Abstract
Background:
Both euthyroid sick syndrome and myocardial ischemia-reperfusion injury are common and have been significantly associated with morbidity and mortality after pediatric cardiac surgery with cardiopulmonary bypass. This single-center, prospective, double-blind, randomized placebo-controlled clinical pilot trial was designed to assess if preoperative oral thyroid hormone therapy could prevent the occurrence of euthyroid sick syndrome (ESS) and attenuate myocardial ischemia-reperfusion injury (IRI) after cardiac surgery with cardiopulmonary bypass (CPB) in children.
Methods:
Forty children aged 3 to 12 year, scheduled for elective congenital heart disease repair surgery with CPB, were randomized into 2 groups of equal size to receive the following treatments in a double-blind manner: placebo (control group) and thyroid tablet 0.4 mg/kg (trial group) taken orally once a day for 4 days before surgery. The perioperative serum thyroid hormone levels and hemodynamic variables were determined. The extubation time, duration of intensive care unit (ICU) stay, and use of inotropic drugs in the ICU were recorded. The myocardial expressions of heat shock protein 70 (HSP70), myosin heavy chain (MHC) mRNA, and thyroid hormone receptor (TR) mRNA were detected. The serum creatine kinase-MB (CK-MB) activity and troponin I (TnI) positive ratio at 24 hour after surgery were assessed.
Results:
There were no significant differences in hemodynamic variables at all observed points, extubation time, and duration of ICU stay between groups. As compared with baselines on administration, serum triiodothyronine (T3) and free T3 (FT3) levels on the first, second, and fourth postoperative day, and serum thyrotropic-stimulating hormone (TSH), tetraiodothyronine (T4), and free T4 (FT4) levels on the first postoperative day were significantly decreased in the 2 groups. Serum T3, FT3, and T4 levels on the first and second postoperative day, and serum FT4 level on the first postoperative day were significantly higher in the trial group than in control group. As compared with the control group, the number of patients requiring inotropic drugs in the ICU, serum CK-MB activity, serum positive TnI ratio, and myocardial expression of MHCβ mRNA were significantly decreased, and myocardial expressions of both HSP70 and MHCα mRNA were significantly increased in the trial group.
Conclusions:
In children undergoing cardiac surgery with CPB, preoperative oral small-dose thyroid hormone therapy reduces severity of postoperative ESS and provides a protection against myocardial IRI by increasing HSP70 and MHCα expression.
Keywords: cardiac surgery, cardiopulmonary bypass, children, euthyroid sick syndrome, ischemia-reperfusion injury, thyroid hormone
1. Introduction
The heart is a major target of thyroid hormones, with maintenance of euthyroid hormone balance critical for proper function.[1] Moreover, thyroid hormones have been shown a vital role in cardiac repair after injury beyond their roles in development and metabolism homeostasis.[2] After cardiac surgery with cardiopulmonary bypass (CPB), however, serum thyroid hormone levels are often decreased, especially in pediatric patients.[3–5] This is called as the euthyroid sick syndrome (ESS). It is reported that after cardiac surgery with CPB, 50% to 75% of adult patients present a decreased serum level of triiodothyronine (T3) (type 1 ESS) and 100% of pediatric patients display decreased serum levels of both T3 and tetraiodothyronine (T4) (type 2 ESS).[6] Because ESS has been associated with increased morbidity after cardiac surgery,[3–5,7] it is deemed that intravenous supplementation of thyroid hormones in postoperative period is benefit to infants and small children.[8] This view is supported by the largest randomized clinical trial so far, the Triiodothyronine for Infants and Children Undergoing CPB (TRICC) study, in which subgroup analysis shows a significant reduction in extubation time, less use of inotropic drugs, and better cardiac function with intravenous T3 supplementation after surgery in pediatric patients aged <5 months.[9] However, the most serious concern on intravenous administration of thyroid hormones involves the potential of accidental overdose leading to increased arrhythmias and sudden death.[1]
Ischemia and reperfusion occur during almost every cardiac surgery and myocardial ischemia/reperfusion injury (IRI) is an important cause of morbidity and mortality in the early postoperative period subsequent to cardiac surgery. It has been shown that thyroid hormones can limit myocardial IRI via a fine balance between proapoptotic and prosurvival signaling pathways.[2] Furthermore, thyroid hormones can provide a protection against myocardial IRI by inducing pharmacological preconditioning.[10] The available evidence also indicates that perioperative oral T3 therapy can significantly attenuate the postoperative decline in serum T3 level or maintain total and free serum T3 levels within normal limits in adult and pediatric patients undergoing cardiac surgery.[11–13] Given that oral administration is a safe, convenient and feasible route of perioperative medication, we speculate that preoperative oral thyroid hormone therapy can not only increase therapeutic controllability, but also may provide a protection against myocardial IRI and prevent the occurrence of ESS after cardiac surgery. However, there has been no study to determine the preventive efficacy of preoperative oral thyroid hormone therapy on postoperative ESS and myocardial IRI in pediatric patients undergoing cardiac surgery with CPB. Therefore, the purposes of this single-center, prospective, double-blind, randomized placebo-controlled clinical pilot trial were to determine whether preoperative oral thyroid hormone therapy could prevent the occurrence of ESS and attenuate postoperative myocardial IRI in children undergoing congenital heart surgery with CPB; to assess if this therapy could improve clinical outcomes, such as use of inotropic drugs in the ICU, extubation time, and duration of ICU stay.
2. Methods
2.1. Study population
After institutional ethics committee approval, children aged 3 to 12 years and scheduled for elective congenital heart disease repair surgery with CPB under general anesthesia were recruited from Henan Provincial People's Hospital between May and September, 2015. Following written informed consent from the parents was obtained, 40 children (20 boys and 20 girls) were enrolled into this study. Of them, 23 had simple ventricular septal defects, 7 simple atrial septal defects, 2 simple patent foramen ovale, 3 ventricular septal defects combined with atrial septal defects, 2 ventricular septal defects combined with patent ductus arteriosus, and 3 ventricular septal defects combined with patent foramen ovale. Exclusion criteria were a history of radiation therapy, abnormal baseline thyroid function, thyroid diseases and other endocrine diseases, preoperative thyroid hormone therapy, use of drugs affecting thyroid function, and preoperative use of inotropic drugs for circulatory support, and serum level of creatinine >2.0 mg/dL.
2.2. Randomization and masking
Using a sealed envelope method, children were randomized into 2 groups of equal size to receive one of the following treatments in a double-blind manner: placebo (control group) and thyroid tablet (Shanghai Industrial United Holdings Great Wall Pharmaceutical Co. Ltd., Shanghai, China) 0.4 mg/kg (trial group) (taken orally, once a day) for 4 days before surgery. The medication was prepared by an independent assistant who was not involved in the study. Investigators and participants were blinded to the assigned groups until the end of study.
2.3. Perioperative management
In perioperative period, all children were treated according to the standing guidelines of the hospital. In all children, a peripheral venous access was established and scopolamine 0.006 mg/kg was intravenously given 0.5 hour before anesthesia. After entering operating rooms, noninvasive blood pressure, electrocardiogram, and pulse oxygen saturation were monitored. After routine preoxygenation, anesthesia was induced with ketamine 1 mg/kg and fentanyl 10 μg/kg. Neuromuscular blockade was provided with pipecuronium bromide 0.1 mg/kg administered intravenously. After intubation, the lungs were ventilated with intermittent positive pressure ventilation, with a tidal volume of 8 to 10 mL/kg, a respiratory rate of 22 breaths/min, an inspiratory/expiratory ratio of 1:2, and an oxygen flow of 2.5 L/min. Anesthesia was maintained with 0.5% to 1.5% sevoflurane, and fentanyl, midazolam and pipecuronium bromide were intermittently given intravenously as necessary. The radial artery and central veins were catheterized for monitoring of arterial and central venous pressure during surgery.
All children received standardized surgical and CPB managements. CPB was instituted with a roller pump and a Terumo-05 membrane oxygenator. Surgical procedures were performed under mild hypothermia (30–34 °C) and high-flow CPB (100–130 mL/kg/min). During CPB, hematocrit was maintained at 24% to 28% and a continuous positive airway pressure of 5 to 10 cmH2O with 40% oxygen with air was applied. Also, the cold blood cardioplegia solution (4 °C) was perfused every 30 minutes to provide cardioprotection. The durations of aortic clamp and CPB, and the lowest temperature during CPB were recorded. After the completion of surgery and systemic re-warming, appropriate doses of protamine sulfate were used for recovery from anticoagulation and children were then weaned from CPB. Afterwards, children with tracheal intubation were transferred to the ICU, in where they received standardized postoperative managements according to institutional guidelines.
2.4. Measured variables
2.4.1. Primary outcome measure
For each child, the peripheral venous blood samples on admission, immediately before anesthesia, at the lowest temperature during CPB, and on the first, second, and fourth postoperative day were obtained to measure perioperative thyroid function. Serum was separated from blood by centrifugation and stored at –70 °C until assayed. The serum levels of T3, free T3 (FT3), T4, free T4 (FT4), and thyrotropic-stimulating hormone (TSH) were determined with a semi-quantitative microplate reader by enzyme-linked immunosorbent assay BIO-RAD MODEL 550 (Bio-Rad Laboratories, CA).
At 24 hour after surgery, a peripheral venous blood sample (5 mL) was taken to determine serum creatine kinase-MB (CK-MB) activity and troponin I (TnI) positive rate with an Abbott AXSYM automatic immune analyzer (Abbott Laboratories, Chicago, IL).
During surgery, right atria samples were taken from 6 children randomly chosen from each group to detect myocardial expressions of heat shock protein 70 (HSP70), thyroid hormone receptor (TR) isoforms (TRα1, TRα2, and TRβ1) mRNA, and myosin heavy chain α and β (MHCα and MHCβ) mRNA. The myocardial expression of HSP70 was detected by Western-blotting. The myocardial cells were collected and lysed with the buffer (1% sodium dodecylsulfate, 10 mmol/L Tris-Cl [pH 7.6], 20 Ag/AL aprotinin, 20 Ag/AL leupeptin, and 1 mmol/L 4-(2-aminoethyl) benzenosulfonyl fluoride). The protein concentrations were determined using the Bicinchoninic Acid Protein Assay kit (Pierce, Rockford, IL). Ten micrograms of protein were separated on 12% of sodium dodecyl sulfate polyacrylamide gel electropheresis gels and transferred to the polyvinylidene difluoride membranes. After blocking, the membranes were incubated with the appropriate primary antibody, anti-HSP70 antibody (1:1000 dilution) or β-actin (1:1000 dilution; Santa Cruz Biotechnology), at 4 °C overnight. After washing, the membranes were incubated with secondary antibody at a dilution of 1:3000 at room temperature for 1 hour. Proteins were detected with the enhanced chemiluminescence kit (Amersham Pharmacia Biotechnology, Inc., Piscataway, NJ). The images were analyzed using the Bandscan software (Glyko, Novato). The ratios of protein stripe grey value between target protein and β-actin protein provided a measurement of myocardial HSP70 expression.
The myocardial expressions of TR isoforms mRNA, and MHCα and MHCβ mRNA were detected by a reverse transcription-polymerase chain reaction (RT-PCR) technique. The total RNA in the myocardium was extracted according to the instruction manual of Trizol kit. RNA concentration was determined with an ultraviolet spectrophotometer (Eppendorf Biophotometer, Eppendorf AG. Hamburg, German). Total RNA (5 μg) and cDNA obtained by reverse transcription synthesis were taken according to the instruction manual of RT-PCR kit, and Sybr Green was served as a fluorescent marker to carry out RT-PCR with PCR instrument (GeneAmp 7500, Perkin-Elmer Corp., MA). PCR primers: TRα1 upstream was 5′-GCT TCC TCC ACA TGA AAG TCG-3′ and downstream 5′-GTG GGA GCT GAA TCT ATC CAA G-3′, TRα2 upstream was 5′-GTC ACC TCC CAT CCC GTA AGA-3′ and downstream 5′-GGG CCT CAA AGG ACA AGT AAG-3′, TRβ1 upstream was 5′-GGT GGC CTC CAA TAG CTC-3′ and downstream 5′-CAG GAA TGG GCA CAT GAC TGA-3′, MHCα upstream was 5′-GTC ATT GCT GAAACC GAG AAT G-3′ and downstream 5′-GC AAA GT A CTG GATGAC ACG CT-3′, and MHCβ upstream was 5′-GTC ACT GCC GAG ACC GAG TA-3′ and downstream 5′-GAT CAT CCA GGA GCC GTA G-3′. The reaction system was as follows: pre-denaturing at 95 °C for 5 minute, denaturing at 94 °C for 1 minute, reannealing at 60 °C for 30 second, elongation at 72 °C for 30 second, 40 cycles. Melting curve was analyzed with GenApm 7500 SDS software (Perkin-Elmer Corp., MA). The mRNA expression level of each gene in the myocardium was determined by 2−ΔΔCt method.
2.4.2. Secondary outcome measures
Blood pressure and heart rate were recorded on admission, immediately after surgery, and on days 1 and 2 after surgery when children were in rest condition for more than 0.5 hour. The extubation time, duration of ICU stay, and use of inotropic drugs in the ICU were recorded.
2.5. Sample size and statistical analysis
According to our preliminary study, power analysis showed that 18 children would be required in each group for a 90% power to detect a 0.15 ng/mL difference in serum T3 level between groups with a standard deviation of 0.2 ng/mL and α level of 0.05 using a non-paired t test. Allowing for possible dropouts attributable to different causes, we chose to examine 20 children in each group.
The data obtained were analyzed with SPSS statistical software (Version 12.0, SPSS Inc., Chicago, IL). Continuous variables were tested for normality of distribution using the Kolmogorov–Smirnov test with Lilliefors correction and for homogeneity of variance using the Levene test. If the data from the 2 groups were normally distributed and had homogeneous variance, they were compared by using the unpaired t test; otherwise the Kruskal–Wallis test was used. The comparisons of nonparametric data between groups were done using the Fisher exact test. For intragroup comparisons of hemodynamic variables and serum thyroid hormone levels, repeated measures analysis of variance followed by Dunnett test was used. Unless otherwise stated, data are expressed as mean ± SD or number of patients. A value of P < .05 was considered significant.
3. Results
A CONSORT (Consolidated Standards of Reporting Trials) diagram detailing the recruitment and analysis of patients is presented in Fig. 1. Patient demographics and intraoperative details are summarized in Table 1. There were no significant differences in age, height, weight, lowest temperature during CPB, durations of CPB, and aortic clamp between groups (Table 1). Heart beats were automatically recovered in all children after opening the ascending aorta. There were no significant differences in hemodynamic variables at all observed points between groups (Table 2).
Figure 1.
Flow diagram.
Table 1.
Demographic and clinical data in 2 groups.

Table 2.
Perioperative hemodynamic variables in 2 groups.

3.1. Serum CK-MB activity and TnI positive rate
In the 2 groups, serum CK-MB activity at 24 hour after surgery was higher than the normal value (0–25 U/L). However, both serum CK-MB activity and TnI positive rate were significantly lower in the trial group than in the control group (34.4 ± 9.1 vs 47.8 ± 17.6 U/L and 35% vs 70%; P < .05).
3.2. Serum thyroid hormone levels
As compared with baselines on admission, serum T3 and FT3 levels on the first, second, and fourth postoperative day, and serum TSH, T4, and FT4 levels on the first postoperative day were significantly decreased in the 2 groups. As compared with control group, serum T3, FT3, and T4 levels on the first and second postoperative day and serum FT4 level on the first postoperative day were significantly increased in the trial group (Table 3). However, serum TSH level immediately before anesthesia was significantly decreased compared with baselines on admission, and serum TSH level at lowest temperature during CPB was lower in the trial group than in the control group.
Table 3.
Serum of thyroid hormone levels in 2 groups.

3.3. Clinical outcomes
The extubation time and duration of ICU stay were 6.5 ± 4.7 and 48.7 ± 13.1 hour in the control group, respectively, 6.4 ± 4.3 and 46.1 ± 11.4 hour in the trial group, without significantly statistical differences between groups. However, the number of children requiring inotropic drugs in the ICU was significantly decreased in the trial group compared with the control group (1/20 vs 6/20; P < .05).
3.4. Myocardial expression of HSP70, TR isoforms, myosin heavy chain α and β
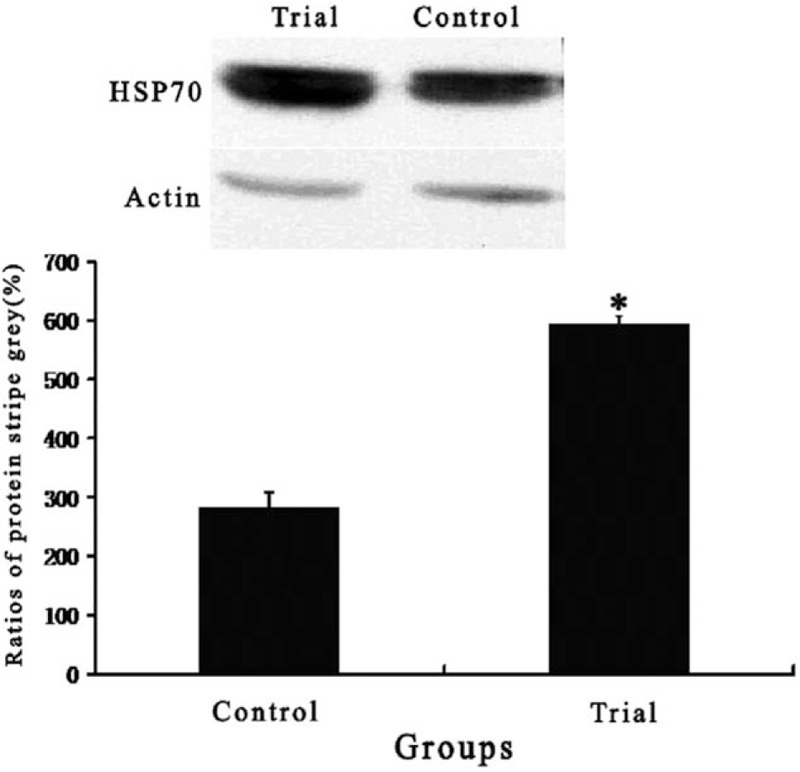
Myocardial expression of HSP70 was significantly stronger in the trial group compared with the control group (Fig. 2). There was no significant difference in myocardial expression of each TR isoform mRNA between groups. Myocardial expression of MHCα mRNA was significantly higher in the trial group than in the control group. However, myocardial expression of MHCβ mRNA was significantly lower in the trial group than in the control group (Table 4).
Figure 2.
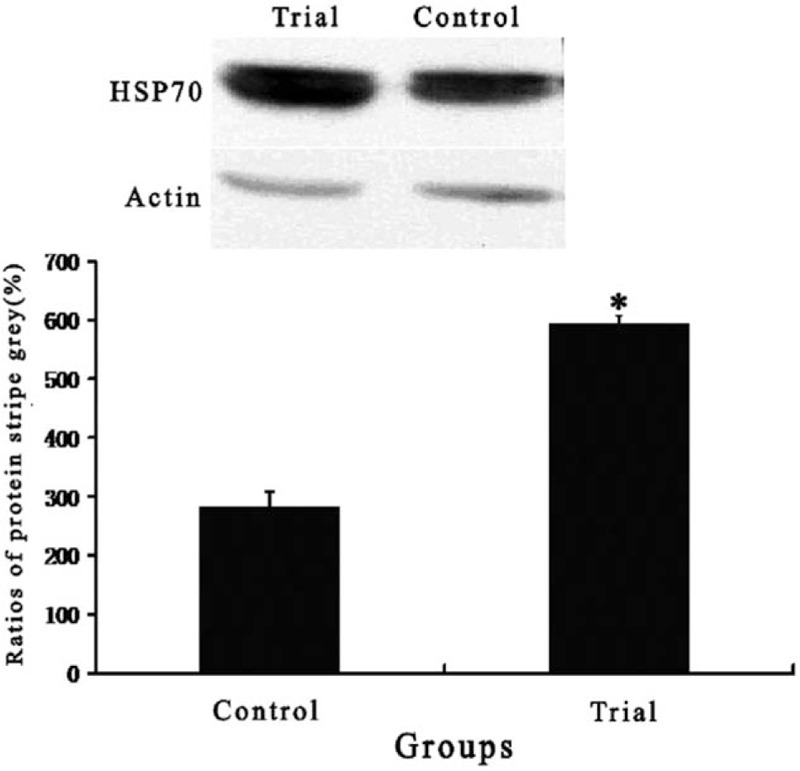
Myocardial expression of heat shock protein 70 in 2 groups. Data are expressed as means ± SD. Compared with control group, ∗P < .05.
Table 4.
Myocardial expressions of myosin heavy chain α and β (MHCα and MHCβ) mRNA, and thyroid hormone receptor (TR) isoforms (TRα1, TRα2, and TRβ1) mRNA in 2 groups (2−ΔΔCt).

3.5. Adverse reaction
During preoperative oral thyroid hormone therapy, no patient had clinically significant changes in blood pressure, heart rate, arrhythmias, or temperature to suggest toxicity.
4. Discussion
Pediatric cardiac surgery with CPB can result in a significant and persistent depression of circulating thyroid hormones. Suppression often involves the entire thyroid axis, including hypothalamic, pituitary, thyroid, and peripheral metabolisms, etc.[3–6,14,15] After pediatric cardiac surgery, moreover, the most common pattern of thyroid function suppression is a decrease in total and unbound T3 with normal levels of TSH and T4 (type 1 ESS). Very sick patients may show a significant decrease in total T3 and T4 levels; this state is called the low-T4 syndrome or type 2 ESS. In both types 1 and 2 ESS, serum TSH levels are impaired and do not increase in reaction to low T3 or T4 level.[8] Possible reasons for thyroid function suppression include hemodilution, hypothermia, non-pulsatile flow, alterations in peripheral hormone metabolism, increased inflammatory mediators (tumor necrosis factor and interleukins), and use of various drugs (dopamine, glucocorticoids, and opiates).[6] Because of extensive expression of TR, wide effects of thyroid hormones in the body[16] and possible association of ESS with increased morbidity after pediatric cardiac surgery,[3–5,7] supplement therapy with extraneous thyroid hormones has been suggested by many authors but is controversial.[8]
In previous studies, perioperative oral thyroid hormone therapy in adult and pediatric patients undergoing cardiac surgery with CPB often used levothyroxine (Euthyrox),[11–13] which is a synthetic tetraiodothyronine sodium that can be transformed into T3 by deiodination in liver.[17] Considering that postoperative decline of serum T3 and T4 is common in children undergoing cardiac surgery with CPB,[3,4,7,15] oral preparation of thyroid hormones used in this study is thyroid tablet including T3 and T4. Thyroid tablet is made from thyroid gland of sheep or pig, and is frequently applied for treatment of hypothyroidism in children.[18,19] Furthermore, thyroid tablet has been used for treatment of hypothyroxinemia in pediatric patients with cardiac failure,[20,21] and for improvement of perioperative hemodynamics in infants and children undergoing cardiac surgery with CPB.[22] In available literature, there is no report about the use of thyroid tablet for the prevention of ESS after cardiac surgery. Given that hyperthyroxinemia can facilitate the occurrence of myocardial IRI[23,24] and preoperative long-term thyroid hormone supplementation may adversely alter the thyroid-pituitary axis,[13] smallest dose of thyroid tablet and shortest duration of drug use were selected in this study, according to the previous studies.[20,21]
The results of this study showed that after pediatric cardiac surgery with CPB, serum thyroid hormone levels were significantly decreased in the control group compared with the baselines on admission, with the mostly significant reduction in serum FT3. Furthermore, serum FT3, FT4, T4, and TSH levels reached the lowest values on the first postoperative day. These results are consistent with the findings of previous studies.[3–7,14,15] This study also showed that in the trial group, serum T3 and FT3 levels on the first, second, and fourth postoperative day were significantly lower than the baselines, but postoperative serum T4 and FT4 levels did not differ from the baselines. According to the diagnostic criteria of ESS,[6,8,25] type 2 ESS occurred in the control group and only type 1 ESS in the trial group. These results suggest that in children undergoing cardiac surgery with CPB, preoperative short-term oral small-dose thyroid tablet can attenuate the suppression of circulating thyroid hormones and decrease the severity of ESS after surgery. Given that serum thyroid hormone levels immediately before anesthesia were within normal range in the trial group, moreover, we believe that a better preventive effect of postoperative ESS would have obtained, if a larger dose of thyroid tablet was given before surgery.
In this study, serum TSH level immediately before anesthesia in the trial group were significantly decreased compared with baselines, and serum TSH level at lowest temperature during CPB were significantly reduced in the trial group compared with control group. However, serum T3 and FT3 levels at lowest temperature during CPB, and postoperative serum thyroid hormone levels were higher in the trial group compared with the control group, and postoperative serum TSH levels and myocardial expression of TR isoforms were not significantly different between groups. These results suggest that dose and duration of preoperative oral thyroid hormones used in this study may result in a slight depression on the thyroid-pituitary axis, but do not adversely affect the responsive ability of the thyroid-pituitary axis to surgical stress.
During cardiac surgery with CPB, other than mechanical trauma to myocardium, both CPB and aortic cross-clamping can also trigger myocardial injury. CPB provokes a vigorous systemic inflammatory response, induced by the exposure of blood elements to non-physiological surfaces, resulting in myocardial injury. During the CPB, moreover, the heart is isolated from the circulation, and as a consequence suffers from an ischemic insult followed by an additional hit upon reperfusion, the so-called myocardial IRI.[26] The evidence from basic researches shows that thyroid hormones can limit myocardial IRI via a fine balance between pro-apoptotic and pro-survival signaling pathways.[2] This response is TRα1 dependent. An interaction between stress-induced growth kinase signaling and TRα1 has been shown to occur and determine postischemic remodeling and cardiac recovery depending on the availability of thyroid hormones. In addition, thyroid hormones can induce pharmacological preconditioning to provide a protection against myocardial IRI.[10] However, it is unclear whether these favorable effects of thyroid hormones on myocardial IRI can be translated clinical benefit of cardioprotection during cardiac surgery with CPB. This study showed that serum CK-MB activity at 24 hour after surgery was significantly increased in 2 groups, suggesting the occurrence of myocardial IRI. However, both postoperative serum CK-MB activity and TnI positive rate at 24 hour after surgery were significantly decreased and number of patients requiring positive inotropic drugs in ICU was significantly reduced in the trial group compared with the control group. These results suggest that preoperative short-term oral small-dose thyroid hormones may provide a protection against myocardial IRI during cardiac surgery with CPB in children.
This study also showed that myocardial expressions of HSP70 and MHCα were stronger in the trial group than in the control group. HSP70, a group of protein molecule family produced by cells under stress, plays an important physiological role in cell growth and survival under stress.[27] It has been shown that upregulation of myocardial HSP70 expression can attenuate myocardial IRI by multiple mechanisms, such as GSK-3β pathway, Akt/endothelial nitric oxide synthase pathway, reperfusion injury salvage kinase and survivor activating factor enhancement pathways.[28–30] Moreover, MHCα has 3-fold higher ATPase activity than MHCβ, and thyroid hormone is a main factor which enhances myocardial expression of MHCα. Thus, MHCα plays an important role in mechanism of cardiac contraction, and characteristics of myocardial contraction.[31] It has been shown that after myocardial infarction, MHCα expression is significantly downregulated and MHCβ expression is reciprocally upregulated.[32] This shift, which is attributed to low thyroid hormone levels, contributes to myocardial systolic dysfunction. When myocardial MHCα expression increases, the ability to convert ATP into ADP increases, improving myocardial contractility.[12,32,33] Based on these results, we believe that in children undergoing cardiac surgery with CPB, cardioprotective effects of preoperative short-term oral small-dose thyroid hormones are probably attributable to increased myocardial expression of HSP70 and MHCα.
However, this study did not show any differences in extubation time and duration of ICU stay associated with the preoperative thyroid hormone administration. Given that most of patients included in this study underwent simple closure of a ventricular or atrial septal defect and that this procedure is not usually associated with a prolonged or complicated postoperative course, it is not surprising that clinical considerations outweigh preoperative oral therapy of thyroid hormones in dictating clinical outcomes in this patient cohort.[13] In fact, the available evidence indicates that degree of postoperative hypothyroxinemia is closely related to the severity of illness and the postoperative course in children undergoing cardiac surgery with CPB.[6,7] Furthermore, perioperative supplementation of thyroid hormones has been associated with improved hemodynamics, reduced peripheral vascular resistance, increased cardiac output, shortened extubation time and other effects after cardiac surgery with CPB in infants and children.[5,9,13,34–38] When considered together with findings from our and other studies regarding therapy of thyroid hormones in pediatric patients undergoing cardiac surgery with CPB, thus, the demonstrated beneficial effect on clinical outcomes and the clear lack of negative effects make us to recommend that preoperative short-term oral thyroid hormone therapy may be a useful option for improvement of clinical outcomes.
It must be pointed out that several aspects of this study design deserve special attention. First, as a pilot study, only a smallest dose of medication and a shortest duration of drug use were selected in this study based on the recommendation of previous studies.[20,21] The results of the trial group showed that serum T3 and FT3 levels on the first, second and fourth postoperative day were significantly lower than baseline values, suggesting an inadequate normalization of thyroid hormone levels. Thus, optimizing dose and duration of oral thyroid hormone therapy before pediatric cardiac surgery is still needed. Second, as discussed above, this study mainly included pediatric patients undergoing simple cardiac surgery. To achieve good compliance for oral medication, moreover, only children aged 3 to 12 years were recruited. Thus, the findings of this study may not be generalized to the infants undergoing complex cardiac surgery with CPB. Third, the sample size was calculated based on possible difference in serum T3 levels between groups. The biomarkers of myocardial injury including serum CK-MB activity and TnI positive rate were significantly decreased in the trial group, but this study was not powered to show differences between groups in clinical outcomes, such as extubation time and duration of ICU stay. Thus, it is unclear whether favorable effects of preoperative oral thyroid hormones on postoperative ESS and myocardial IRI can be translated to postoperative clinical benefit in pediatric patients undergoing cardiac surgery with CPB. To address this issue, the large-scale clinical trials are still required, and these new studies should have enough power for postoperative outcomes. If further studies show consistent beneficial effect of preoperative oral thyroid hormones on clinical outcomes following pediatric cardiac surgery, the implications for practice are immense. Finally, this study only assessed the possible roles of HSP70, MHCα, and MHCβ in mechanisms of cardioprotection by preoperative oral thyroid hormones. In fact, thyroid hormones have been shown to provide a protection against myocardial IRI by multiple mechanisms, such as regulation of prosurvival pathways, including activation of the PI3K/AKT and PKC signaling cascade, enhancement of HSP27 expression, phosphorylation and translocation, suppression of p38MAPK, rescue of the mitochondrial integrity and mitochondrial quality control, optimization of substrate utilization, etc.[39] Evidently, this study cannot provide any clue for possible contributions of these factors to cardioprotection of preoperative oral thyroid hormones.
In summary, this pilot study demonstrates that children after cardiac surgery with CPB are at a high risk to develop postoperative ESS. Preoperatively short-term oral thyroid hormones can reduce severity of postoperative ESS without negative effects and provides a protection against myocardial IRI by increasing HSP70 and MHCα expression. We recommend that preoperative oral thyroid hormone therapy may be a useful option for improvement of clinical outcomes in children undergoing cardiac surgery with CPB.
Author contributions
Jia-Qiang Zhang and Quan-Yong Yang significantly contributed to the design and implementation of the study and data acquisition, analysis and interpretation and drafted the manuscript. Fu-Shan Xue contributed considerably to the conception and design of the study, helped draft the manuscript and critically revised it. Wei Zhang, Gui-Zhen Yang, Xu Liao and Fan-Min Meng participated substantially in data acquisition and interpretation. All authors approved the final manuscript.
Conceptualization: Jia-Qiang Zhang, Quan-Yong Yang, Fu-Shan Xue, Xu Liao, Fan-Min Meng.
Data curation: Jia-Qiang Zhang, Quan-Yong Yang, Wei Zhang, Gui-Zhen Yang.
Formal analysis: Jia-Qiang Zhang, Quan-Yong Yang, Fu-Shan Xue.
Funding acquisition: Fu-Shan Xue.
Investigation: Jia-Qiang Zhang, Quan-Yong Yang, Wei Zhang, Gui-Zhen Yang, Xu Liao, Fan-Min Meng.
Methodology: Jia-Qiang Zhang, Wei Zhang, Gui-Zhen Yang, Xu Liao, Fan-Min Meng.
Supervision: Fu-Shan Xue, Fan-Min Meng.
Validation: Wei Zhang, Gui-Zhen Yang, Xu Liao, Fan-Min Meng.
Writing – original draft: Jia-Qiang Zhang, Quan-Yong Yang.
Writing – review & editing: Fu-Shan Xue, Fan-Min Meng.
Footnotes
Abbreviations: CK-MB = creatine kinase-MB, CPB = cardiopulmonary bypass, ESS = euthyroid sick syndrome, FT3 = free T3, HSP70 = heat shock protein 70, ICU = intensive care unit, MHC = myosin heavy chain, RT-PCR = reverse transcription-polymerase chain reaction, T3 = triiodothyronine, FT4 = free T4, T4 = tetraiodothyronine, TnI = troponin I, TR = thyroid hormone receptor, TSH = thyrotropic-stimulating hormone.
This study was supported by the National Natural Science Foundation of China (No. 81170128). The funders had no role in study design, data collection or analysis, decision to publish, or preparation of the manuscript.
All authors have no financial support and potential conflicts of interest for this work.
References
- [1].Gerdes AM, Ojamaa K. Thyroid hormone and cardioprotection. Compr Physiol 2016;6:1199–219. [DOI] [PubMed] [Google Scholar]
- [2].Pantos C, Mourouzis I. Translating thyroid hormone effects into clinical practice: the relevance of thyroid hormone receptor α1 in cardiac repair. Heart Fail Rev 2015;20:273–82. [DOI] [PubMed] [Google Scholar]
- [3].Talwar S, Khadgawat R, Sandeep JA, et al. Cardiopulmonary bypass and serum thyroid hormone profile in pediatric patients with congenital heart disease. Congenit Heart Dis 2012;7:433–40. [DOI] [PubMed] [Google Scholar]
- [4].Babazadeh K, Tabib A, Eshraghi P, et al. Non-thyroidal illness syndrome and cardiopulmonary bypass in children with congenital heart disease. Caspian J Intern Med 2014;5:235–42. [PMC free article] [PubMed] [Google Scholar]
- [5].Bettendorf M, Schmidt KG, Grulich-Henn J, et al. Tri-iodothyronine treatment in children after cardiac surgery: a double-blind, randomised, placebo-controlled study. Lancet 2000;356:529–34. [DOI] [PubMed] [Google Scholar]
- [6].Plumpton K, Haas NA. Identifying infants at risk of marked thyroid suppression post-cardiopulmonary bypass. Intensive Care Med 2005;31:581–7. [DOI] [PubMed] [Google Scholar]
- [7].Dagan O, Vidne B, Josefsberg Z, et al. Relationship between changes in thyroid hormone level and severity of the postoperative course in neonates undergoing open-heart surgery. Paediatr Anaesth 2006;16:538–42. [DOI] [PubMed] [Google Scholar]
- [8].Haas NA, Camphausen CK, Kececioglu D. Clinical review: thyroid hormone replacement in children after cardiac surgery--is it worth a try. Crit Care 2006;10:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Portman MA, Slee A, Olson AK, et al. TRICC investigators triiodothyronine supplementation in infants and children undergoing cardiopulmonary bypass (TRICC): a multicenter placebo-controlled randomized trial: age analysis. Circulation 2010;122(11 suppl):S224–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kumar A, Taliyan R, Sharma PL. Evaluation of thyroid hormone induced pharmacological preconditioning on cardiomyocyte protection against ischemic-reperfusion injury. Indian J Pharmacol 2012;44:68–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Choi YS, Shim JK, Song JW, et al. Efficacy of perioperative oral triiodothyronine replacement therapy in patients undergoing off-pump coronary artery bypass grafting. J Cardiothorac Vasc Anesth 2013;27:1218–23. [DOI] [PubMed] [Google Scholar]
- [12].Choi YS, Kwak YL, Kim JC, et al. Peri-operative oral triiodothyronine replacement therapy to prevent postoperative low triiodothyronine state following valvular heart surgery. Anaesthesia 2009;64:871–7. [DOI] [PubMed] [Google Scholar]
- [13].Marwali EM, Boom CE, Sakidjan I, et al. Oral triiodothyronine normalizes triiodothyronine levels after surgery for pediatric congenital heart disease. Pediatr Crit Care Med 2013;14:701–8. [DOI] [PubMed] [Google Scholar]
- [14].Bettendorf M, Schmidt KG, Tiefenbacher U, et al. Transient secondary hypothyroidism in children after cardiac surgery. Pediatr Res 1997;41:375–9. [DOI] [PubMed] [Google Scholar]
- [15].Bartkowski R, Wojtalik M, Korman E, et al. Thyroid hormones levels in infants during and after cardiopulmonary bypass with ultrafiltration. Eur J Cardiothorac Surg 2002;22:879–84. [DOI] [PubMed] [Google Scholar]
- [16].Kinugawa K, Yonekura K, Ribeiro RC, et al. Regulation of thyroid hormone receptor isoforms in physiological and pathological cardiac hypertrophy. Circ Res 2001;89:591–8. [DOI] [PubMed] [Google Scholar]
- [17].Singh YN, Gleysteen AL, Ganschow SL. 5′-Deiodinase type 1 activity in liver and brain of the thyroxine-treated dystrophic hamster. J Pharm Pharmacol 2004;56:769–74. [DOI] [PubMed] [Google Scholar]
- [18].Wei MM. Study on the efficacy of Thyroxine Sodium and Euthyrox for hpothyroidism in children. Jilin Med J 2015;36:1078–9. [Google Scholar]
- [19].Ma XH, Wang CX, Wang L, et al. Observation on short-term curative effect of low dose thyroxine for treatment of transient hypothyroxinemia in premature infants. Maternal Child Health Care China 2013;28:447–50. [Google Scholar]
- [20].Fu SY, Lian LS, Zhang H. The clinical efficacy of thyroid hormones for refractory heart failure in children. Fujian Med J 2008;30:44–6. [Google Scholar]
- [21].Chen P, Lin YS, Wu BY. The clinical use of thyroid hormones in children with refractory heart failure. Chin J Pediatr 2000;6:42–3. [Google Scholar]
- [22].Wang WM, Cui CY, Guo SY, et al. Effects of thyroid hormone on cardiac function during open heart surgery in infants. J Clin Pediatr Surg 2007;6:13–4. [Google Scholar]
- [23].Pavón N, Hernández-Esquivel L, Buelna-Chontal M, et al. Antiarrhythmic effect of tamoxifen on the vulnerability induced by hyperthyroidism to heart ischemia/reperfusion damage. J Steroid Biochem Mol Biol 2014;143:416–23. [DOI] [PubMed] [Google Scholar]
- [24].Hernández-Esquivel L, Pavón N, Buelna-Chontal M, et al. Cardioprotective properties of citicoline against hyperthyroidism-induced reperfusion damage in rat hearts. Biochem Cell Biol 2015;93:185–91. [DOI] [PubMed] [Google Scholar]
- [25].Peeters RP, Wouters PJ, van TH, et al. Serum 3,3′,5′-triiodothyronine (rT3) and 3,5,3′-triiodothyronine/rT3 are prognostic markers in critically ill patients and are associated with postmortem tissue deiodinase activities. J Clin Endocrinol Metab 2005;90:4559–65. [DOI] [PubMed] [Google Scholar]
- [26].De Hert S, Moerman A. Myocardial injury and protection related to cardiopulmonary bypass. Best Pract Res Clin Anaesthesiol 2015;29:137–49. [DOI] [PubMed] [Google Scholar]
- [27].Guisasola MC, Desco MM, Gonzalez FS, et al. Heat shock proteins, end effectors of myocardium ischemic preconditioning. Cell Stress Chaperones 2006;11:250–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Pasqua T, Filice E, Mazza R, et al. Cardiac and hepatic role of r-AtHSP70: basal effects and protection against ischemic and sepsis conditions. J Cell Mol Med 2015;19:1492–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Chen HB, Zhang XC, Cheng YF, et al. Association of heat shock protein 70 expression with rat myocardial cell damage during heat stress in vitro and in vivo. Genet Mol Res 2015;14:1994–2005. [DOI] [PubMed] [Google Scholar]
- [30].Yadav HN, Singh M, Sharma PL. Pharmacological inhibition of GSK-3β produces late phase of cardioprotection in hyperlipidemic rat: possible involvement of HSP 72. Mol Cell Biochem 2012;369:227–33. [DOI] [PubMed] [Google Scholar]
- [31].Hart DL, Heidkamp MC, Iyengar R, et al. CRNK gene transfer improves function and reverses the myosin heavy chain isoenzyme switch during post-myocardial infarction left ventricular remodeling. J Mol Cell Cardiol 2008;45:93–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Wan W, Xu X, Zhao W, et al. Exercise training induced myosin heavy chain isoform alteration in the infarcted heart. Appl Physiol Nutr Metab 2014;39:226–32. [DOI] [PubMed] [Google Scholar]
- [33].Miyata S, Minobe W, Bristow MR, et al. Myosin heavy chain isoform expression in the failing and nonfailing human heart. Circ Res 2000;86:386–90. [DOI] [PubMed] [Google Scholar]
- [34].Mackie AS, Booth KL, Newburger JW, et al. A randomized, double-blind, placebo-controlled pilot trial of triiodothyronine in neonatal heart surgery. J Thorac Cardiovasc Surg 2005;130:810–6. [DOI] [PubMed] [Google Scholar]
- [35].Carrel T, Eckstein F, Englberger L, et al. Thyronin treatment in adult and pediatric heart surgery: clinical experience and review of the literature. Eur J Heart Fail 2002;4:577–82. [DOI] [PubMed] [Google Scholar]
- [36].Bialkowski J. Use of thyroid hormones after cardiopulmonary bypass in children. Cardiol Young 1998;8:139–40. [DOI] [PubMed] [Google Scholar]
- [37].Chowdhury D, Parnell VA, Ojamaa K, et al. Usefulness of triiodothyronine (T3) treatment after surgery for complex congenital heart disease in infants and children. Am J Cardiol 1999;84:1107–9. A10. [DOI] [PubMed] [Google Scholar]
- [38].Portman MA, Fearneyhough C, Ning XH, et al. Triiodothyronine repletion in infants during cardiopulmonary bypass for congenital heart disease. J Thorac Cardiovasc Surg 2000;120:604–8. [DOI] [PubMed] [Google Scholar]
- [39].Pingitore A, Nicolini G, Kusmic C, et al. Cardioprotection and thyroid hormones. Heart Fail Rev 2016;21:391–9. [DOI] [PubMed] [Google Scholar]


