Abstract
Background The assessment of pituitary tumor (PT) volume is important in the treatment and follow-up of patients with PT. Previously, PT volume estimation has been performed by conventional geometric equations (CGE) such as abc/2 (simplified ellipsoid volume equation) and 4πr 3 /3 (sphere), both presuming a symmetric tumor shape, which occurs uncommonly in patients with PT. In contrast, three-dimensional (3D) voxel-based software segmentation takes the irregular and asymmetric shapes that PTs often possess into account and might be a more accurate method for PT volume segmentation.
The purpose of this study is twofold. (1) To compare 3D segmentation with CGE for PT volume estimation. (2) To assess inter-rater reliability in 3D segmentation of PTs.
Methods Nineteen high-resolution (1mm slice thickness) T1-weighted MRI examinations of patients with PT were independently analyzed and manually segmented, using the software ITK-SNAP, by two certified neuroradiologists. Concurrently, the volumes of the PTs were estimated with abc/2 and 4πr 3 /3 by a clinician, and the results were compared with the corresponding segmented volumes.
Results There was a significant decrease in PT volume attained from the segmentations compared with the calculations made with abc/2 ( p < 0.001, mean volume 18% higher than segmentation) and 4πr 3 /3 ( p < 0.001, mean volume 28% higher than segmentation). The intraclass correlation coefficient (ICC) for the two sets of segmented PTs was 0.99.
Conclusion CGE ( abc/2 and 4πr 3 /3 ) significantly overestimates PT volume compared with 3D volumetric segmentation. The inter-rater agreement on manual 3D volumetric software segmentation is excellent.
Keywords: pituitary tumor volume, volumetric analysis, segmentation, abc/2
Introduction
Pituitary tumors (PTs) comprise around 9 to 16% of all primary brain and CNS tumors. 1 2 3 They appear as mass lesions in the sella turcica and the sellar region in close vicinity to important anatomical structures such as the optic chiasm, internal carotid arteries, and the cavernous sinuses. Tumor volume and shape are important in the diagnosis and staging of PTs 4 5 6 and patients with known PT are often followed many years with magnetic resonance imaging (MRI) to estimate tumor growth. Furthermore, the volume is an important factor in determining medical, surgical, and oncological intervention and has been shown to have an important prognostic value. 7 8 9
In common clinical practice, the size of a PT is often expressed as the largest diameter or three perpendicular measurements giving the tumors length, width, and height. Based on these measurements, the tumors are divided into microadenomas (largest diameter < 1 cm) and macroadenomas (largest diameter > 1 cm). 10 The volume of PTs can be approximated by conventional geometric equations (CGE) based on the tumor measurements. However, CGE assume a totally symmetric shape of the PTs, which rarely is the case. Three-dimensional (3D) segmentation takes the irregular and asymmetric shape of the PT into account and might be a more accurate method of tumor volume estimation. 11 However, this method is time consuming and might be user dependent.
A common CGE to estimate various types of intracerebral lesions, including brain tumors and intracerebral hemorrhages, is abc/2 (abc corresponding to length [a], width [b], and height [c] of the lesion). 12 13 14 15 16 17 This equation is initially derived from the formula of an ellipsoid, (length)*(width)*(height)*π/6 . If π is approximated to 3, the equation can be simplified as abc/2 . 18 Fig. 1 illustrates the shape of an ellipsoid. Another CGE, corresponding to the volume of a sphere ( (mean radius) 3 *4* π/3 ) might also be used to appreciate the volume of mass lesions. 19
Fig. 1.
Illustration of an ellipsoid. The abc/2 equation for volume estimation is derived from the equation for an ellipsoid's volume, (length)*(width)*(height)*π/6, by approximating π to 3. Picture published under the Creative Commons Attribution Share Alike 3.0 License, attained from Wikimedia Commons, author Peter Mercator.
During the past decade, different computer softwares have been developed to estimate the volume of PTs based on 3D voxel-based segmentation on MRI sequences. The segmentation can be performed manually or in a semi-/fully automatic way. Commonly used softwares for intracranial volumetry are ITK-SNAP , 20 3D-Slicer , 21 FreeSurfer , 22 and OsiriX . 23
In this study, we compared the 3D volumetric segmentation with CGE for estimating PT volumes and assessed inter-rater reliability for manual segmentation of PTs.
Materials and Methods
Patients and MRI Scans
Between January 2013 and October 2015, 126 trans-sphenoidal endoscopic pituitary operations were performed on 123 patients at the Sahlgrenska University Hospital, Gothenburg, Sweden. In 24 cases (19%), the patient underwent a gadolinium enhanced T1-weighted MRI (Sagittal 3D, Fast Field Echo, TR 8.3 milliseconds, TE 3.8 milliseconds, flip angle 8, number of slices 200, FOV 251 mm, image acquisition matrix 252 × 222 reconstructed to 560 × 560, acquired vx size 1 × 1 × 1 mm, reconstructed vx size 0.5 × 0.5 × 1) on a 3T Achieva dStream (Philips Medical Systems; Best, the Netherlands). The sequence was intended for neuronavigation during surgery.
Of these 24 patients, one case was excluded from the volumetric analysis due to disturbing artifacts on the MRI after a previous PT operation. Three other cases were excluded because of poor image quality, i.e., movement artifacts. One examination was lost in the image storing process. The remaining 19 examinations were included in this study.
Volume Segmentation
All 3D volumetric segmentations were performed independently by two certified neuroradiologists (A.K., D.Z.) blinded from each other. The DICOM image series were imported to the open-source software ITK-SNAP software (Version 3.4.0, University of Pennsylvania, United States). Using the paintbrush mode in the ITK-SNAP software, the tumor tissue was manually outlined in each axial and/or coronal image slice, excluding encased vessels and intact pituitary tissue. The volume of each PT was automatically calculated in ITK-SNAP in the “volume and statistics” window, where the value represents the product of the number of segmented voxels multiplied by the voxel volume. Fig. 2 shows the workspace within ITK-SNAP.
Fig. 2.
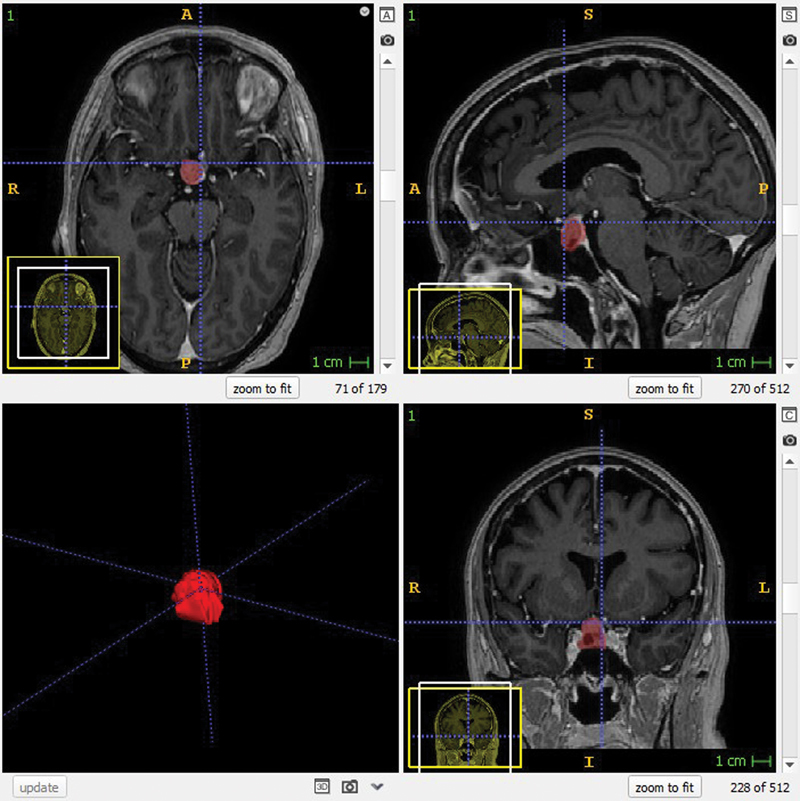
View of three-dimensional segmentation of a pituitary tumor. The tumor is marked in red on axial (upper left), sagittal (upper right), and coronal views (lower right) on magnetic resonance images.
Equations for Volume Approximation
Using the segmentations as overlay (i.e., the tissue the neuroradiologists interpreted as tumor) and the ruler tool in ITK-SNAP, the diameters of the PTs were measured by one clinician (K.L.). First, a line was drawn through the PT to measure the maximal diameter found in the axial slices (a, length). In the same slice, a line perpendicular to (a) marked the largest width (b). The height (c) was measured by multiplying the number of slices with visible tumor tissue by the slice thickness (1 mm).
All diameters were estimated twice in each case and a mean value was calculated for (a), (b), and (c).
PT volumes were generated according to the equations:
abc/2
4πr 3 /3 (sphere, r = the mean radii of the measurements a, b, and c).
Statistical Analysis
The systematic differences between the segmented volumes and the volumes attained from equations 1 and 2 were tested by the Wilcoxon Signed rank test. A mean value of the two segmented volumes for each PT was used. All tests were two tailed and conducted at 0.05 significance level.
The agreement for volumetric segmentation between our two raters was described by 95% confidence interval (CI) as limits of agreement, intra-individual SD (IISD) and by intra-class correlation coefficient (ICC, Shrout–Fleiss, random set ICC 1 2 ) and graphically presented by a Bland–Altman plot. IISD was interpreted as the difference between subject's measurement and the true value was expected to be less than 1.96*IISD.
Results
There was a significant difference between 3D volumetric segmentations and the volumes attained by abc/2 ( p < 0.001) and 4πr 3 /3 ( p < 0.001), respectively. The mean volume calculated by abc/2 was 18% larger and the mean volume calculated by 4πr 3 /3 was 28% larger than the mean volume attained from the 3D volumetric segmentations.
The mean volume calculated with 4πr 3 /3 was 9% larger ( p < 0.001) than the mean volume calculated with abc/2.
The data are presented in Table 1 and illustrated in Fig. 3 .
Table 1. Patients' age, sex, and pituitary tumor are presented. Measurements from pituitary tumors presurgery and results from volumetric segmentation and volume estimation by abc/2 and 4πr 3 /3 equations are listed .
| Age at op. | Sex | Diameters (a,b,c), mm | Knosp classification, dx; sin |
Segmentation, cm 3 (mean) | abc/2 , cm 3 | 4πr 3 /3 , cm 3 |
|---|---|---|---|---|---|---|
| 61 | M | 20.3, 12.5, 11.0 | 0, 1 | 1.06 | 1.39 | 1.63 |
| 56 | M | 17.6, 13.5, 12.0 | 1, 0 | 1.07 | 1.43 | 1.56 |
| 55 | F | 19.8, 13.4, 11.0 | 2, 0 | 1.15 | 1.46 | 1.67 |
| 30 | M | 14.4, 11.7, 19.0 | 0, 0 | 1.52 | 1.60 | 1.78 |
| 62 | M | 18.8, 13.3, 15.0 | 0, 1 | 1.58 | 1.88 | 2.03 |
| 68 | F | 22.0, 17.2, 22.5 | 2, 1 | 3.34 | 4.25 | 4.55 |
| 63 | F | 24.3, 15.8, 19.5 | 0, 1 | 3.47 | 3.74 | 4.11 |
| 68 | M | 27.0, 21.3, 21.0 | 2, 0 | 4.11 | 6.02 | 6.44 |
| 69 | F | 26.3, 20.1, 21.0 | 2, 1 | 4.17 | 5.54 | 5.92 |
| 34 | F | 25.8, 22.3, 26.0 | 0, 2 | 5.55 | 7.48 | 7.89 |
| 61 | M | 31.2, 23.1, 29.5 | 3a, 1 | 7.52 | 10.63 | 11.41 |
| 74 | M | 25.5, 18.0, 30.5 | 2, 2 | 7.56 | 7.00 | 7.86 |
| 55 | F | 28.6, 21.2, 34.0 | 3a, 1 | 9.09 | 10.30 | 11.40 |
| 74 | F | 32.5, 23.5, 36.0 | 1, 1 | 10.01 | 13.75 | 15.11 |
| 58 | M | 28.8, 22.1, 32.0 | 1, 1 | 10.15 | 10.18 | 11.04 |
| 83 | M | 35.5, 22.7, 28.0 | 3a, 3a | 10.37 | 11.29 | 12.43 |
| 36 | M | 35.3, 24.3, 37.0 | 2, 0 | 13.96 | 15.91 | 17.52 |
| 60 | M | 30.4, 29.7, 43.0 | 3a, 2 | 18.63 | 19.43 | 21.27 |
| 11 | M | 49.1, 38.9, 49.0 | 1, 4 | 38.30 | 46.75 | 49.82 |
| Mean | 8.03 | 9.48 | 10.29 | |||
| p -Value | <0.001 | <0.001 |
Fig. 3.
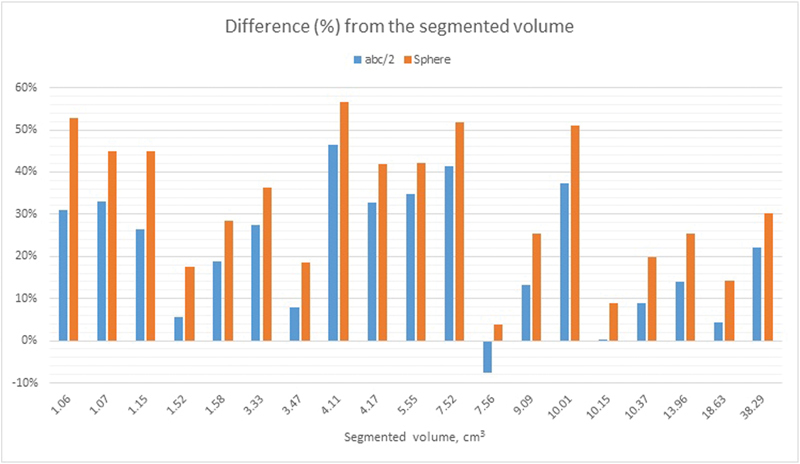
The diagram illustrates the difference (in %) between the volumes attained by segmentations and the equations abc/2 and 4πr 3 /3. The x-axis shows the segmented volume (the mean value of two independent segmentations per PT, performed by two certified neuroradiologists) of the pituitary tumors in increasing order. PT, pituitary tumor.
No significant difference was found between the segmentations by our two raters ( p = 0.541). The mean difference in volume between the two raters was − 36.4 mm 3 (limits of agreement − 943.4– 870.6). IISD was 319.52. ICC was 0.99. Fig. 4 shows the difference in the segmentations between the two raters in a Bland–Altman plot.
Fig. 4.
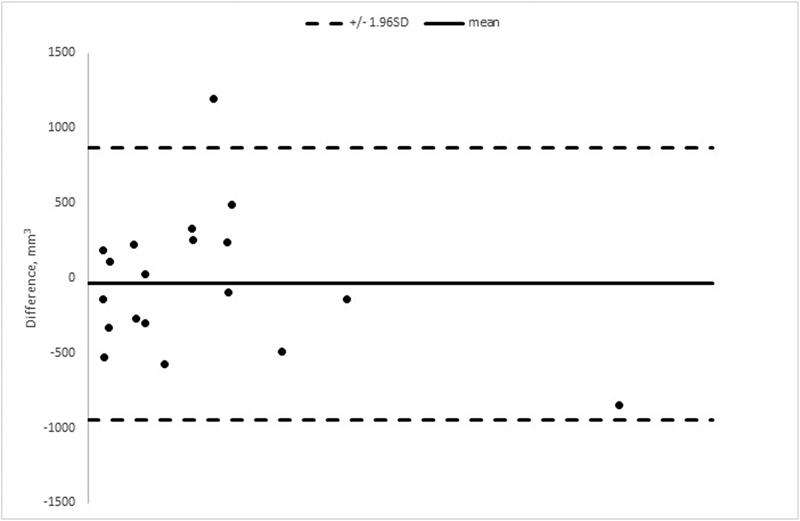
The Bland–Altman plot showing the difference in measured pituitary tumor volume in 19 patients. The volumetric segmentations were performed independently by two certified neuroradiologists.
Discussion
This study shows that the pituitary tumor volumes estimated by CGE are significantly larger in comparison to the results of 3D volumetric segmentations, which is in agreement with a recent study. 11 One reason for this difference ought to be that CGE assumes a symmetric shape of PTs, while segmentation accounts for the frequent asymmetric and irregular appearance of PTs. Therefore, 3D volumetric segmentation is believed to represent a closer estimate of the true PT volume compared with CGE.
Previous studies evaluating volumetric measurements using the abc/2 equation for cerebral hemorrhages have shown diverse results. 12 18 24 25 Yu et al showed a high correlation between abc/2 and manual slice-by-slice segmentation for volumetric assessment of acoustic neuromas. 26 Sreenivasan et al similarly found good agreement between abc/2 and segmentation for smaller masses. However, for larger tumors this correlation was poorer, 27 which is in concordance with our study that only included pituitary macroadenomas, i.e., tumors with the largest diameter > 1cm.
Davies et al recently compared PT volumes calculated by an ellipsoid equation with volumes attained from manual slice-by-slice segmentation 28 and found a good correlation between the two methods. However, the correlation decreased with increased tumor size. In contrary to our results, in their study the ellipsoid equation tended to underestimate the volume compared with segmentation for larger PTs. These divergent results could partially be due to different methods of measuring the diameters (a), (b), and (c). Further, the slice thickness of the MRI scans is an important variable and is not mentioned by Davies et al.
An assumption may be that PTs with strict intrasellar growth (Knosp grade: 1–2) have a more symmetric tumor shape compared with PTs that extend into the cavernous sinus (Knosp grade: 3–4). As CGEs assume a symmetrical tumor shape, CGE estimated volumes of Knosp grade 1–2 may be closer to the segmented volumes compared with Knosp grade 3–4 tumors, which may be more irregular in shape. In this study, 14 patients had a Knosp grade 1–2 tumor and 5 patients had a Knosp grade 3–4 tumor.
For Knosp grade 1–2 tumors, abc/2 overestimated the volumes by 22% compared with the segmentations; for Knosp grade 3–4 tumors, abc/2 overestimated the volumes by 18% compared with the segmentations. For Knosp grade 1–2 tumors, 4πr 3 /3 overestimated the volumes by 34% compared with the segmentations; for Knosp grade 3–4 tumors, 4πr 3 /3 overestimated the volumes by 28% compared with the segmentations. However, when comparing the overestimation of PT volumes by abc/2 or by 4πr 3 /3 there was no significant difference between Knosp grade 1–2 and Knosp grade 3–4 tumors.
The 3D volumetric segmentations in this study were performed using ITK-SNAP, a well-documented software that has been used in numerous research articles for volumetric segmentation. 29 30 31 32 The inter-rater agreement of the 3D segmentations was very high suggesting that it is a reproducible method for volume estimation. In general, our two observers had a good agreement per PT. The relative difference in volume tended to be larger for the smaller PTs and, considering that our study consists of macroadenomas, it is possible that there is a considerable intervariability for 3D segmentation of microadenomas. Previously John et al evaluated the inter-rater reliability for manual segmentation of the superior, inferior, and middle frontal gyri with a high correlation between their observers. 33
The MRI scans we used were of high resolution with thin slice thickness (1 mm) and voxel sizes of ≤ 1 mm 3 . MRI with considerably higher slice thickness is common. Luft et al examined the impact of slice thickness on MRI-based segmentation by using Ni-doped agarose gel phantoms with known volume and concluded that volumetric error positively correlated to the slice thickness. 34
Software segmentation most likely results in a more accurate volume estimation for PTs compared with CGEs, but it requires initial learning and is time consuming. ITK-SNAP and similar modern segmentation software provide various algorithms to segment structures automatically. In this study, we initially tried to segment the PTs automatically in ITK-SNAP but observed that nearby structures (carotids, intact pituitary gland, and parts of the cavernous sinuses) were erroneously included by the automatic segmentations.
Reliable automatic/semiautomatic segmentation is, due to time restraints, a prerequisite for a viable introduction of volumetric segmentation into clinical routine. It is currently an important field of research. Boers et al presented a fully automatic method for segmentation of subarachnoid hemorrhage on whole brain CT scans and validated it by comparing it to manual segmentation done in ITK SNAP 2.4.0 on 30 patients. 35 Gaonkar et al proposed a method of semiautomatic tumor segmentation based on geodesic distance transform. 36 Ambarki et al evaluated an automatic method to measure intracranial volume in the software SyMRI and found it reliable. 37 Qui et al developed an algorithm for volumetric measurement of the ventricles with good results. 38
Segmentation of PTs, using high-quality MRI scans, may be a good estimate of the true PT volume as compared with traditional geometrical equations. However, the true PT volume can only be attained by anatomical dissection or by measurement of the tumor volume resected in the operating room. The volume and shape of PTs are important variables that can directly affect the surgical and radiosurgical treatment. Therefore, fast and precise volumetric techniques can facilitate the surgical and radiosurgical planning in the management of patients with PT. Our findings highlight the need for further research as well as further development of volumetric techniques.
Conclusion
CGE ( abc/2 and 4πr 3 /3 ) significantly overestimates PT volume compared with 3D volumetric segmentation. The inter-rater agreement on manual 3D volumetric software segmentation is excellent.
CGE can be a time-effective way to get an approximation of the PT volume, but based on the inaccuracy of CGE compared with 3D segmentation found in this study, we would not recommend the use of CGE to estimate PT volume.
Funding Statement
Funding None.
Conflict of Interest None.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
The study was approved by the Regional Ethical Review Board in Gothenburg (No. 2015:100–15).
Informed Consent
Formal consent for this type of study is not required.
References
- 1.Ostrom Q T, Gittleman H, Farah P et al. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2006-2010. Neuro-oncol. 2013;15 02:ii1–ii56. doi: 10.1093/neuonc/not151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johannesen T B, Angell-Andersen E, Tretli S, Langmark F, Lote K. Trends in incidence of brain and central nervous system tumors in Norway, 1970-1999. Neuroepidemiology. 2004;23(03):101–109. doi: 10.1159/000075952. [DOI] [PubMed] [Google Scholar]
- 3.Materljan E, Materljan B, Sepcić J, Tuskan-Mohar L, Zamolo G, Erman-Baldini I. Epidemiology of central nervous system tumors in Labin area, Croatia, 1974-2001. Croat Med J. 2004;45(02):206–212. [PubMed] [Google Scholar]
- 4.Hardy J. New York, NY: Elsevier; 1973. Transsphenoidal surgery of hypersecreting pituitary tumors; pp. 179–194. [Google Scholar]
- 5.Di Ieva A, Rotondo F, Syro L V, Cusimano M D, Kovacs K. Aggressive pituitary adenomas--diagnosis and emerging treatments. Nat Rev Endocrinol. 2014;10(07):423–435. doi: 10.1038/nrendo.2014.64. [DOI] [PubMed] [Google Scholar]
- 6.Knosp E, Steiner E, Kitz K, Matula C.Pituitary adenomas with invasion of the cavernous sinus space: a magnetic resonance imaging classification compared with surgical findings Neurosurgery 19933304610–617.; discussion 617–618 [DOI] [PubMed] [Google Scholar]
- 7.Mortini P, Losa M, Barzaghi R, Boari N, Giovanelli M.Results of transsphenoidal surgery in a large series of patients with pituitary adenoma Neurosurgery 200556061222–1233.; discussion 1233 [DOI] [PubMed] [Google Scholar]
- 8.Milker-Zabel S, Debus J, Thilmann C, Schlegel W, Wannenmacher M. Fractionated stereotactically guided radiotherapy and radiosurgery in the treatment of functional and nonfunctional adenomas of the pituitary gland. Int J Radiat Oncol Biol Phys. 2001;50(05):1279–1286. doi: 10.1016/s0360-3016(01)01535-8. [DOI] [PubMed] [Google Scholar]
- 9.Schwyzer L, Starke R M, Jane J A, Jr, Oldfield E H. Percent reduction of growth hormone levels correlates closely with percent resected tumor volume in acromegaly. J Neurosurg. 2015;122(04):798–802. doi: 10.3171/2014.10.JNS14496. [DOI] [PubMed] [Google Scholar]
- 10.Heaney A P, Fernando M, Yong W H, Melmed S. Functional PPAR-γ receptor is a novel therapeutic target for ACTH-secreting pituitary adenomas. Nat Med. 2002;8(11):1281–1287. doi: 10.1038/nm784. [DOI] [PubMed] [Google Scholar]
- 11.Chohan M O, Levin A M, Singh R et al. Three-dimensional volumetric measurements in defining endoscope-guided giant adenoma surgery outcomes. Pituitary. 2016;19(03):311–321. doi: 10.1007/s11102-016-0709-2. [DOI] [PubMed] [Google Scholar]
- 12.Wang C W, Juan C J, Liu Y J et al. Volume-dependent overestimation of spontaneous intracerebral hematoma volume by the ABC/2 formula. Acta Radiol. 2009;50(03):306–311. doi: 10.1080/02841850802647039. [DOI] [PubMed] [Google Scholar]
- 13.Pekarek L A, Starr B A, Toledano A Y, Schreiber H. Inhibition of tumor growth by elimination of granulocytes. J Exp Med. 1995;181(01):435–440. doi: 10.1084/jem.181.1.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee Y, Auh S L, Wang Y et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood. 2009;114(03):589–595. doi: 10.1182/blood-2009-02-206870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flaherty M L, Tao H, Haverbusch M et al. Warfarin use leads to larger intracerebral hematomas. Neurology. 2008;71(14):1084–1089. doi: 10.1212/01.wnl.0000326895.58992.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paluzzi A, Fernandez-Miranda J C, Tonya Stefko S, Challinor S, Snyderman C H, Gardner P A. Endoscopic endonasal approach for pituitary adenomas: a series of 555 patients. Pituitary. 2014;17(04):307–319. doi: 10.1007/s11102-013-0502-4. [DOI] [PubMed] [Google Scholar]
- 17.Karppinen A, Kivipelto L, Vehkavaara S et al. Transition From Microscopic to Endoscopic Transsphenoidal Surgery for Nonfunctional Pituitary Adenomas. World Neurosurg. 2015;84(01):48–57. doi: 10.1016/j.wneu.2015.02.024. [DOI] [PubMed] [Google Scholar]
- 18.Kothari R U, Brott T, Broderick J P et al. The ABCs of measuring intracerebral hemorrhage volumes. Stroke. 1996;27(08):1304–1305. doi: 10.1161/01.str.27.8.1304. [DOI] [PubMed] [Google Scholar]
- 19.Nobels F R, de Herder W W, van den Brink W M et al. Long-term treatment with the dopamine agonist quinagolide of patients with clinically non-functioning pituitary adenoma. Eur J Endocrinol. 2000;143(05):615–621. doi: 10.1530/eje.0.1430615. [DOI] [PubMed] [Google Scholar]
- 20.Yushkevich P A, Piven J, Hazlett H C et al. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage. 2006;31(03):1116–1128. doi: 10.1016/j.neuroimage.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 21.Fedorov A, Beichel R, Kalpathy-Cramer J et al. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn Reson Imaging. 2012;30(09):1323–1341. doi: 10.1016/j.mri.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reuter M, Schmansky N J, Rosas H D, Fischl B. Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage. 2012;61(04):1402–1418. doi: 10.1016/j.neuroimage.2012.02.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosset A, Spadola L, Ratib O. OsiriX: an open-source software for navigating in multidimensional DICOM images. J Digit Imaging. 2004;17(03):205–216. doi: 10.1007/s10278-004-1014-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gebel J M, Sila C A, Sloan M A et al. Comparison of the ABC/2 estimation technique to computer-assisted volumetric analysis of intraparenchymal and subdural hematomas complicating the GUSTO-1 trial. Stroke. 1998;29(09):1799–1801. doi: 10.1161/01.str.29.9.1799. [DOI] [PubMed] [Google Scholar]
- 25.Huttner H B, Steiner T, Hartmann M et al. Comparison of ABC/2 estimation technique to computer-assisted planimetric analysis in warfarin-related intracerebral parenchymal hemorrhage. Stroke. 2006;37(02):404–408. doi: 10.1161/01.STR.0000198806.67472.5c. [DOI] [PubMed] [Google Scholar]
- 26.Yu Y L, Lee M S, Juan C J, Hueng D Y. Calculating the tumor volume of acoustic neuromas: comparison of ABC/2 formula with planimetry method. Clin Neurol Neurosurg. 2013;115(08):1371–1374. doi: 10.1016/j.clineuro.2012.12.029. [DOI] [PubMed] [Google Scholar]
- 27.Sreenivasan S A, Madhugiri V S, Sasidharan G M, Kumar R V. Measuring glioma volumes: A comparison of linear measurement based formulae with the manual image segmentation technique. J Cancer Res Ther. 2016;12(01):161–168. doi: 10.4103/0973-1482.153999. [DOI] [PubMed] [Google Scholar]
- 28.Davies B M, Carr E, Soh C, Gnanalingham K K. Assessing size of pituitary adenomas: a comparison of qualitative and quantitative methods on MR. Acta Neurochir (Wien) 2016;158(04):677–683. doi: 10.1007/s00701-015-2699-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang H, Schneider T, Wheeler-Kingshott C A, Alexander D C. NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain. Neuroimage. 2012;61(04):1000–1016. doi: 10.1016/j.neuroimage.2012.03.072. [DOI] [PubMed] [Google Scholar]
- 30.Mosconi M W, Cody-Hazlett H, Poe M D, Gerig G, Gimpel-Smith R, Piven J. Longitudinal study of amygdala volume and joint attention in 2- to 4-year-old children with autism. Arch Gen Psychiatry. 2009;66(05):509–516. doi: 10.1001/archgenpsychiatry.2009.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shi F, Fan Y, Tang S, Gilmore J H, Lin W, Shen D. Neonatal brain image segmentation in longitudinal MRI studies. Neuroimage. 2010;49(01):391–400. doi: 10.1016/j.neuroimage.2009.07.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yushkevich P A, Avants B B, Pluta J et al. A high-resolution computational atlas of the human hippocampus from postmortem magnetic resonance imaging at 9.4 T. Neuroimage. 2009;44(02):385–398. doi: 10.1016/j.neuroimage.2008.08.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.John J P, Wang L, Moffitt A J, Singh H K, Gado M H, Csernansky J G.Inter-rater reliability of manual segmentation of the superior, inferior and middle frontal gyri Psychiatry Res 2006148(2–3):151–163. [DOI] [PubMed] [Google Scholar]
- 34.Luft A R, Skalej M, Welte D, Kolb R, Klose U. Reliability and exactness of MRI-based volumetry: a phantom study. J Magn Reson Imaging. 1996;6(04):700–704. doi: 10.1002/jmri.1880060421. [DOI] [PubMed] [Google Scholar]
- 35.Boers A M, Zijlstra I A, Gathier C S et al. Automatic quantification of subarachnoid hemorrhage on noncontrast CT. Am J Neuroradiol. 2014;35(12):2279–2286. doi: 10.3174/ajnr.A4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gaonkar B, Macyszyn L, Bilello M et al. Automated tumor volumetry using computer-aided image segmentation. Acad Radiol. 2015;22(05):653–661. doi: 10.1016/j.acra.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ambarki K, Lindqvist T, Wåhlin A et al. Evaluation of automatic measurement of the intracranial volume based on quantitative MR imaging. Am J Neuroradiol. 2012;33(10):1951–1956. doi: 10.3174/ajnr.A3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qiu W, Yuan J, Rajchl M et al. 3D MR ventricle segmentation in pre-term infants with post-hemorrhagic ventricle dilatation (PHVD) using multi-phase geodesic level-sets. Neuroimage. 2015;118:13–25. doi: 10.1016/j.neuroimage.2015.05.099. [DOI] [PubMed] [Google Scholar]


