclinical Brain Lesions in Hong Kong; EDIS = Epidemiology of Dementia in Singapore; RS = Rotterdam Study; SHIP = Study of Health in Pomerania.
Figure 2. Forest plots of the association of region-specific ePVS counts with MMSE score and G-factor.
Effect estimates adjusted for age, sex, ethnicity, education, systolic blood pressure, diastolic blood pressure, total cholesterol, type 2 diabetes mellitus, lacunes, white matter hyperintensities, intracranial volume, gray matter volume, and white matter volume for the association between region-specific enlarged perivascular spaces (ePVS) counts and Mini-Mental State Examination (MMSE) and general fluid cognitive ability factor (G-factor). ASPS-Fam = Austrian Stroke Prevention Family Study; CI = confidence interval; CU-RISK = Chinese University of Hong Kong–Risk Index for Subclinical Brain Lesions in Hong Kong; EDIS = Epidemiology of Dementia in Singapore; RS = Rotterdam Study; SHIP = Study of Health in Pomerania.
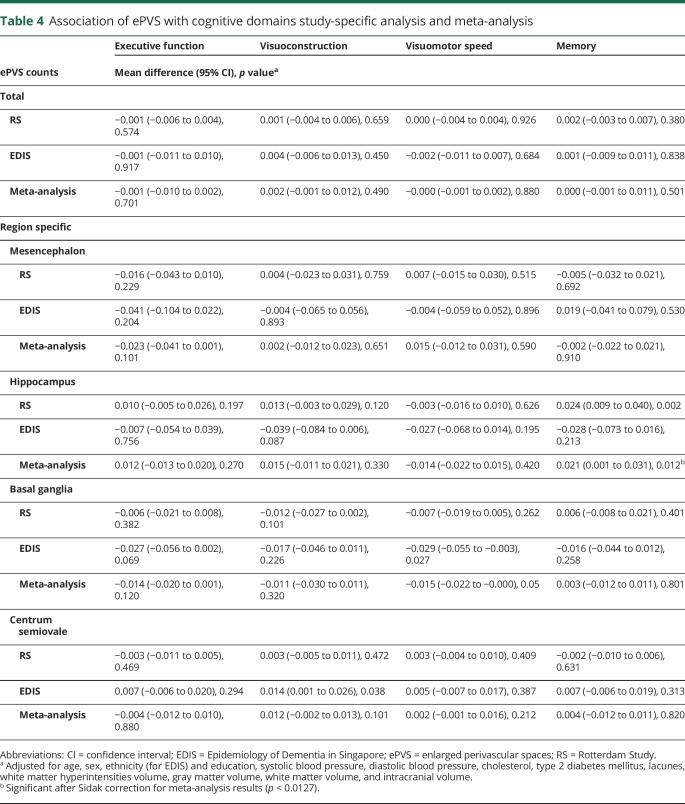
With respect to the cognitive domain–specific analyses within the RS and EDIS studies, our meta-analysis result showed that higher ePVS counts in the basal ganglia were significantly associated with worse performance on visuomotor speed (table 4). Conversely, larger ePVS counts in the hippocampus were linked to better performance on memory domain. After application of the Sidak correction (p < 0.0127), only higher ePVS counts in the hippocampus remained significantly associated with better performance on memory.
Table 4.
Association of ePVS with cognitive domains study-specific analysis and meta-analysis
Discussion
In this meta-analysis, total and region-specific ePVS counts were not associated with poor cognition in the elderly population without dementia. Adjustment for vascular risk factors and MRI markers did not alter these associations.
A few prior studies have investigated the effects of ePVS on cognition, but the results were inconsistent. It was reported that total ePVS counts were significantly associated with worse performance on nonverbal reasoning and visuospatial testing in a small sample of cognitively normal elderly.6 Another study of population-based elderly reported a link between the highest-severity degree of ePVS with incident dementia during a 4-year follow-up period.26 This was further corroborated by another recent study in which ePVS measuring ≥3mm was associated with cognitive decline and incident vascular dementia.6 In contrast, a hospital-based study of TIA and ischemic stroke reported no association with cognitive performance, although an association was suggested between ePVS and other SVD markers.7 Furthermore, a study in stroke- and dementia-free participants showed that hippocampal ePVS were not related to cognition and the occurrence of dementia in 8 years of follow-up.8
In the present meta-analysis of 5 population-based studies, no suggestive trends or borderline associations were observed between ePVS counts and cognitive function. We also explored the analysis between region-specific ePVS and cognition, but again no relationship was observed between ePVS in 4 regions of the brain and overall cognitive functioning. Although no direct conclusions can be drawn with respect to risk of cognitive decline, the cross-sectional results from the present study suggest 2 possibilities. First, the elderly populations studied in this meta-analysis might be in a more advanced stage of cerebrovascular damage in the brain, which may explain the lack of association between ePVS and cognition because ePVS may be an early feature of SVD. Conversely, because all the participating sites are population-based studies in which individuals may not have significant cognitive impairment, there is a chance that the likelihood of identifying a significant association between ePVS and cognitive dysfunction may be diminished. However, it is still conceivable that localization of ePVS in 4 brain regions due to different underlying pathophysiologic mechanisms27 might be relevant in relation to cognition, but such an effect is not reflected in the overall MMSE and G-factor results. Study-specific analysis within the present study revealed that ePVS in basal ganglia were associated with worse cognitive performance in the domain of visuomotor speed. However, this result was not significant after correction for multiple comparisons. Contrary to expectations, our results also showed that higher ePVS counts in the hippocampus were linked to better cognitive performance in memory. The focal damage resulting from the exact location of ePVS remains controversial, but it has been suggested that the presence of at least 1 hippocampal ePVS decreases the risk of severe hippocampal atrophy at 4 years of follow-up.8 We speculate that this may explain the relationship between ePVS and better performance in memory. However, such results should be interpreted with caution because the presence of 1 ePVS in the hippocampus may have a nonsignificant effect on cognition owing to their low number and size.
Among elderly, ePVS are considered part of the spectrum of SVD that are associated with WMH, lacunes, and cognitive impairment.5 Neuropathologic studies have shown a significant overlap among WMH, infarcts, and ePVS. However, it is difficult to infer a sequence of events often late in their time course from a static picture obtained postmortem.2 It is not clear, therefore, if ePVS precede, follow, or appear concurrently with SVD. We cannot exclude the possibility that ePVS (1 mm) may have a subtle effect on cognitive functioning; however, the clinical significance of such an effect may be limited. Indeed, it is shown that only large ePVS ≥ 3mm are associated with cognitive decline, which represents a more severe form of the SVD spectrum.6 We postulate that ePVS may influence cognition in other disease populations with extensive SVD burden and in patients with Alzheimer disease.
Strengths of the study include a large sample size, a harmonized method to grade ePVS among studies, the use of 4 different brain regions to explore the effects of ePVS on cognition, and the ability to investigate several explanatory variables with multivariate regression to correct for confounders, including those identified with multisequence MRI. However, there are several limitations to this study. First, this is a cross-sectional analysis that can determine only associations, not causation or sequence of development of cerebral SVD. Second, a single slice was used to grade ePVS in the basal ganglia and centrum semiovale, which may underestimates ePVS counting in those brain regions. However, we have previously shown a high correlation between a single slice and the whole-brain approach for grading ePVS.9 Third, the common outcome measure in all studies was MMSE score, which is a crude measure for cognitive functioning. This was dealt with by use of the G-factor (present mainly in 3 studies) to reflect overall cognition and all aspects of disease severity. Finally, because ePVS grading was not available in some individuals from the ASPS-Fam, SHIP-2, and EDIS studies due to poor-quality scans, the absence of T2-weighted images, or the presence of motion artifacts, these persons were excluded from the analysis, which may result in selection bias in that the excluded persons may be older, may be less educated, and may carry more risk factors. Future studies with longitudinal designs are warranted to examine whether ePVS contribute to cognitive decline independently of other age-related brain abnormalities or co-occur with other SVD to produce cognitive dysfunction.
Glossary
- ASPS-Fam
Austrian Stroke Prevention Family Study
- CI
confidence interval
- CU-RISK
Chinese University of Hong Kong–Risk Index for Subclinical Brain Lesions in Hong Kong
- EDIS
Epidemiology of Dementia in Singapore
- ePVS
enlarged perivascular spaces
- FLAIR
fluid-attenuated inversion recovery
- FUPC
first unrotated principal component
- G-factor
general fluid cognitive ability factor
- LGT
Lern- und Gedächtnistest
- MMSE
Mini-Mental State Examination
- RS
Rotterdam Study
- SEED
Singapore Epidemiology of Eye Disease
- SHIP
Study of Health in Pomerania
- SVD
small vessel disease
- UNIVRSE
Uniform Neuro-Imaging of Virchow-Robin Spaces Enlargement
- WMH
white matter hyperintensities
- WMS
Wechsler Memory Scale
Author contributions
S. Hilal and C.S. Tan participated in data acquisition, performed statistical analyses, and drafted and revised the manuscript. N. Venketasubramanian, M.K. Ikram, J. Abrigo, M.W. Vernooij, C. Chen, N. Hosten, and H. Volzke participated in data acquisition and revised the manuscript for intellectual content. H.H.H. Adams, M. Habes, and E. Hofer participated in data analysis and interpretation and revised the manuscript for intellectual content. H.J. Grabe, V. Mok, and R. Schmidt were responsible for obtaining funding and revising the manuscript for intellectual content. M.A. Ikram was responsible for the study concept and design, obtaining funding, and drafting and revising the manuscript.
Study funding
The ASPS-Fam was funded by Austrian Science Fund (FWF) grants PI904, P20545-P05, and P13180. The Medical University of Graz and the Steiermärkische Krankenanstalten Gesellschaft support the databank of the ASPS-Fam. SHIP-2 is part of the Community Medicine Research net of the University of Greifswald, Germany, which is funded by the Federal Ministry of Education and Research (grants 01ZZ9603, 01ZZ0103, and 01ZZ0403), the Ministry of Cultural Affairs and the Social Ministry of the Federal State of Mecklenburg-West Pomerania. MRI scans in SHIP have been supported by a joint grant from Siemens Healthcare, Erlangen, Germany, and the Federal State of Mecklenburg-West Pomerania. Dr. Habes was supported by “Alfried Krupp von Bohlen und Halbach” foundation. RS is supported by the Erasmus Medical Center and Erasmus University Rotterdam; the Netherlands Organization for Scientific Research; the Netherlands Organization for Health Research and Development (ZonMw); the Research Institute for Diseases in the Elderly; the Ministry of Education, Culture and Science; the Ministry of Health, Welfare and Sports; the European Commission; and the Municipality of Rotterdam. EDIS is supported by the National Medical Research Council (NMRC), Singapore (NMRC/CG/NUHS/2010 [grant R-184-006-184-511]) and Singapore Ministry of Health's NMRC (NMRC/CSA/038/2013). CU-RISK was funded by General Research Fund, Hong Kong (grant CUHK 471911).
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Greenberg SM. Small vessels, big problems. N Engl J Med 2006;354:1451–1453. [DOI] [PubMed] [Google Scholar]
- 2.Doubal FN, MacLullich AM, Ferguson KJ, Dennis MS, Wardlaw JM. Enlarged perivascular spaces on MRI are a feature of cerebral small vessel disease. Stroke 2010;41:450–454. [DOI] [PubMed] [Google Scholar]
- 3.Potter GM, Doubal FN, Jackson CA, et al. Enlarged perivascular spaces and cerebral small vessel disease. Int J Stroke 2015;10:376–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braffman BH, Zimmerman RA, Trojanowski JQ, Gonatas NK, Hickey WF, Schlaepfer WW. Brain MR: pathologic correlation with gross and histopathology, 1: lacunar infarction and Virchow-Robin spaces. AJR Am J Roentgenol 1988;151:551–558. [DOI] [PubMed] [Google Scholar]
- 5.Maclullich AM, Wardlaw JM, Ferguson KJ, Starr JM, Seckl JR, Deary IJ. Enlarged perivascular spaces are associated with cognitive function in healthy elderly men. J Neurol Neurosurg Psychiatry 2004;75:1519–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ding J, Sigurethsson S, Jonsson PV, et al. Large perivascular spaces visible on magnetic resonance imaging, cerebral small vessel disease progression, and risk of dementia: the Age, Gene/Environment Susceptibility-Reykjavik Study. JAMA Neurol 2017;74:1105–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hurford R, Charidimou A, Fox Z, Cipolotti L, Jager R, Werring DJ. MRI-visible perivascular spaces: relationship to cognition and small vessel disease MRI markers in ischaemic stroke and TIA. J Neurol Neurosurg Psychiatry 2014;85:522–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yao M, Zhu YC, Soumare A, et al. Hippocampal perivascular spaces are related to aging and blood pressure but not to cognition. Neurobiol Aging 2014;35:2118–2125. [DOI] [PubMed] [Google Scholar]
- 9.Adams HH, Hilal S, Schwingenschuh P, et al. A priori collaboration in population imaging: the Uniform Neuro-Imaging of Virchow-Robin Spaces Enlargement consortium. Alzheimers Dement 2015;1:513–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seiler S, Pirpamer L, Hofer E, et al. Magnetization transfer ratio relates to cognitive impairment in normal elderly. Front Aging Neurosci 2014;6:263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghadery C, Pirpamer L, Hofer E, et al. R2* mapping for brain iron: associations with cognition in normal aging. Neurobiol Aging 2015;36:925–932. [DOI] [PubMed] [Google Scholar]
- 12.Schmidt R, Fazekas F, Kapeller P, Schmidt H, Hartung HP. MRI white matter hyperintensities: three-year follow-up of the Austrian Stroke Prevention Study. Neurology 1999;53:132–139. [DOI] [PubMed] [Google Scholar]
- 13.Schmidt R, Lechner H, Fazekas F, et al. Assessment of cerebrovascular risk profiles in healthy persons: definition of research goals and the Austrian Stroke Prevention Study (ASPS). Neuroepidemiology 1994;13:308–313. [DOI] [PubMed] [Google Scholar]
- 14.Ikram MA, Brusselle GGO, Murad SD, et al. The Rotterdam Study: 2018 update on objectives, design and main results. Eur J Epidemiol 2017;32:807–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hilal S, Tan CS, Xin X, et al. Prevalence of cognitive impairment and dementia in Malays: Epidemiology of Dementia in Singapore study. Curr Alzheimer Res 2017;14:620–627. [DOI] [PubMed] [Google Scholar]
- 16.Hilal S, Mok V, Youn YC, Wong A, Ikram MK, Chen CL. Prevalence, risk factors and consequences of cerebral small vessel diseases: data from three Asian countries. J Neurol Neurosurg Psychiatry 2017;88:669–674. [DOI] [PubMed] [Google Scholar]
- 17.Habes M, Erus G, Toledo JB, et al. White matter hyperintensities and imaging patterns of brain ageing in the general population. Brain 2016;139:1164–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adams HH, Cavalieri M, Verhaaren BF, et al. Rating method for dilated Virchow-Robin spaces on magnetic resonance imaging. Stroke 2013;44:1732–1735. [DOI] [PubMed] [Google Scholar]
- 19.Schmidt R, Ropele S, Enzinger C, et al. White matter lesion progression, brain atrophy, and cognitive decline: the Austrian Stroke Prevention Study. Ann Neurol 2005;58:610–616. [DOI] [PubMed] [Google Scholar]
- 20.Vrooman HA, Cocosco CA, van der Lijn F, et al. Multi-spectral brain tissue segmentation using automatically trained k-Nearest-Neighbor classification. Neuroimage 2007;37:71–81. [DOI] [PubMed] [Google Scholar]
- 21.Hilal S, Sikking E, Shaik MA, et al. Cortical cerebral microinfarcts on 3T MRI: a novel marker of cerebrovascular disease. Neurology 2016;87:1583–1590. [DOI] [PubMed] [Google Scholar]
- 22.Davies G, Armstrong N, Bis JC, et al. Genetic contributions to variation in general cognitive function: a meta-analysis of genome-wide association studies in the CHARGE consortium (N = 53949). Mol Psychiatry 2015;20:183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prins ND, van Dijk EJ, den Heijer T, et al. Cerebral small-vessel disease and decline in information processing speed, executive function and memory. Brain 2005;128:2034–2041. [DOI] [PubMed] [Google Scholar]
- 24.Van Buuren S, Groothuis-Oudshoorn K. Mice: multivariate imputation by chained equations in R. J Stat Softw 2011;45:1–67. [Google Scholar]
- 25.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Soft 2010;46:1–48. [Google Scholar]
- 26.Zhu YC, Dufouil C, Soumare A, Mazoyer B, Chabriat H, Tzourio C. High degree of dilated Virchow-Robin spaces on MRI is associated with increased risk of dementia. J Alzheimers Dis 2010;22:663–672. [DOI] [PubMed] [Google Scholar]
- 27.Zhu YC, Tzourio C, Soumare A, Mazoyer B, Dufouil C, Chabriat H. Severity of dilated Virchow-Robin spaces is associated with age, blood pressure, and MRI markers of small vessel disease: a population-based study. Stroke 2010;41:2483–2490. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Studies participating in this meta-analysis have separate and specific data request and approval policies, depending on local, national, and international laws and regulations. Because of restrictions based on such privacy laws and regulations and informed consent of the participants, data cannot be made freely available in a public repository for any of the participating studies. Requests for information on procedures and formal data requests can be submitted to investigators from the respective studies (M.A.I., H.J.G., V.M., C.C, and R.S.).