Abstract
Objective The main purpose of this article is to assess the effectiveness and safety of surgery via the endoscopic endonasal approach (EEA) for cavernous sinus (CS) lesion in patients with nonfunctioning pituitary adenomas (NFPA).
Design Retrospective study.
Setting Keio University Hospital.
Participants Thirty patients who underwent CS surgery via the EEA between 2009 and 2017 for Knosp grade 4 NFPA with pre- and postoperative magnetic resonance imaging available for volumetric analysis.
Main Outcome Measures Clinical presentation, extent of resection, and surgical complications.
Results Gross total and near total resection of CS tumors was achieved in 12/30 (40%) cases of Knosp grade 4 NFPA. The average resection rate of CS lesions in these 30 patients was 73.5%; 77.3% in primary cases and 70.1% in recurrent cases that did not vary significantly. Preoperative visual disturbance and oculomotor nerve palsy improved in 12/19 (63.1%) and ⅗ (60%) cases, respectively. Complications associated with CS via the EEA were postoperative cerebrospinal leakage (1/30, 3.3%), meningitis (1/30, 3.3%), and transient cranial nerve palsy (2/30, 6.7%). These complications except a case of mild transient abducens nerve palsy occurred in recurrent cases with subdural lesions.
Conclusions Although the optimal management of CS lesions in NFPA is controversial, debulking via the EEA is an effective and safe option that improves neurological symptoms and enables effective adjuvant radiotherapy. Recurrent cases with subdural invasion are technically challenging, even using the EEA, and special care is required to avoid complications.
Keywords: skull base, complications, pituitary adenoma
Introduction
Cavernous sinus (CS) lesions are difficult to manage surgically because of the involvement of the internal carotid artery (ICA) and many cranial nerves. Parkinson first reported a surgical approach for CS lesions, 1 and the neurovascular structures in the CS have subsequently been clarified by numerous anatomical studies. 2 3 4 5 Transcranial skull base approaches to the CS were basically performed through the lateral and superior walls of the CS. 2 5 Technological progress, including electrophysiological monitoring, has improved the surgical capacity for CS lesions; however, surgery in this area remains risky, even for experienced surgeons. CS surgery is associated with high rates of complications including new cranial nerve palsies, and with low rates of improvement in preoperative cranial nerve palsies.
Stereotactic radiotherapy was developed as a noninvasive treatment of brain lesions using highly focused irradiation. 6 In particular, the development of the Gamma Knife has led to good long-term results in recent years. 7 8 9 10 Although the rate of tumor control varies depending on lesion size and histopathology, many studies have showed the success of stereotactic radiosurgery in the treatment of CS lesions, 7 9 11 12 13 with acceptable morbidity rates. 8 9 10 12 A more conservative surgical management of CS lesions is performed, followed by adjuvant stereotactic radiotherapy.
The microscopic transsphenoidal approach to the CS was first performed in the 1990s. 14 15 The transsphenoidal approach is an excellent, logical route for removing CS tumors through the medial CS wall, especially pituitary adenomas. In the transsphenoidal approach, tumors invading the CS through its medial wall are approached inferomedially following the direction of tumor growth, thus sparing the cranial nerves. However, the limitations of the transsphenoidal approach are the narrow surgical corridor and insufficient visualization. With the development of new endoscopic instruments, improved visualization provided by endoscopes has expanded the limitations of the traditional microscopic transsphenoidal approach, enabling safe resection of CS lesions. Moreover, a better surgical outcome can potentially be achieved using a two-surgeon, bimanual technique in a team collaborating with an otolaryngologist. 16
Although the efficacy of surgical management of CS tumors via the endoscopic endonasal approach (EEA) has been reported in cases of invasive pituitary adenoma, 17 18 19 20 21 22 23 limited data have been focused on nonfunctioning pituitary adenoma (NFPA). The removal of CS invasion in NFPA cases is still controversial, and we intentionally excluded functioning tumors and focused on the surgical results via the EEA of Knosp grade 4 NFPA, in which the CS lateral extension was clear. The benefits of surgical resection must be weighed against the complications associated with surgery and the need to respond to patient expectations.
Materials and Methods
Study Selection
This retrospective study was approved by the Keio University School of Medicine review board. We reviewed patients with NFPA, involving the CS who underwent surgery via the EEA. CS invasion was reported according to the Knosp criteria, 24 and only Knosp grade 4 cases were included in the present study.
Data Analyses
We analyzed tumor size, symptoms, extent of resection, and complications. All pre- and postoperative MRI images were retrospectively reviewed by an independent neurosurgeon (KK), who calculated the extent of surgical resection of the total tumor and the CS. Volumetric analysis was completed using the SYNAPSE VINCENT imaging system (Fujifilm Medical Co., Tokyo, Japan) with three-dimensional visualization, and the segmentation tool function on gadolinium-enhanced T1-weighted images obtained close to the date of the operation. The degree of surgical resection of the CS was classified retrospectively as: (1) gross total resection (GTR; 100% volume reduction); (2) near total resection (NTR; 80–99% volume reduction); (3) subtotal resection (50–80% volume reduction); or (4) partial resection (< 50% volume reduction) according to a previous report on the analysis of CS surgery. 23
Surgical Procedure
Surgery via the EEA was performed using the binostril, two-surgeon technique in collaboration with otolaryngologists. An outfracture of the bilateral middle turbinate was performed to create a surgical corridor, and the right middle turbinate was resected if necessary. A modified rescue flap was raised in cases when cerebrospinal fluid (CSF) leakage was not expected; 25 if CSF leakage was expected, a vascularized nasoseptal flap was prepared. 26 After resection of the posterior segment of the nasal septum for the binostril approach, a wide sphenoidotomy was performed. The sellar floor was opened in most cases, and the bone of the ventral CS was removed. The extent of the bone removal was dependent on the tumor location. For lesions occupying both the sella and the CS, the transsellar and medial approach to the CS was used. For lesions involving the CS but not the sella, the direct CS approach was used. For lesions in the lateral part of the CS, the transpterygoid approach was needed in some cases. If needed, additional bony decompression of the optic canal was performed before tumor removal. In the transsellar and medial approach, resection of the CS tumor proceeded from medial to lateral, with opening of the medial wall of the CS. In the direct CS approach/transpterygoid approach, the dura of the ventral CS was opened after confirmation of the location of the ICA using both navigation and Doppler. During tumor removal, electrostimulation was used to identify the course of cranial nerves III and VI, especially lateral to the intracavernous ICA.
After tumor removal, when the intracavernous ICA was broadly exposed, the dural defect was covered with a nasoseptal flap. The large dural defect with CSF leakage was reconstructed in a multilayered fashion. The fascia was positioned as a subdural inlay, and a nasoseptal flap was used to cover. Abdominal fat and oxidized cellulose with fibrin glue were used at the dural and osseous edges.
Statistical Analyses
Statistical analyses of categorical variables were performed using the chi-square and Fisher's exact tests, as appropriate. Statistical analyses of means were performed using the unpaired Student's t -test with equal variance and without equal variance (Welch's t -test) as necessary. A p value of less than 0.05 was considered statistically significant.
Results
Patient Characteristics
The EEA was performed for CS lesions in 62 patients at Keio University Hospital between November 2009 and May 2017. Of these 62 patients, pre- and postoperative MRI studies were available for volumetric analysis for 30 patients with Knosp grade 4 NFPA. The average age is 64.8 (37–81) and there was a clear sex predilection in NFPA, with females comprising 76.7% of patients (23/30).
Table 1 summarizes the clinical presentations. The most common preoperative symptom related to the CS and visual function was visual disturbance ( n = 19), followed by oculomotor nerve palsy ( n = 5). There were postoperative improvements in 12/19 (63.1%) cases of visual disturbance, and in ⅗ (60%) cases of oculomotor nerve palsy. There was no apparent postoperative improvement in one patient with abducens nerve palsy, whereas two cases experienced transient abducens nerve paralysis postoperatively.
Table 1. Pre- and postoperative cranial nerve deficits.
| Pituitary adenomas ( n = 30) | |||
|---|---|---|---|
| Preop | Postoperative | ||
| Improved | Worse | ||
| CN II | 19 | 12 | 0 |
| CN III | 5 | 3 | 0 |
| CN IV | 0 | 0 | 0 |
| CN V | 0 | 0 | 0 |
| CN VI | 1 | 0 | 0 (2 a ) |
Abbreviation: CN, cranial nerve.
Transient deterioration.
Surgical Outcomes
The outcomes assessed included the extent of resection ( Table 2 ) and complications ( Table 3 ). The goal of surgery was relief of symptoms and avoidance of complications with maximum possible tumor resection. GTR and NTR was achieved in three (10%) and nine (30%) cases, respectively. The average resection rate did not significantly differ between primary cases and recurrent cases ( p = 0.14).
Table 2. Outcomes of resection of cavernous sinus lesions by the endoscopic endonasal approach.
| Pituitary adenoma n =14 |
Primary recurrent n = 16 |
|
|---|---|---|
| Degree of resection | ||
| Partial (< 50%) | 1(7%) | 2(12.5%) |
| Subtotal (50–80%) | 5(36%) | 10(62.5%) |
| Near total (80–95%) | 7(50%) | 2(12.5%) |
| Gross total (> 95%) | 1(7%) | 2(12.5%) |
| Average of resection | 77.3% | 70.1% |
| 73.5% | ||
Table 3. Surgical complications.
| Pituitary adenomas ( n = 30) | |
| Primary ( n = 14) | |
| Transient palsy of cranial nerve VI | 1 |
| Recurrent ( n = 16) | |
| CSF leakage | 1 |
| Meningitis | 1 |
| Transient palsy of cranial nerve VI | 1 |
Abbreviation: CSF, cerebrospinal fluid.
Table 3 presents the summary of surgical complications. No new permanent cranial palsies occurred in any of the total 30 cases. Transient abducens palsy occurred in two patients, which was fully resolved within 6 months after surgery. Postoperative CSF leakage occurred in one patient (3.3%) and meningitis in one patient (3.3%), who were recurrent cases with subdural lesions. No primary cases experienced severe complications, although one patient had mild transient abducens nerve palsy.
Discussion
After Jho and Ha 27 reported the endonasal approach to CS lesions, the EEA to the CS has been performed by other groups, mainly for the treatment of invasive pituitary adenomas. In most reports, the extent of tumor removal in cases of pituitary adenoma invasion of the CS has been discussed in relation to the Knosp grade. 20 28 29 30 31 32 33 34 35 36 37 38 39 However, a recent systematic review revealed that intraoperative CS invasion as judged during endoscopic surgery was different to the preoperative radiographic estimation of Knosp grade. 18 Hence, it may not be appropriate to discuss the removal rate of CS invasion in pituitary tumor cases with an estimated Knosp grade. Therefore, in the present study, the surgical outcome of pituitary adenoma was analyzed only for cases with Knosp grade 4 extension, in which the CS lateral extension was clear. In addition, the removal of CS invasion in NFPA cases is still controversial, and we intentionally excluded functioning tumors and focused on the analysis of CS invasion in NFPA.
It is difficult to compare the surgical results between open craniotomy and the EEA because of patient selection bias and surgeon familiarity. However, many articles report that the EEA is the surgical method currently preferred for CS lesions, rather than open craniotomy; this is because the concept of the “medial-to-lateral” endonasal route to the CS has been accepted as a safe approach, because the cranial nerves are located on the far side of the surgical corridor. In particular, pituitary adenomas invade from medial to lateral, and then generally push the contents of the CS in a medial to lateral direction. Therefore, the EEA is suitable not only for sellar and suprasellar lesions but also for CS invasion by pituitary adenomas. A recent review of the surgical results of CS invasion in pituitary adenoma suggests that morbidity rates are lower with the EEA than open craniotomy, reporting permanent morbidity rates of the EEA and craniotomy of 3 to 6% and 6 to 10%, respectively. 23 In addition, a systematic review of surgical methods for CS invasion in Knosp grade 3 to 4 pituitary adenoma showed that endoscopic surgery had significantly higher GTR rates compared with microscopic surgery performed by the same team. 18 This indicates the advantage of the panoramic views provided by endoscopy, as the improved visualization may allow more aggressive resection.
Our purpose of the surgery for CS invasion in NFPA was to reduce the tumor size to enable the performance of effective stereotactic radiotherapy ( Fig. 1 ), which is similar to the concept of the “medial-to-lateral endonasal approach” reported by Woodworth et al. 23 Their surgical concept was to aggressively remove the tumor in the medial CS, while the tumor in the lateral CS was debulked in preparation for radiotherapy. 23 In their report, GTR (> 95%) for Knosp grades 3 to 4 was achieved in 8.7% of 23 cases, and the average extent of tumor removal was 66.6%. 23 Furthermore, all four patients with preoperative cranial neuropathies improved postoperatively. 23 Surgical complications in 36 cases, including Knosp grade 1 to 2, were transient postoperative cranial neuropathy (5.6%), postoperative CSF leak (2.8%), reoperation for mucocele (2.8%), and infection (2.8%). 23 The GTR rates reported by Woodworth et al 23 were lower than in other reports, 17 40 but the risk of permanent cranial neuropathy was also low, with a high chance of improvement in pre-existing deficits. In our study, GTR was achieved in 10% of Knosp grade 4 cases, and the average extent of tumor removal was 73.5%; surgical complications were transient abducens palsy (6.7%), postoperative CSF leak (3.3%), and meningitis (3.3%), similarly to the results of Woodworth et al. 23
Fig. 1.
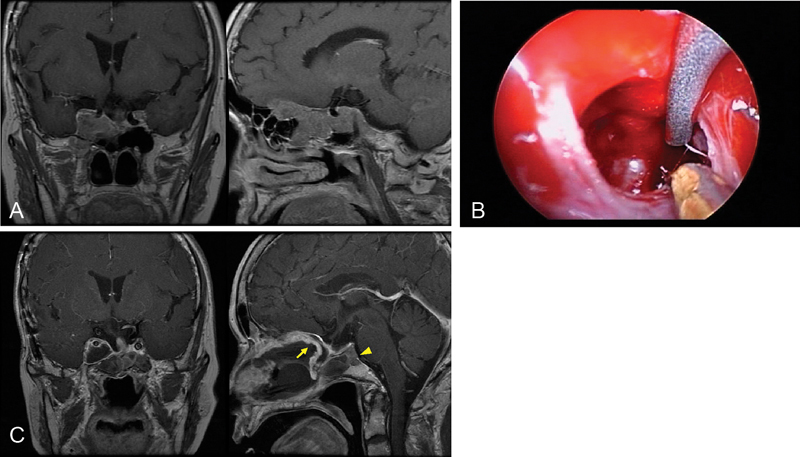
Recurrent nonfunctioning pituitary adenoma involving the cavernous sinus (CS). ( A ) Preoperative magnetic resonance imaging (MRI) demonstrating a pituitary adenoma in the right CS extending to the anterior cranial fossa. ( B ) Exposure of the internal carotid artery (ICA) in the right CS. The medial part of the CS tumor is being removed. ( C ) Postoperative MRI showing near total resection, with a small residual tumor (arrowhead) behind the right ICA. The arrow indicates a nasoseptal flap for the dural reconstruction.
Fernandez-Miranda et al 41 described anatomy-based CS compartments from the EEA perspective for pituitary adenoma. These four CS compartments are based on their relationship with the cavernous ICA: the superior compartment relates to the interclinoidal ligament and oculomotor nerve, the posterior compartment contains the gulfar segment of the abducens nerve and the inferior hypophyseal artery, the inferior compartment contains the sympathetic nerve and distal cavernous abducens nerve, and the lateral compartment includes all cavernous cranial nerves and the inferolateral arterial trunk. 41 Abducens nerve palsy is one of the highest risks in CS surgery via the EEA. These proposed CS compartments can increase pre- and intraoperative understanding of the surgical anatomy by the EEA, leading to better surgical results.
Conclusion
Considering the current good treatment outcomes of radiotherapy for CS tumors, a safe tumor debulking procedure via the EEA with minimum complications is preferable to attempting CS tumor removal in patients of NFPA, as these tumors are almost impossible to remove completely.
References
- 1.Parkinson D. A surgical approach to the cavernous portion of the carotid artery. Anatomical studies and case report. J Neurosurg. 1965;23(05):474–483. doi: 10.3171/jns.1965.23.5.0474. [DOI] [PubMed] [Google Scholar]
- 2.Hakuba A, Tanaka K, Suzuki T, Nishimura S.A combined orbitozygomatic infratemporal epidural and subdural approach for lesions involving the entire cavernous sinus J Neurosurg 198971(5 Pt 1):699–704. [DOI] [PubMed] [Google Scholar]
- 3.Harris F S, Rhoton A L. Anatomy of the cavernous sinus. A microsurgical study. J Neurosurg. 1976;45(02):169–180. doi: 10.3171/jns.1976.45.2.0169. [DOI] [PubMed] [Google Scholar]
- 4.Inoue T, Rhoton A L, Jr, Theele D, Barry M E. Surgical approaches to the cavernous sinus: a microsurgical study. Neurosurgery. 1990;26(06):903–932. doi: 10.1097/00006123-199006000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Perneczky A, Knosp E, Matula C.Cavernous sinus surgery. Approach through the lateral wall Acta Neurochir (Wien) 198892(1-4):76–82. [DOI] [PubMed] [Google Scholar]
- 6.Leksell L. Stereotactic radiosurgery. J Neurol Neurosurg Psychiatry. 1983;46(09):797–803. doi: 10.1136/jnnp.46.9.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iwai Y, Yamanaka K, Ishiguro T.Gamma knife radiosurgery for the treatment of cavernous sinus meningiomas Neurosurgery 20035203517–524., discussion 523–524 [DOI] [PubMed] [Google Scholar]
- 8.Kim M, Paeng S, Pyo S, Jeong Y, Lee S, Jung Y.Gamma knife surgery for invasive pituitary macroadenoma J Neurosurg 2006105(Suppl):26–30. [DOI] [PubMed] [Google Scholar]
- 9.Kuo J S, Chen J C, Yu Cet al. Gamma knife radiosurgery for benign cavernous sinus tumors: quantitative analysis of treatment outcomes Neurosurgery 200454061385–1393., discussion 1393–1394 [DOI] [PubMed] [Google Scholar]
- 10.Roche P H, Régis J, Dufour H et al. Gamma knife radiosurgery in the management of cavernous sinus meningiomas. J Neurosurg. 2000;93 03:68–73. doi: 10.3171/jns.2000.93.supplement. [DOI] [PubMed] [Google Scholar]
- 11.Chang S D, Adler J R, Jr, Martin D P. LINAC radiosurgery for cavernous sinus meningiomas. Stereotact Funct Neurosurg. 1998;71(01):43–50. doi: 10.1159/000029647. [DOI] [PubMed] [Google Scholar]
- 12.Lee J Y, Niranjan A, McInerney J, Kondziolka D, Flickinger J C, Lunsford L D. Stereotactic radiosurgery providing long-term tumor control of cavernous sinus meningiomas. J Neurosurg. 2002;97(01):65–72. doi: 10.3171/jns.2002.97.1.0065. [DOI] [PubMed] [Google Scholar]
- 13.Nicolato A, Foroni R, Alessandrini F, Bricolo A, Gerosa M.Radiosurgical treatment of cavernous sinus meningiomas: experience with 122 treated patients Neurosurgery 200251051153–1159., discussion 1159–1161 [DOI] [PubMed] [Google Scholar]
- 14.Fraioli B, Esposito V, Santoro A, Iannetti G, Giuffrè R, Cantore G. Transmaxillosphenoidal approach to tumors invading the medial compartment of the cavernous sinus. J Neurosurg. 1995;82(01):63–69. doi: 10.3171/jns.1995.82.1.0063. [DOI] [PubMed] [Google Scholar]
- 15.Hashimoto N, Kikuchi H. Transsphenoidal approach to infrasellar tumors involving the cavernous sinus. J Neurosurg. 1990;73(04):513–517. doi: 10.3171/jns.1990.73.4.0513. [DOI] [PubMed] [Google Scholar]
- 16.Kikuchi R, Toda M, Tomita T, Ogawa K, Yoshida K. Surgical outcome of endoscopic endonasal surgery for non-functional pituitary adenoma by a team of neurosurgeons and otolaryngologists adenoma by a team of neurosurgeons and otolaryngologists. Turk Neurosurg. 2017;27(01):1–7. doi: 10.5137/1019-5149.JTN.14354-15.0. [DOI] [PubMed] [Google Scholar]
- 17.Ceylan S, Koc K, Anik I. Endoscopic endonasal transsphenoidal approach for pituitary adenomas invading the cavernous sinus. J Neurosurg. 2010;112(01):99–107. doi: 10.3171/2009.4.JNS09182. [DOI] [PubMed] [Google Scholar]
- 18.Dhandapani S, Singh H, Negm H M, Cohen S, Anand V K, Schwartz T H. Cavernous sinus invasion in pituitary adenomas: systematic review and pooled data meta-analysis of radiologic criteria and comparison of endoscopic and microscopic surgery. World Neurosurg. 2016;96:36–46. doi: 10.1016/j.wneu.2016.08.088. [DOI] [PubMed] [Google Scholar]
- 19.Ferreli F, Turri-Zanoni M, Canevari F R et al. Endoscopic endonasal management of non-functioning pituitary adenomas with cavernous sinus invasion: a 10-year experience. Rhinology. 2015;53(04):308–316. doi: 10.4193/Rhino14.309. [DOI] [PubMed] [Google Scholar]
- 20.Micko A S, Wöhrer A, Wolfsberger S, Knosp E. Invasion of the cavernous sinus space in pituitary adenomas: endoscopic verification and its correlation with an MRI-based classification. J Neurosurg. 2015;122(04):803–811. doi: 10.3171/2014.12.JNS141083. [DOI] [PubMed] [Google Scholar]
- 21.Taniguchi M, Hosoda K, Akutsu N, Takahashi Y, Kohmura E. Endoscopic endonasal transsellar approach for laterally extended pituitary adenomas: volumetric analysis of cavernous sinus invasion. Pituitary. 2015;18(04):518–524. doi: 10.1007/s11102-014-0604-7. [DOI] [PubMed] [Google Scholar]
- 22.Vellutini EdeA, Beer-Furlan A, Stamm A C. Endoscopic transsellar approach to pituitary adenomas with cavernous sinus invasion: is this just a matter of lateral extension? Pituitary. 2016;19(03):342–343. doi: 10.1007/s11102-014-0625-2. [DOI] [PubMed] [Google Scholar]
- 23.Woodworth G F, Patel K S, Shin B et al. Surgical outcomes using a medial-to-lateral endonasal endoscopic approach to pituitary adenomas invading the cavernous sinus. J Neurosurg. 2014;120(05):1086–1094. doi: 10.3171/2014.1.JNS131228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knosp E, Steiner E, Kitz K, Matula C.Pituitary adenomas with invasion of the cavernous sinus space: a magnetic resonance imaging classification compared with surgical findings Neurosurgery 19933304610–617., discussion 617–618 [DOI] [PubMed] [Google Scholar]
- 25.Ozawa H, Tomita T, Watanabe Y et al. Sigmoid incision rescue nasoseptal flap technique for endoscopic endonasal skull base surgery. Acta Otolaryngol. 2016;136(06):636–640. doi: 10.3109/00016489.2016.1143122. [DOI] [PubMed] [Google Scholar]
- 26.Hadad G, Bassagasteguy L, Carrau R L et al. A novel reconstructive technique after endoscopic expanded endonasal approaches: vascular pedicle nasoseptal flap. Laryngoscope. 2006;116(10):1882–1886. doi: 10.1097/01.mlg.0000234933.37779.e4. [DOI] [PubMed] [Google Scholar]
- 27.Jho H D, Ha H G. Endoscopic endonasal skull base surgery: Part 2--The cavernous sinus. Minim Invasive Neurosurg. 2004;47(01):9–15. doi: 10.1055/s-2004-818346. [DOI] [PubMed] [Google Scholar]
- 28.Bao X, Deng K, Liu X et al. Extended transsphenoidal approach for pituitary adenomas invading the cavernous sinus using multiple complementary techniques. Pituitary. 2016;19(01):1–10. doi: 10.1007/s11102-015-0675-0. [DOI] [PubMed] [Google Scholar]
- 29.Dallapiazza R F, Grober Y, Starke R M, Laws E R, Jr, Jane J A., JrLong-term results of endonasal endoscopic transsphenoidal resection of nonfunctioning pituitary macroadenomas Neurosurgery 2015760142–52., discussion 52–53 [DOI] [PubMed] [Google Scholar]
- 30.de Paiva Neto M A, Vandergrift A, Fatemi N et al. Endonasal transsphenoidal surgery and multimodality treatment for giant pituitary adenomas. Clin Endocrinol (Oxf) 2010;72(04):512–519. doi: 10.1111/j.1365-2265.2009.03665.x. [DOI] [PubMed] [Google Scholar]
- 31.Hofstetter C P, Nanaszko M J, Mubita L L, Tsiouris J, Anand V K, Schwartz T H. Volumetric classification of pituitary macroadenomas predicts outcome and morbidity following endoscopic endonasal transsphenoidal surgery. Pituitary. 2012;15(03):450–463. doi: 10.1007/s11102-011-0350-z. [DOI] [PubMed] [Google Scholar]
- 32.Karppinen A, Kivipelto L, Vehkavaara S et al. Transition from microscopic to endoscopic transsphenoidal surgery for nonfunctional pituitary adenomas. World Neurosurg. 2015;84(01):48–57. doi: 10.1016/j.wneu.2015.02.024. [DOI] [PubMed] [Google Scholar]
- 33.Koutourousiou M, Gardner P A, Fernandez-Miranda J C, Paluzzi A, Wang E W, Snyderman C H. Endoscopic endonasal surgery for giant pituitary adenomas: advantages and limitations. J Neurosurg. 2013;118(03):621–631. doi: 10.3171/2012.11.JNS121190. [DOI] [PubMed] [Google Scholar]
- 34.Messerer M, De Battista J C, Raverot G et al. Evidence of improved surgical outcome following endoscopy for nonfunctioning pituitary adenoma removal. Neurosurg Focus. 2011;30(04):E11. doi: 10.3171/2011.1.FOCUS10308. [DOI] [PubMed] [Google Scholar]
- 35.Nishioka H, Fukuhara N, Horiguchi K, Yamada S. Aggressive transsphenoidal resection of tumors invading the cavernous sinus in patients with acromegaly: predictive factors, strategies, and outcomes. J Neurosurg. 2014;121(03):505–510. doi: 10.3171/2014.3.JNS132214. [DOI] [PubMed] [Google Scholar]
- 36.Paluzzi A, Fernandez-Miranda J C, Tonya Stefko S, Challinor S, Snyderman C H, Gardner P A. Endoscopic endonasal approach for pituitary adenomas: a series of 555 patients. Pituitary. 2014;17(04):307–319. doi: 10.1007/s11102-013-0502-4. [DOI] [PubMed] [Google Scholar]
- 37.Stofko D L, Nickles T, Sun H, Dehdashti A R.The value of immediate postoperative MR imaging following endoscopic endonasal pituitary surgery Acta Neurochir (Wien) 201415601133–140., discussion 140 [DOI] [PubMed] [Google Scholar]
- 38.Torales J, Halperin I, Hanzu F et al. Endoscopic endonasal surgery for pituitary tumors. Results in a series of 121 patients operated at the same center and by the same neurosurgeon. Endocrinol Nutr. 2014;61(08):410–416. doi: 10.1016/j.endonu.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 39.Yamada S, Fukuhara N, Horiguchi K et al. Clinicopathological characteristics and therapeutic outcomes in thyrotropin-secreting pituitary adenomas: a single-center study of 90 cases. J Neurosurg. 2014;121(06):1462–1473. doi: 10.3171/2014.7.JNS1471. [DOI] [PubMed] [Google Scholar]
- 40.Frank G, Pasquini E. Endoscopic endonasal cavernous sinus surgery, with special reference to pituitary adenomas. Front Horm Res. 2006;34:64–82. doi: 10.1159/000091573. [DOI] [PubMed] [Google Scholar]
- 41.Fernandez-Miranda J C, Zwagerman N T, Abhinav K et al. Cavernous sinus compartments from the endoscopic endonasal approach: anatomical considerations and surgical relevance to adenoma surgery. J Neurosurg. 2017 doi: 10.3171/2017.2.JNS162214. [DOI] [PubMed] [Google Scholar]


